Abstract
Neurodegenerative diseases affect millions of people worldwide, and as the global population ages, there is a critical need to improve our understanding of the molecular and cellular mechanisms that drive neurodegeneration. At the molecular level, neurodegeneration involves the activation of complex signaling pathways that drive the active destruction of neurons and their intracellular components. Here, we use an in vivo motor neuron injury assay to acutely induce neurodegeneration in order to follow the temporal order of events that occur following injury in Drosophila melanogaster. We find that sites of injury can be rapidly identified based on structural defects to the neuronal cytoskeleton that result in disrupted axonal transport. Additionally, the neuromuscular junction accumulates ubiquitinated proteins prior to the neurodegenerative events, occurring at 24 hours post injury. Our data provide insights into the early molecular events that occur during axonal and neuromuscular degeneration in a genetically tractable model organism. Importantly, the mechanisms that mediate neurodegeneration in flies are conserved in humans. Thus, these studies have implications for our understanding of the cellular and molecular events that occur in humans and will facilitate the identification of biomedically relevant targets for future treatments.
Keywords: Drosophila melanogaster, neurodegeneration, neuronal injury, neuromuscular junction
Introduction
Neurodegenerative diseases are incurable and severely debilitating conditions resulting from the progressive degeneration of nerve cells. These diseases affect millions of individuals, and as the global population ages, it has been predicted by the World Health Organization that neurodegenerative diseases will overtake cancer to become the second leading cause of death.1 Extensive research efforts are aimed at tackling the challenges of finding causes, developing cures, and identifying ways to treat those with neurodegenerative diseases. In order to discover cures of neurodegenerative diseases, we must first understand the basic cellular and molecular mechanisms that occur during degeneration of nerve cells.
Neurodegeneration is a tightly controlled and well-orchestrated process resulting in the progressive and nonre-versible deterioration of neurons, often culminating in cell death. It requires the function of specific molecular programs that involve cell death machinery and is activated in response to injury, stress, or genetic lesions that induce neurodegenerative diseases.2 Similar mechanisms also control neurodegeneration during normal synaptic development. An outstanding challenge in the biomedical sciences is to understand the molecular mechanisms that drive neurodegeneration and to discover novel ways to prevent or suppress the progressive nature of neuronal elimination.
In vivo molecular research involving neurodegeneration and neuronal injury inevitably utilizes animal models and historically has focused on vertebrate systems. These models, such as the SOD1 mouse model and chronic sciatic nerve banding, often require complex genetic engineering or surgical manipulations, which have propelled researchers to explore neurodegeneration in more experimentally tractable model organisms, including Drosophila melanogaster. The molecular and cellular hallmarks of neurodegeneration in Drosophila and humans, including a disrupted cytoskeleton, defects in axonal transport, reduced firing capabilities, and subsequent deterioration of the neuron, are similar.3 Moreover, precise genetic lesions in homologous genes cause comparable disease phenotypes in human beings and flies. Examples include mutations in dynactin, which cause amyotrophic lateral sclerosis in humans and similar severe motor neuron degeneration in Drosophila and mutations in the spectrin/ankyrin skeleton causing motor neuron degeneration in Drosophila and Spinocerebellar ataxia 5 (SCA5) in humans.4–6 The ability to perform large systematic genetic screens in Drosophila has aided in the identification of genes involved in axon and synaptic degeneration.7,8 Drosophila has additionally facilitated in the identification of genes that suppress the neurodegenerative phenotype, which is of great biomedical relevance.9
Nerve cells do not undergo cell division and are therefore susceptible to injury via damage by pressure, stretching, or cutting. Neuronal injury can cause defects in axonal transport, synaptic transmission, and/or a complete loss of signal transmission from soma to nerve terminal. When nerve fibers are completely transected, the distal section undergoes sudden and catastrophic disintegration after an initial delay via a process called Wallerian degeneration. Although the timing of axonal fragmentation and disintegration is heterogeneous among the entire axon population, once initiated it is rapid and irreversible.9 Despite immense efforts directed at understanding the precise molecular pathways giving rise to Wallerian degeneration and ways to suppress it, we still do not know the full molecular mechanisms driving degeneration. Recently, Drosophila has emerged as a good model system to unravel the complex molecular events occurring after neuronal injury.10,11 However, there is no comprehensive analysis of the temporal order of events that occurs in Drosophila in response to injury that could provide the foundation for future screening/studies.
In this study, we report on an in vivo motor neuron injury assay in Drosophila, which reproducibly induces neurodegeneration by 24 hours post injury. Previously, a similar assay has been utilized to identify specific genes required for neurodegeneration and to dissect transcriptional responses to neuronal injury, but to our knowledge, a foundational temporal observation at the cellular level within axons and at the neuromuscular junction (NMJ) has not been conducted.12–14 In our assay, a simple mechanical injury of segmental nerves causes immediate impairment of the microtubule cytoskeleton and visible disruption of neuroglian, a cell adhesion protein known to play a role in stabilizing neurons.15 Approximately six hours after injury, there is a buildup of mitochondria indicative of axonal transport defects. Twelve hours post injury, we see an accumulation of ubiquitinated proteins at the NMJ that proceeds synaptic neurodegeneration, which is evident by 24 hours. Our overarching goal is to dissect the spatial and temporal cellular events that occur after neuronal injury, which will lay the framework for future studies in the identification of molecules that prevent or alleviate neurodegeneration.
Materials and Methods
Fly stocks
Drosophila were raised on standard culture media at 25 °C. Strains used in these studies include Oregon-R, w1118 (wild-type); elavC155-GAL416 (neuron-specific); and UAS-mito-GFP (w1118; P{UAS-mito-HA-GFP.AP}3, e1 stock #8442 from Bloomington Stock Center).
