Abstract
We present a 42-year-old man with a 1-month history of painless jaundice, dark urine, clay-colored stools, and a 13.5-kg weight loss. Laboratory tests revealed elevated liver enzymes and CA19-9. Imaging showed dilation of both the intra- and extrahepatic bile ducts, narrowing of the bile duct at the junction of the common bile duct and common hepatic duct, and a hypoechoic mass involving the neck of the gallbladder and the muscularis propria of the duodenum. Examination of the resected gallbladder and perihilar nodes ruled out malignancy and revealed a diffuse inflammatory infiltrate of giant histiocytes with clear, lipid-containing cytoplasm (xanthoma cells), consistent with xanthogranulomatous cholecystitis.
Introduction
Xanthogranulomatous cholecystitis (XGC) is a rare condition that causes severe chronic inflammation of the gallbladder and is thought to be caused by an inflammatory response to extravasated bile from blocked or ruptured Rokitansky-Aschoff sinuses.1,2 Despite being a benign disease, it progressively invades adjacent organs mimicking biliary tract malignancy that often leads to unnecessary radical surgery.1,2
Case Report
A 42-year-old otherwise healthy man presented with 1-month history of painless jaundice, dark urine, claycolored stools, and a 13.5-kg weight loss. He denied significant alcohol use or recent exposure to hepatotoxic drugs. Physical examination was remarkable for scleral icterus, jaundice, and excoriations on his extremities. Laboratory work-up revealed elevation of aspartate aminotransferase (118 U/L), alanine aminotransferase (265 U/L), alkaline phosphatase (282 U/L), total bilirubin (6.1 U/L), conjugated bilirubin (4.3 U/L), and CA 19-9 (381 U/mL).
Abdominal ultrasound showed a 2.7-cm gallstone impacted in the gallbladder neck, with dilation of both the intra- and extrahepatic bile ducts. Endoscopic retrograde cholangiopancreatogram (ERCP) revealed narrowing of the bile duct at the junction of the common bile duct and common hepatic duct with dilation of the biliary tree proximal to the narrowing (Figure 1). Mirizzi syndrome was suspected and a magnetic resonance cholangiopancreatography (MRCP) showed an enhancing, infiltrative, mass-like process centered on the neck of the gallbladder and the cystic duct. An endoscopic ultrasound (EUS) demonstrated a 2.6 x 1.6-cm hypoechoic mass involving the neck of the gallbladder and the muscularis propria layer of the duodenum, raising suspicion of a gastrointestinal stromal tumor (Figure 2). There was a 1-cm lymph node in the periportal region. Fine-needle aspiration (FNA) was performed on both the mass and the lymph node. Cytology results from the mass showed chronic inflammation with numerous histiocytes, and cytology results from the lymph node showed benign lymph node tissue.
Figure 1.
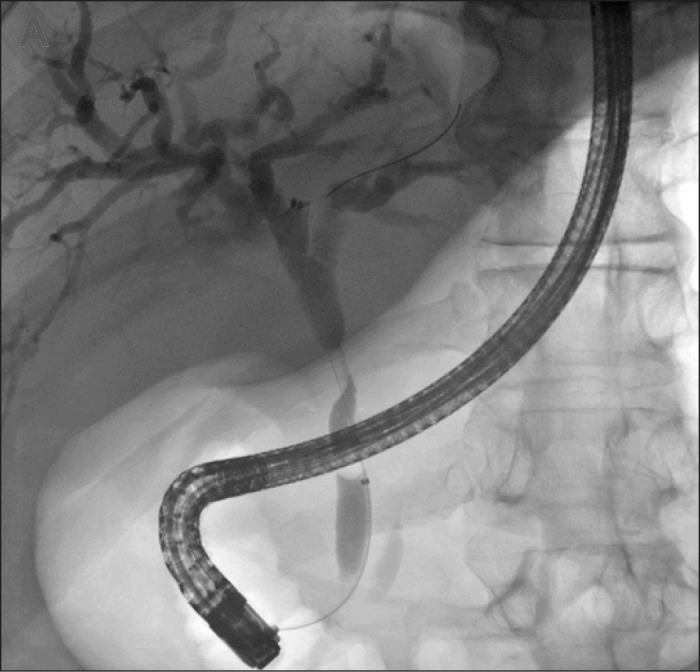
ERCP of bile duct narrowing at the junction of the common bile and common hepatic ducts, with dilation of the biliary tree proximal to the narrowing.
Figure 2.