Neuronal injury and recovery
Second- or early third-instar larvae were injured according to the study by Xiong et al with the following modifications.12 Larvae were rinsed in cold saline to remove food debris and to decelerate motility. Under a dissecting microscope, larvae were carefully rolled onto their dorsal sides to visualize the segmental nerves through the cuticle prior to pinching ~1/3 of the dorsal cuticle containing the segmental nerves while taking care to avoid the majority of the ventral body wall musculature. After injury, larvae were transferred to a yeasted grape plate covered with a moist Kimwipe and kept alive for specified periods of time at 25 °C.
Imaging and analysis
Images were digitally captured using EZ-C1 Nikon software on a Nikon D-Eclipse C1 laser scanning confocal microscope and analyzed using Fiji software. Individual nerves and synapses were optically sectioned at 0.5 μm with a Plan Apo 100×/1.40 oil immersion objective. Using Fiji software, Z-stacks were combined into a single maximum projection image. Accumulations of ubiquitinated proteins were analyzed in Fiji using the Particle Analyzer function, set to pick up puncta >10 pixels in size. Two-tailed unpaired t-tests and nonparametric two-tailed Wilcoxon–Mann–Whitney tests were performed with 95% confidence intervals using GraphPad Software.
Immunohistochemistry
At 0, 6, 12, 24, and 48 hours after mechanical neuronal injury, larvae were dissected in phosphate-buffered saline and fixed in either 4% paraformaldehyde in PBS for 20 minutes to visualize green fluorescent protein or in Bouin’s fixative for two minutes for all other antibodies and stained according to standard procedures.8,17 Primary antibodies were used at the following dilutions: 1:100 anti-Bruchpilot (nc82; Developmental Studies Hybridoma Bank), 1:100 anti-BP104 (neuroglian; Developmental Studies Hybridoma Bank), 1:20 anti-Futsch (22C10; Developmental Studies Hybridoma Bank), 1:10,000 anti-Dlg18 (anti-discs large), and 1:500 anti-FK2 (Millipore). Secondary Alexa Fluor antibodies (goat anti-mouse 488, goat anti-rabbit 555, and goat anti-rabbit 647) were obtained from Jackson ImmunoResearch Laboratories, Inc. Cy3- and Cy5- conjugated horseradish peroxidase (HRP) were also obtained from Jackson ImmunoResearch Laboratories, Inc. and were used at a 1:300 dilution.
NMJ degeneration quantification
The neurodegenerative phenotype was analyzed according to standard protocols.8,17,19–23 Neurodegeneration at the NMJ was scored at 400× magnification with the observer being blind to the genotype and injury conditions. Muscle 6/7 of segments A2–A6 were quantified with a degenerative event being defined as an area of the NMJ containing clearly defined postsynaptic Dlg staining without any apposing presynaptic Brp staining. Two-tailed unpaired t-tests and nonparametric two-tailed Wilcoxon–Mann–Whitney tests were performed with 95% confidence intervals using GraphPad Software.
Larval motility movies
Drosophila third-instar larval motility was recorded in 30-second intervals using Moticam 2.0 software connected to a Moticam 3.0 MP digital camera mounted on a dissecting microscope set at 40× magnification. Using QuickTime player software, the video clips were cropped to demonstrate the crawling behavior of a single larva across one field of view.
Results
Mechanical injury causes immediate disruption of the microtubule cytoskeleton and the L1-type cell adhesion molecule neuroglian resulting in disrupted axonal transport
Neuronal injury was induced by mechanically damaging peripheral nerves in Drosophila larvae with size 5 forceps. To ensure that each animal experienced comparable mechanical injury, we first examined the characteristic crawling behavior of each larva. Uninjured larvae move by sequentially executing a single mouth hook extension followed by an initiation of a peristalsis-like body wall contraction, which drives the animal forward (Supplementary Movie 1). Immediately after mechanical injury, however, larvae showed distinctive paralysis of all body wall musculature posterior to the site of injury and were thus unable to initiate posterior body wall contractions (Supplementary Movie 2). Despite the severe paralysis, injured larvae went through normal pupation and were able to survive into adulthood.
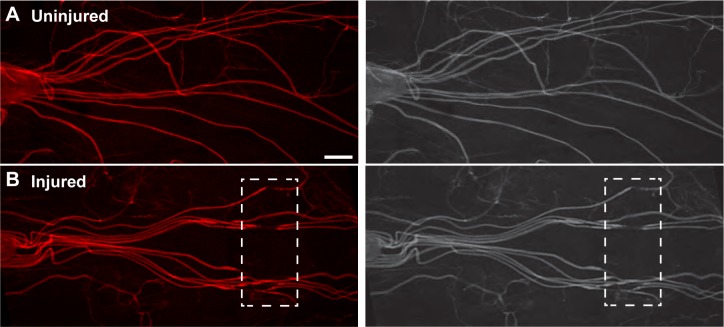
In Drosophila, antibodies against HRP act as specific neuronal membrane markers allowing for visualization of all neurons, including peripheral motor neuron axons.24 In uninjured third instar larvae, fluorescently conjugated HRP antibodies were employed to visualize the series of paired segmental nerves exiting both sides of the ventral nerve cord and extending along the length of the animal. Our neuronal injury assay resulted in damage to ~6–12 segmental nerves depending on the precise location of the crush site, as indicated by a reduction in the amount of HRP staining immediately after injury (Fig. 1, boxed area). Sites of injury range from ~100 to 200 μm in length.
Figure 1.
Mechanical injury of Drosophila larvae demonstrates damage to the majority of segmental peripheral nerves. (A) Fluorescently conjugated antibodies against HRP indicate intact segmental nerves in wild-type uninjured animals extending from the dorsal nerve cord down to their respective muscle targets. (B) After mechanical injury, HRP staining demonstrates ~8–12 damaged segmental nerves. The boxed region shows the location of the crush site. Scale bar = 100 μm.
Each segmental nerve in Drosophila third instar larvae contains between 60 and 80 individual axons that extend from their cell bodies, which reside in the ventral nerve cord.25 Motor neuron axons within segmental nerves can be visualized by staining for neuronal membranes using HRP and for axonal microtubules using antibodies against the Drosophila specific gene, Futsch (MAP1B). Futsch is expressed only in neuronal cells, has sequence similarity to vertebrate neurofilament proteins, and has been used extensively to visualize neuronal projections in Drosophila.26 Uninjured animals show numerous continuous axonal microtubules with intact neuronal membranes within each segmental nerve extending from the dorsal nerve cord. However, immediately after injury, both ends of each neuronal crush site contain severely disrupted axonal microtubules. Although some individual axonal microtubules extend farther into the crush site, the majority of microtubules become completely disintegrated as they extend into the epicenter of the neuronal crush site (Fig. 2).
Figure 2.
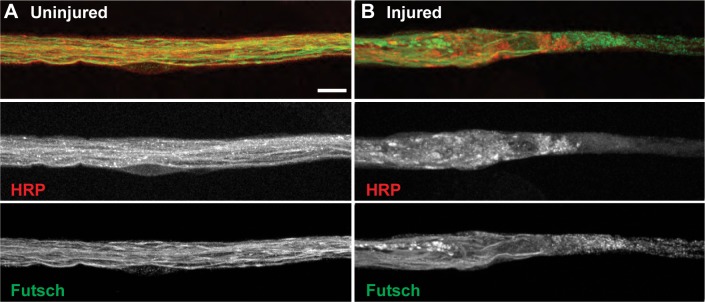
The microtubule cytoskeleton is severely disrupted upon mechanical injury to motor neurons. (A) Uninjured segmental nerves show continuous axonal microtubules (green, bottom panel) and neuronal membranes (red, middle panel) as shown by staining with antibodies against Futsch and HRP, respectively (n = 30 segmental nerves). (B) Mechanical injury of motor neurons induces disruption of both neuronal membranes (red, middle panel) and axonal microtubules (green, bottom panel; n = 55 injured segmental nerves). Scale bar = 10 μm.
In addition to severe disruption of the axonal microtubule cytoskeleton, neuroglian, an L1-type cell adhesion molecule (L1-CAM), is altered immediately after mechanical injury to segmental nerves.27,28 Drosophila neuroglian was first discovered in 1989 as an essential integral membrane protein localizing to neuronal cell bodies and all along neuronal axons and more recently has been shown to be essential for synaptic stabilization.28,29 In wild-type uninjured segmental nerves, neuroglian extends the length of each individual axon as visualized by colocalization with the neuronal membrane marker, HRP. However, after neuronal injury, there is a complete breakdown of the organized axonal neuroglian near the ends of each crush site, resulting in a complete obliteration of neuroglian within the actual injury site (Fig. 3).
Figure 3.
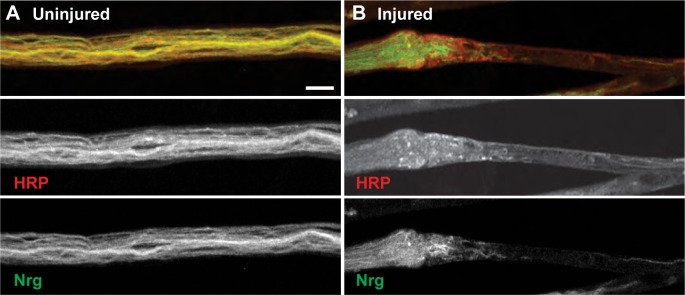
Injured segmental nerves display disordered neuroglian organization at sites of injury. (A) Uninjured segmental nerves show continuous axonal neuroglian staining (green, bottom panel) within the HRP-positive neuronal membranes (red, middle panel; n = 30 segmental nerves). (B) Segmental nerves that have been mechanically injured demonstrate severe disruption of both neuronal membranes (red, middle panel) and the neuron-specific protein neuroglian (green, bottom panel; n = 45 injured segmental nerves). Scale bar = 10 μm.
Severe disruption of microtubules within motor neuron axons prevents typical anterograde and retrograde axonal transportation. Interestingly, axonal transport defects are observed in, and thought to contribute to, the pathologies of neuronal injury and a diverse range of neurodegenerative diseases.30 Drosophila have long served as an excellent model system to study the mechanisms of axonal transport due to the presence of larval segmental nerves containing individual axons extending from the central nervous system down the length of the animal to their synaptic terminals along the body wall musculature.25,31 To examine the effects of mechanical injury on axonal transport, we focused on mitochondria, which are transported along larval motor axons in the anterograde direction by kinesin-1 and to the synapse in the retrograde direction by cytoplasmic dynein where they regulate synaptic strength.32,33 Uninjured segmental nerves contain a relatively uniform number of mitochondria dispersed along motor neuron axons as demonstrated by examining mitochondria tagged with green fluorescent protein (mito-GFP). However, after injury of segmental nerves, there is a buildup of mito-GFP at the proximal and distal ends of the crush site within six hours indicating that axonal transport is inhibited. Additionally, there appears to be no mito-GFP within the crush site itself at any point after the initial injury (Fig. 4). These results are consistent with a previous study in Drosophila demonstrating that neuronal injury causes vesicular cargo accumulations at sites of axonal injury with an absence of cargo within the crush site itself.12
Figure 4.