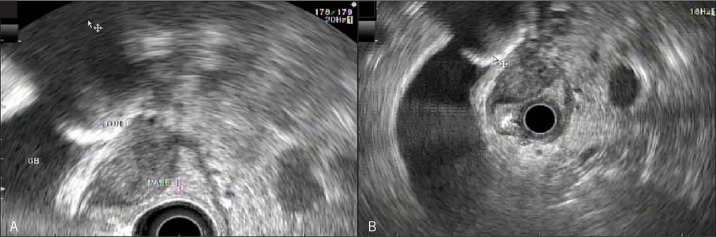
EUS view of hypoechoic mass involving (A) the neck of the gallbladder and (B) the muscularis propria layer of the duodenum.
Due to the atypical appearance of the mass and diagnostic uncertainty, an exploratory laparotomy was performed. Intraoperative frozen section of the resected gallbladder and perihilar nodes revealed chronic inflammation and fibrosis without malignancy, and further resection was not performed. Final pathologic evaluation revealed a diffuse inflammatory infiltrate of giant histiocytes with clear, lipid-containing cytoplasm (xanthoma cells) consistent with xanthogranulomatous tumor-like inflammation (Figure 3).
Figure 3.
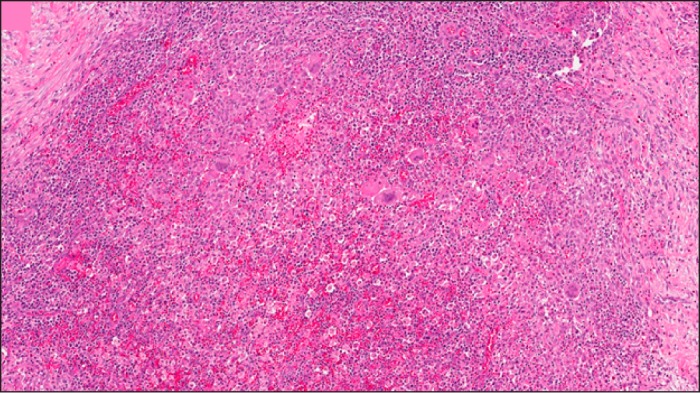
Pathologic examination of resected gallbladder showing a diffuse inflammatory infiltrate of giant histiocytes with clear, lipid-containing cytoplasm (xanthoma cells).
Discussion
Clinically differentiating XGC from gallbladder cancer is difficult, as both can have a similar presentation with jaundice and weight loss. CA 19-9 has been shown to be elevated in XGC, thus is not a reliable marker to differentiate XGC from cancer.3,4 Both computed tomography (CT) and magnetic resonance imaging (MRI) have not been shown to be useful in differentiating XGC from cancer.5
EUS with FNA is useful if results are positive for malignancy, but negative results leave diagnostic uncertainty. Additionally, if cholangiocarcinoma is of concern, performing FNA on the primary mass may remove potential treatment options due to risk of tumor seeding, and caution must be used in making this decision.6 Treatment of choice for XGC is cholecystectomy, but because gallbladder cancer has been reported concomitantly in patients with XGC, frozen section can help guide the optimum resection.7,8
Disclosures
Author contributions: All authors wrote and revised the manuscript. A. Bhatt is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
Previous Presentation: This case report was presented at the 2014 ACG Annual Scientific Meeting; October 17-22, 2014; Philadelphia, Pennsylvania.
References
- 1.Robert KM and Parsons MA. Xanthogranulomatous cholecystitis: Clinicopathological study of 13 cases. J Clin Pathol. 1987;40(4):412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang T, Zhang BH, Zhang J, et al. Surgical treatment of xanthogranulomatous cholecystitis: Experience in 33 cases. Hepatobiliary Pancreat Dis Int. 2007;6(5):504–508. [PubMed] [Google Scholar]
- 3.Yoshida J, Chijiiwa K, Shimura H, et al. Xanthogranulomatous cholecystitis versus gallbladder cancer: Clinical differentiating factors. Am Surg. 1997;63(4):367–371. [PubMed] [Google Scholar]
- 4.Adachi Y, Iso Y, Moriyama M, et al. Increased serum CA 19-9 in patients with xanthogranulomatous cholecystitis. Hepatogastroenterology. 1998;45(19):77–80. [PubMed] [Google Scholar]
- 5.Chun KA, Ha HK, Yu ES, et al. Xanthogranulomatous cholecystitis: CT features with emphasis on differentiation from gallbladder carcinoma. Radiology. 1997;203(1):93–7. [DOI] [PubMed] [Google Scholar]
- 6.Heimbach JK, Sanchez W, Rosen CB, Gores GJ. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB (Oxford). 2011;13:356–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodman ZD, Ishak KG. Xanthogranulomatous cholecystitis. Am J Surg Pathol. 1981;5(7):653–659. [DOI] [PubMed] [Google Scholar]
- 8.Kwon AH, Sakaida N. Simultaneous presence of xanthogranulomatous cholecystitis and gallbladder cancer. J Gastroenterol. 2007;42(8):703–704. [DOI] [PubMed] [Google Scholar]