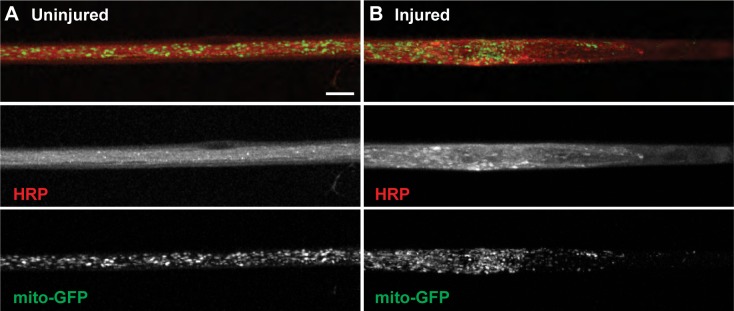
Mechanical injury of segmental nerves disrupts axonal transport and leads to a buildup of mitochondria at the proximal end of the crush site. (A) Uninjured animals show mitochondria (green, bottom panel) uniformly spread along the axons of segmental nerves within the neuronal membranes stained by HRP (red, middle panel; n = 65 segmental nerves). (B) Larvae that underwent mechanical injury show an absence of mitochondria within the crush site itself and a buildup of mitochondria at the proximal end of the crush site (n = 125 injured segmental nerves). Scale bar = 10 μm.
Ubiquitinated proteins accumulate at the NMJ ~12 hours after mechanical injury prior to neurodegeneration
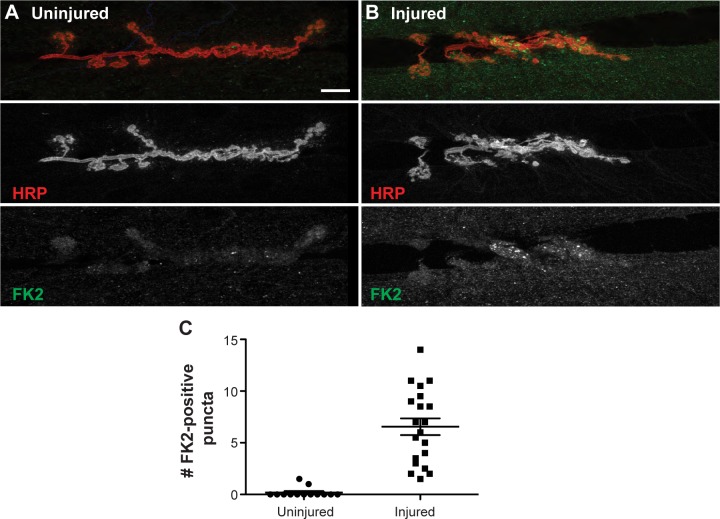
Ubiquitinated protein accumulations have long been known to be associated with neurodegenerative diseases.34 We sought to examine if neuronal injury of Drosophila motor neurons could induce increases in the amount of ubiquitinated proteins at the NMJ using an antibody (α-FK2) against both mono-and poly-ubiquitinated proteins. Quantification of numbers of FK2-positive puncta within the NMJ at muscle 6/7 revealed a significant increase 12 hours post injury. Uninjured larval NMJs contained an average of 0.19 puncta per synapse (n = 13 NMJs), whereas injured larvae had an average of 6.55 puncta per synapse (n = 20 NMJs; Fig. 5; P < 0.0001). These data are consistent with previous reports of autopsies from various human spinal cord traumas signifying that ubiquitin accumulations mark an early event in neuronal injury.33
Figure 5.
Injury to motor neuron axons induces a significant increase in ubiquitinated proteins at the NMJ. (A) The NMJs at muscle 6/7 in uninjured third instar larvae show well-defined neuronal membranes stained with HRP (red, middle panel) and very few accumulations of ubiquitinated proteins stained with FK2 (green, bottom panel). (B) Approximately 12 hours after neuronal injury, animals appear to have disrupted neuronal membranes (red, middle panel) and accumulations of ubiquitinated proteins at the NMJs of muscle 6/7 (green, bottom panel). (C) The number of FK2-positive puncta at each NMJ of muscle 6/7 was significantly increased 12 hours postneuronal injury from 0.1923 (n = 13 NMJs) to 6.55 (n = 20 NMJs; P < 0.0001). Error bars represent SEM. Scale bar = 10 μm.
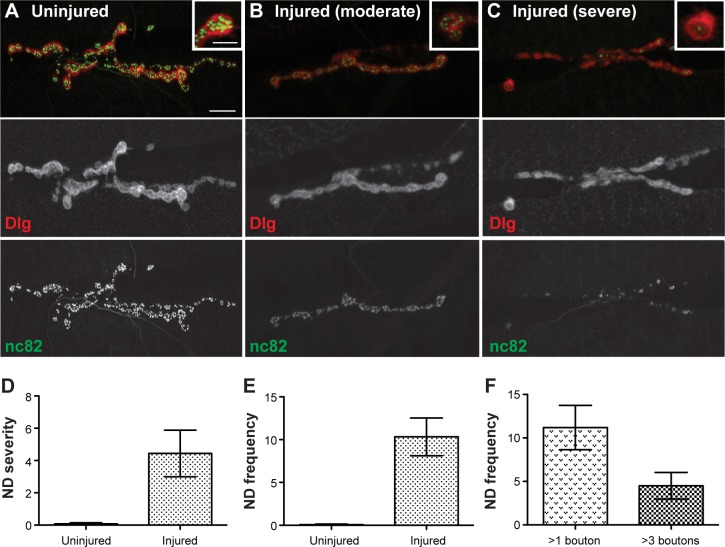
It has been previously reported in Drosophila that segmental nerve injury causes a significant loss of presynaptic vesicular glutamate transporter (VGlut) proteins at NMJ synapses.13 We wanted to examine if other presynaptic markers were lost after injury and to examine the extent of neurodegeneration using our previously established method for quantitatively investigating neurodegeneration at the Drosophila NMJ.2,17,19–23 In this assay, NMJs were stained for the presynaptic active zone marker Bruchpilot (Brp; stained with nc82 antibody) and the postsynaptic marker discs large (Dlg) at various time points after mechanical injury. Boutons clearly stained with Dlg but lacking nc82 immunoreactivity signify a neurodegenerative event. The NMJs from uninjured animals show the presynaptic marker Brp in perfect apposition with the postsynaptic marker Dlg (Fig. 6A). However, 24 hours post injury, NMJs exhibited various degrees of missing presynaptic Brp staining at the NMJ ranging from moderate neurodegeneration (<10 boutons retracted) to severe neurodegeneration (>10 boutons retracted; Fig. 6B and C). The highly compact muscle membrane folds that create the postsynaptic part of the NMJ degenerate more slowly then their presynaptic counterpart and thus persist beyond the neurodegenerative events that we are characterizing.19 The severity of neurodegeneration was quantified as the average number of boutons per NMJ exhibiting degeneration and was significantly higher in animals with neuronal injury (average = 4.44, n = 237 NMJs) compared to uninjured animals (average = 0.07, n = 140 NMJs; Fig. 6D; t-test: P = 0.030; significance remains with Wilcoxon–Mann–Whitney test: P = 0.0007). The frequency of neurodegeneration was quantified as the average percentage of NMJs per animal with degeneration and was determined to be significantly higher after neuronal injury in animals (average = 10.33, n = 25 animals) compared to in uninjured animals (average = 0.14, n = 14 animals; Fig. 6E; t-test: P = 0.0018; significance remains with Wilcoxon–Mann–Whitney test: P = 0.0016). To more fully understand the neurodegenerative frequency, we also measured the percentage of NMJs with degeneration of >1 bouton retracted (average = 11.20) and >3 boutons retracted (average = 4.49; Fig. 6F; t-test: P = 0.0286; significance remains with Wilcoxon–Mann–Whitney test: P = 0.0429). This neurodegenerative phenotype is less severe than what is seen by genetic modifications but is similar to previously published results examining the loss of presynaptic VGlut proteins at the NMJ after neuronal injury.2,13,17,19–23 Together, these data suggest a spatial and temporal sequence of cellular events originating at the site of axonal injury with immediate cytoskeletal defects inducing axonal transport dysfunction by six hours, followed by accumulations of ubiquitinated proteins by 12 hours and subsequent neurodegeneration at the NMJ by 24 hours (Supplementary Table 1).
Figure 6.
Neuronal injury induces moderate-to-severe neurodegeneration at the NMJ of muscle 6/7. (A) Wild-type uninjured NMJs show the presynaptic active zone maker Brp stained with nc82 (green, bottom panel) in apposition to the postsynaptic marker Dlg (red, middle panel) throughout the entire NMJ. (B) Neuronal injury can induce moderate neurodegeneration (<10 boutons retracted) in which the majority of the NMJ has Brp (green, bottom panel) and Dlg (red, middle panel) in perfect apposition. However, some of the boutons stained with Dlg lack the presynaptic active zone marker Brp. (C) Neuronal injury can also induce severe neurodegeneration in which >10 boutons lack Brp staining (green, bottom panel), suggesting that the neuron has retracted from the muscle as shown by the remaining postsynaptic Dlg staining without any accompanying Brp (red, middle panel). (D) Quantification of neurodegeneration severity was measured as the number of boutons per NMJ that were retracted (averages: uninjured = 0.0714 [n = 140 NMJs]; injured = 4.44 [n = 237 NMJs]; P = 0.030). (E–F) Quantification of neurodegeneration frequency was measured as the average percentage of NMJs with any retractions (E) or with >1 and >3 boutons retracted (F) (averages: uninjured = 0.1429; injured = 10.33; P = 0.0018). Error bars represent SEM. Scale bar = 10 μm. Inset scale bar = 5 μm.
Discussion
In humans, injury of peripheral motor neurons results in reduced sensation, reduction of innervation density, and often a very poor prognosis for functional outcomes. In order to develop novel therapeutic interventions following neuronal injury, we must first gain a better understanding of the cellular and molecular mechanisms leading to the observed neurodegenerative phenotypes. This work provides a spatial and temporal framework for the cellular events following neuronal injury in a simple reproducible Drosophila model system. We have demonstrated that after mechanical injury there is an immediate disruption of both microtubules and neuroglian followed by a buildup of mitochondria at six hours signifying axonal transport defects. Accumulations of ubiquitinated proteins at the NMJ occur by 12 hours, which proceeds neurodegeneration observed by 24 hours (Supplementary Table 1). Providing this initial groundwork will allow for a more comprehensive dissection of the molecular mechanisms triggering these processes and for the exploration of novel means to suppress the devastating outcomes resulting from neuronal injury.
The peripheral nervous system contains axons that extend great distances from their cell bodies and are therefore susceptible to damage in multiple cellular compartments. In most cases, injury of neurons occurs within axons but activates molecular signaling pathways at a distance (i.e. within the cell body or at the synaptic terminal). In humans, axonal injury is universally found in spinal and head trauma and has been recognized as a key predictor of patient prognosis.35 One of the major consequences of axonal injury is disruption of axonal transport, which contributes to the pathologies of many neurodegenerative diseases.30 In peripheral nerves, an intact axonal microtubule cytoskeleton is crucial for normal axonal transportation and synaptic transmission at downstream NMJs. In our model, mechanical injury induces an immediate disruption of the microtubule cytoskeleton extending the distance of the actual crush site itself. We observe, as others have, that a primary effect of axonal injury is the deformation of axonal microtubules resulting in the interruption of axonal transport and the buildup of transported materials within hours of trauma.36
In addition to immediate disturbance of the microtubule cytoskeleton, we find that neuroglian is also significantly inhibited at neuronal injury sites. Neuroglian, an L1-CAM, is required for the stabilization of basic neuronal cytoskeletal architecture and for maintenance of the overall stability of neurons and dendrites in Drosophila.15,28,37,38 Mutations in the human L1-CAM gene are also responsible for a range of neurodegenerative disorders, including spastic paraplegia type I.39,40 In Drosophila, neuroglian acts as key regulator of synapse stability in both the central and peripheral nervous systems by linking neuronal membrane proteins to the underlying spectrin skeleton.28 Interestingly, spectrin has previously been demonstrated to be essential for synaptic stability and lack of spectrin results in severe neurodegeneration at NMJs.21,41 It is therefore formally plausible that mechanical injury and subsequent disruption of neuroglian may result in neurodegeneration simply due to the disruption of spectrin at the injury site.
Accumulations of ubiquitinated proteins are known to be a common characteristic of many human neurodegenerative diseases; however, the mechanistic role remains elusive.42,43 It remains unclear if these protein aggregates are toxic or rather are a byproduct of neurons sequestering toxic or damaged proteins.44 In healthy neurons, rapid degradation by the ubiquitin-proteasome system (UPS) prevents high levels of ubiquitinated proteins from accumulating and it has been suggested that neuronal injury and neurodegenerative diseases have reduced UPS activity due to the presence of toxic microenvironments and/or mitochondrial dysfunction.45 Although aggregations of ubiquitinated proteins proceed neurodegeneration in certain genetic backgrounds, the delayed accumulation of ubiquitinated proteins at a distance from the injury site prior to synaptic degeneration is interesting and to our knowledge has not been previously described.46,47 It is known that inhibition of the UPS prevents developmental axon pruning and delays injury-induced axon degeneration, possibly through stabilization of disrupted microtubules.48,49 The UPS, and thus protein turnover, is known to be under precise control at synapses to regulate processes ranging from synaptogenesis to plasticity and remodeling.50 Our simple genetic model for neuronal injury may provide a framework from which to determine: 1) how and why ubiquitinated proteins accumulate at the NMJ after axonal injury, 2) if axonal injury disrupts the UPS or if it is functioning properly but overloaded due to the shear number of damaged proteins, and 3) whether ubiquitinated protein accumulations at the NMJ can serve as a biomarker for neurodegeneration.
This research lays the groundwork for future studies involving the molecular and cellular basis of neurodegeneration after motor neuron injury. Despite the apparent time-scale differences between slowly progressing human neurodegeneration and our acute neuronal injury model, we believe that the model presented here may produce comparable pathological changes as that of humans. Subsequent studies using this simple, genetically tractable model system to study axonal injury will hopefully shed light on numerous aspects that are still unclear such as the involvement and activation of glia, the role of calcium and mitochondria, and the role neurodegeneration plays in muscle atrophy. Recent technological advances in microfluidics may aid in answering these and other remaining questions related to axonal injury and the subsequent degeneration at the NMJ.14
Conclusion
Together our findings and those of others identify Drosophila as an excellent genetically tractable model system to study motor neuron injury and neurodegeneration. The ability to temporally examine the cellular deterioration of neurons after injury provides insight into the molecular mechanisms that drive the degenerative process. In particular, our study highlights the accumulation of ubiquitinated proteins prior to degeneration, which may offer an early indication of neurons that are in peril. We hope that gaining an understanding of how neurons degenerate may also assist in the discovery of novel ways to suppress the active degenerative signaling process. There is precedence for suppression of degeneration in Drosophila motor neurons, which someday may extend to novel therapeutic discoveries in humans.8
Supplementary Materials
Supplementary movie 1. A third instar wild-type larva has a characteristic crawling motility in which a single mouth hook extension is followed by a body wall contraction propelling the larva forward.
Supplementary movie 2. A third instar wild-type larva shortly after being subjected to neuronal injury. The characteristic crawling motility is severely disrupted with the posterior third of the larva unable to perform body wall contractions.
Supplementary table 1. Temporal sequence of cellular events leading to neurodegeneration after mechanical injury at the NMJ.
Acknowledgments
We would like to thank all members of the Keller and Magie Labs at Quinnipiac University for helpful experimental suggestions and for providing a stimulating and interactive laboratory environment for undergraduate research. For fly lines, we thank the Bloomington Stock Center (Indiana University), and for antibodies, we thank the Developmental Studies Hybridoma Bank (University of Iowa).
Footnotes
ACADEMIC EDITOR: Lora Talley Watts, Editor in Chief
PEER REVIEW: Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1,319 words, excluding any confidential comments to the academic editor.
FUNDING: This work was supported by an internal Quinnipiac University Grant-in-Aid to LCK and Dr. Lani Keller’s CAS Grant-in-Aid, 2012–2014. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: BLL and LCK. Analyzed the data: BLL, SHA, and LCK. Wrote the first draft of the manuscript: LCK. Contributed to the writing of the manuscript: LCK. Agreed with manuscript results and conclusions: BLL, SHA, NF, RF, and LCK. Jointly developed the structure and arguments for the paper: BLL, SHA, NF, RF, and LCK. Made critical revisions and approved the final version: BLL, SHA, NF, RF, and LCK. All the authors reviewed and approved the final manuscript.
REFERENCES
- 1.Gammon K. Neurodegenerative diseases: brain windfall. Nature. 2014;515(7526):299–300. doi: 10.1038/nj7526-299a. [DOI] [PubMed] [Google Scholar]
- 2.Keller LC, Cheng L, Locke CJ, Muller M, Fetter RD, Davis GW. Glial-derived prodegenerative signaling in the Drosophila neuromuscular system. Neuron. 2011;72(5):760–775. doi: 10.1016/j.neuron.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Driscoll M, Gerstbrein B. Dying for a cause: invertebrate genetics takes on human neurodegeneration. Nat Rev Genet. 2003;4(3):181–194. doi: 10.1038/nrg1018. [DOI] [PubMed] [Google Scholar]
- 4.Puls I, Jonnakuty C, LaMonte BH, et al. Mutant dynactin in motor neuron disease. Nat Genet. 2003;33(4):455–456. doi: 10.1038/ng1123. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda Y, Dick KA, Weatherspoon MR, et al. Spectrin mutations cause spinocerebellar ataxia type 5. Nat Genet. 2006;38:184–190. doi: 10.1038/ng1728. [DOI] [PubMed] [Google Scholar]
- 6.Lorenzo DN, Li MG, Mische SE, Armbrust KR, Ranum LP, Hays TS. Spectrin mutations that cause spinocerebellar ataxia type 5 impair axonal transport and induce neurodegeneration in Drosophila. J Cell Biol. 2010;189(1):143–158. doi: 10.1083/jcb.200905158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neukomm LJ, Burdett TC, Gonzalez MA, Zuchner S, Freeman MR. Rapid in vivo forward genetic approach for identifying axon death genes in Drosophila. Proc Natl Acad Sci U S A. 2014;111(27):9965–9970. doi: 10.1073/pnas.1406230111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valakh V, Naylor SA, Berns DS, DiAntonio A. A large-scale RNAi screen identifies functional classes of genes shaping synaptic development and maintenance. Dev Biol. 2012;366(2):163–171. doi: 10.1016/j.ydbio.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman MP, Freeman MR. Wallerian degeneration, wld(s) and nmnat. Annu Rev Neurosci. 2010;33:245–267. doi: 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang Y, Bonini NM. Axon degeneration and regeneration: insights from Drosophila models of nerve injury. Annu Rev Cell Dev Biol. 2012;28:575–597. doi: 10.1146/annurev-cellbio-101011-155836. [DOI] [PubMed] [Google Scholar]
- 11.Rooney TM, Freeman MR. Drosophila models of neuronal injury. ILAR J. 2004;54(3):291–295. doi: 10.1093/ilar/ilt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiong X, Wang X, Ewanek R, Bhat P, DiAntonio A, Collins CA. Protein turnover of the Wallenda/DLK kinase regulates a retrograde response to axonal injury. J Cell Biol. 2010;191(1):211–223. doi: 10.1083/jcb.201006039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brace EJ, Chunlai W, Vera V, DiAntonio A. SkpA restrains synaptic terminal growth during development and promotes axonal degeneration following injury. J Neurosci. 2014;34(25):8398–8410. doi: 10.1523/JNEUROSCI.4715-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra B, Ghannad-Rezaie M, Li J, et al. Using microfluidics chips for live imaging and study of injury responses in Drosophila larvae. J Vis Exp. 2014;84:50998. doi: 10.3791/50998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Wang Y, Wong JJL, et al. The endocytic pathways downregulate the L1–type cell adhesion molecule neuroglian to promote dendrite pruning in Drosophila. Dev Cell. 2014;30:463–478. doi: 10.1016/j.devcel.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Lin DM, Goodman CS. Ectopic and increased expression of Fasciclin II alters motoneuron growth cone guidance. Neuron. 1994;13(3):507–523. doi: 10.1016/0896-6273(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 17.Massaro CM, Pielage J, Davis GW. Molecular mechanisms that enhance synapse stability despite persistent disruption of the spectrin/ankyrin/microtubule cytoskeleton. J Cell Biol. 2009;187:101–117. doi: 10.1083/jcb.200903166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lahey T, Goczyca M, Jia XX, Budnik V. The Drosophila tumor suppressor gene dlg is required for normal synaptic bouton structure. Neuron. 1994;13(4):823–835. doi: 10.1016/0896-6273(94)90249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eaton BA, Fetter RD, Davis GW. Dynactin is necessary for synapse stabilization. Neuron. 2002;34(5):729–741. doi: 10.1016/s0896-6273(02)00721-3. [DOI] [PubMed] [Google Scholar]
- 20.Eaton BA, Davis GW. LIM kinase1 controls synaptic stabilities downstream of the type II BMP receptor. Neuron. 2005;47(5):695–708. doi: 10.1016/j.neuron.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Pielage J, Fetter RD, Davis GW. Presynaptic spectrin is essential for synapse stabilization. Curr Biol. 2005;15(10):918–928. doi: 10.1016/j.cub.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 22.Pielage J, Cheng L, Fetter RD, Carlton PM, Sedat JW, Davis GW. A presynaptic giant ankyrin stabilizes the NMJ through regulation of presynaptic microtubules and transsynaptic cell adhesion. Neuron. 2008;58(2):195–209. doi: 10.1016/j.neuron.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pielage J, Bulat V, Zuchero JB, Fetter RD, Davis GW. Hts/Adducin controls synaptic elaboration and elimination. Neuron. 2011;69(6):1114–1131. doi: 10.1016/j.neuron.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jan LY, Jan YN. Antibodies to horseradish peroxidase as specific neuronal markers in Drosophila and in grasshopper embryos. Proc Natl Acad Sci U S A. 1982;79(8):2700–2704. doi: 10.1073/pnas.79.8.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurd DD, Saxton WM. Kinesin mutations cause motor neuron disease phenotypes by disrupting fast axonal transport in Drosophila. Genetics. 1996;144(3):1075–1085. doi: 10.1093/genetics/144.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hummel T, Krukkert K, Roos J, Davis G, Klämbt C. Drosophila Futsch/22C10 is a MAP1B-like protein required for dendritic and axonal development. Neuron. 2000;26(2):357–370. doi: 10.1016/s0896-6273(00)81169-1. [DOI] [PubMed] [Google Scholar]
- 27.Godenschwege TA, Kristiansen LV, Uthaman SB, Hortsch M, Murphey RK. A conserved role for Drosophila neuroglian and human L1-CAM in central-synapse formation. Curr Biol. 2006;16(1):12–23. doi: 10.1016/j.cub.2005.11.062. [DOI] [PubMed] [Google Scholar]
- 28.Enneking EM, Kudumala SR, Moreno E, et al. Transsynaptic coordination of synaptic growth, function, and stability by the L1-type CAM neuroglian. PLoS Biol. 2013;11:e1001537. doi: 10.1371/journal.pbio.1001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bieber AJ, Snow PM, Hortsch M, et al. Drosophila neuroglian: a member of the immunoglobulin superfamily with extensive homology to the vertebrate neural adhesion molecular L1. Cell. 1989;59(3):447–460. doi: 10.1016/0092-8674(89)90029-9. [DOI] [PubMed] [Google Scholar]
- 30.Mandelkow E, Mandelkow EM. Kinesin motors and disease. Trends Cell Biol. 2002;12(12):585–591. doi: 10.1016/s0962-8924(02)02400-5. [DOI] [PubMed] [Google Scholar]
- 31.Lloyd TE. Axonal transport disruption in peripheral nerve disease: from Jack’s discoveries as a resident to recent contributions. J Peripher Nerv Syst. 2012;17(suppl 3):46–51. doi: 10.1111/j.1529-8027.2012.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuro-muscular junctions. Neuron. 2005;47(3):365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 33.Pilling AD, Horiuchi D, Lively CM, Saxton WM. Kinesin-1 and dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol Biol Cell. 2006;17(4):2057–2068. doi: 10.1091/mbc.E05-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dantuma NP, Bott LC. The ubiquitin-proteasome system in neurodegenerative diseases: precipitating factor, yet part of the solution. Front Mol Neurosci. 2014;7:70. doi: 10.3389/fnmol.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medana IM, Esiri MM. Axonal damage: a key predictor of outcome in human CNS diseases. Brain. 2003;126:515–530. doi: 10.1093/brain/awg061. [DOI] [PubMed] [Google Scholar]
- 36.Tang-Shomer MD, Johnson VE, Baas PW, Stewart W, Smith DH. Partial interruption of axonal transport due to microtubule breakage accounts for the formation of periodic varicosities after traumatic axonal injury. Exp Neurol. 2012;233(1):364–372. doi: 10.1016/j.expneurol.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong X, Liu OW, Howell AS, Shen K. An extracellular adhesion molecular complex patterns dendritic branching and morphogenesis. Cell. 2013;155:296–307. doi: 10.1016/j.cell.2013.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salzberg Y, Díaz-Balzac CA, Ramirez-Suarez NJ, et al. Skin-derived cues control arborization of sensory dendrites in Caenorhabditis elegans. Cell. 2013;155:308–320. doi: 10.1016/j.cell.2013.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kenwrick S, Watkins A, DeAngelis E. Neural cell recognition molecule L1: relating biological complexity to human disease mutations. Hum Mol Genet. 2000;9:879–886. doi: 10.1093/hmg/9.6.879. [DOI] [PubMed] [Google Scholar]
- 40.Manness PF, Schachner M. Neural recognition molecules of the immunoglobin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- 41.Hortsch M. Structural and functional evolution of the L1 family: are four adhesion molecules better than one? Mol Cell Neurosci. 2000;15:1–10. doi: 10.1006/mcne.1999.0809. [DOI] [PubMed] [Google Scholar]
- 42.Alves-Rodrigues A, Gregori L, Figueiredo-Pereira ME. Ubiquitin, cellular inclusions and their role in neurodegeneration. Trends Neurosci. 1998;21(12):516–520. doi: 10.1016/s0166-2236(98)01276-4. [DOI] [PubMed] [Google Scholar]
- 43.Ciechanover A, Brundin P. The ubiquitin proteasome system in neurodegenera-tive diseases: sometimes the chicken, sometimes the egg. Neuron. 2003;40(9):427–446. doi: 10.1016/s0896-6273(03)00606-8. [DOI] [PubMed] [Google Scholar]
- 44.Atkin G, Paulson G. Ubiquitin pathways in neurodegenerative disease. Front Mol Neurosci. 2014;7:63. doi: 10.3389/fnmol.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang Q, Figueiredo-Pereira ME. Ubiquitin/proteasome pathway impairment in neurodegeneration: therapeutic implications. Apoptosis. 2010;15(10):1292–1311. doi: 10.1007/s10495-010-0466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Komatsu M, Waguri S, Chiba T, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 47.Hara T, Nakamura K, Matsui M, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 48.Zhai Q, Wang J, Kim A, et al. Involvement of the ubiquitin-proteasome system in the early stages of Wallerian degeneration. Neuron. 2003;39:217–225. doi: 10.1016/s0896-6273(03)00429-x. [DOI] [PubMed] [Google Scholar]
- 49.Feldmeyer D, Lubke J. New Aspects of Axonal Structure and Function. London: Springer Science + Business Media, LLC; 2010. pp. 190–191. [Google Scholar]
- 50.Haas KF, Broadie K. Roles of ubiquitination at the synapse. Biochim Biophys Acta. 2008;1779(8):495–506. doi: 10.1016/j.bbagrm.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary movie 1. A third instar wild-type larva has a characteristic crawling motility in which a single mouth hook extension is followed by a body wall contraction propelling the larva forward.
Supplementary movie 2. A third instar wild-type larva shortly after being subjected to neuronal injury. The characteristic crawling motility is severely disrupted with the posterior third of the larva unable to perform body wall contractions.
Supplementary table 1. Temporal sequence of cellular events leading to neurodegeneration after mechanical injury at the NMJ.