Abstract
Background: Numerous studies have focused on the association between MMP-12-82A>G polymorphism and cancer risk, but produced inconsistent results. Therefore, we performed a meta-analysis of case-control study to evaluate the association of MMP-12-82A>G polymorphism and cancer risk. Methods: A systematic literature search was conducted among PubMed, Web of Science, Science Direct, China National Knowledge Infrastructure (CNKI) and Wangfang databases updated on May 1st, 2015. Crude odds ratios (ORs) with 95% confidence intervals (CIs) were used to evaluate the strength of association between this polymorphism and cancer risk. Results: A total of seventeen case-control studies with 7,450 cases and 7,348 controls were identified and analyzed. Overall, there was no statistically significant association between MMP-12-82A>G polymorphism and increased risk of cancer under all genetic models. Subgroup analysis by ethnicity observed that there is no strong relationship between MMP-12-82A>G polymorphism and cancer risk among Asian and European populations. Furthermore, stratified analysis based on the source of control revealed no statistically significant association between MMP-12-82A>G polymorphism and cancer risk either in hospital-based or population-based studies. However, when we stratified analysis based on cancer type, significant association was found in ovarian cancer, but not in other types of cancer. Conclusion: This meta-analysis suggests that MMP-12-82A>G polymorphism is not significantly associated with overall cancer risk. However, MMP-12-82A>G polymorphism may increase the susceptibility to ovarian cancer.
Keywords: Matrix metalloproteinases-12, polymorphism, cancer risk, meta-analysis
Introduction
Cancer is a leading cause of death and remains to be a major public health problem. About 14.1 million new cancer cases and 8.2 million cancer deaths occurred in 2012 worldwide [1]. It is well known that cancer is a complex disease involving multiple environmental factors and genetic factors. In particular, the association between genetic factors and cancer risk has been studied extensively, recently. Growing epidemiological evidence suggested that host genetic susceptibility plays a vital role in the pathogenesis of various types of cancer [2-4].
Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases that degrades virtually all extracellular matrix components [5]. Several types of MMPs are crucially involved in tumor invasion and metastasis [6-8]. Among them Matrix metalloproteinase-12 (MMP-12) plays an important role in cancer development and progression, including cancer cell growth, migration, invasion, and metastasis [9-11]. Moreover, it has been reported that MMP-12 is highly expressed in a wide range of human cancers, including colorectal cancer, gastric cancer, nasopharyngeal carcinoma and lung cancer [12-15]. In recent years, several single nucleotide polymorphisms (SNPs) in the promoter region of MMP-12 gene have been reported and the SNP of MMP-12-82A>G (rs2276109) is the most extensively studied. The MMP-12-82A>G polymorphism could affect transcriptional activity and lead to alterations in MMP-12 gene expression. A number of studies have focused on the association between MMP-12-82A>G polymorphism and cancer risk, but the results are controversial. Therefore, in this study we performed a meta-analysis to better evaluate the association between MMP-12-82A>G polymorphism and cancer risk.
Methods
Search strategy
To identify all the articles on the association between the MMP-12-82A>G polymorphism and cancer risk, a systematic literature search was performed on electronic databases of PubMed, Web of Science, Science Direct, CNKI and Wanfang databases updated on May 1st, 2015. The search terms used were as follows: “Matrix metalloproteinases-12 or MMP-12”, “SNP or variant or polymorphism” and “cancer or carcinoma or neoplasm”.
Inclusion and exclusion criteria
The following inclusion criteria were applied: (1) case-control studies; (2) investigating the association of MMP-12-82A>G polymorphism with cancer risk; (3) sufficient published data to calculate an odds ratio (OR) with a 95% confidence interval (CI) and P-value; (4) the distribution of genotypes among controls were consistent with the Hardy-Weinberg equilibrium.
The following exclusion criteria were applied: (1) not case-control studies; (2) studies without sufficient date and information; (3) reviews and repeated reports.
Data extraction
The data were extracted by two independent investigators according to the inclusion criteria. In case of conflicts, the disagreements were discussed and resolved with consensus. The following data were extracted from each study: first author’s name, publication year, ethnicity, total numbers of cases and controls, cancer type, source of controls (population-based controls and hospital-based controls), genotyping method, and Hardy-Weinberg equilibrium (HWE) of controls.
Statistical analysis
Odds ratios (ORs) and 95% confidence intervals (95% CIs) were used to estimate the strength of association between MMP-12-82A>G polymorphism and cancer risk for each study. Five different comparison models were calculated for each polymorphism: allele model, homozygote model, heterozygote model, recessive model, and dominant model. The Cochran’s chi-square Q statistic test and I2 statistics test were utilized to measure the potential heterogeneity among the studies. An I2 that represents the percentage value of less than 25% indicates “low”, value of 25% to 50% indicates “moderate”, and value of more than 50% indicates “high”. If P value for heterogeneity test was less than 0.05, ORs were calculated according to the random-effect model [16]. Otherwise, the fixed-effect model was used [17]. Sensitivity analyses were conducted by omitting each study to find potential outliers. The potential publication bias was assessed by Begg’s funnel plot and Egger’s test [18,19]. All statistical analyses were performed using the software STATA version 12.0 (StataCorp LP, College Station, Texas, USA).
Results
Characteristics of eligible studies
According to the search strategy, a total of seventeen relevant case-control studies [20-34] 7,450 cases and 7,348 controls were included in our meta-analysis. The search strategy was illustrated in Figure 1. The detailed characteristics of the included studies were shown in Table 1. Among the twenty studies, eleven studies were conducted in Asian populations and six studies were in European population. In the subgroup analysis by source of control, thirteen studies were performed in hospital-based controls and four were in population-based controls. There were three studies concerning lung cancer, three studies concerning esophageal cancer, two studies concerning colorectal cancer, two studies concerning gastric cancer, two studies concerning ovarian cancer, two studies concerning hepatocellular carcinoma, and the other studies concerning breast cancer, laryngeal carcinoma and bladder cancer. In addition, all the included studies met HWE.
Figure 1.
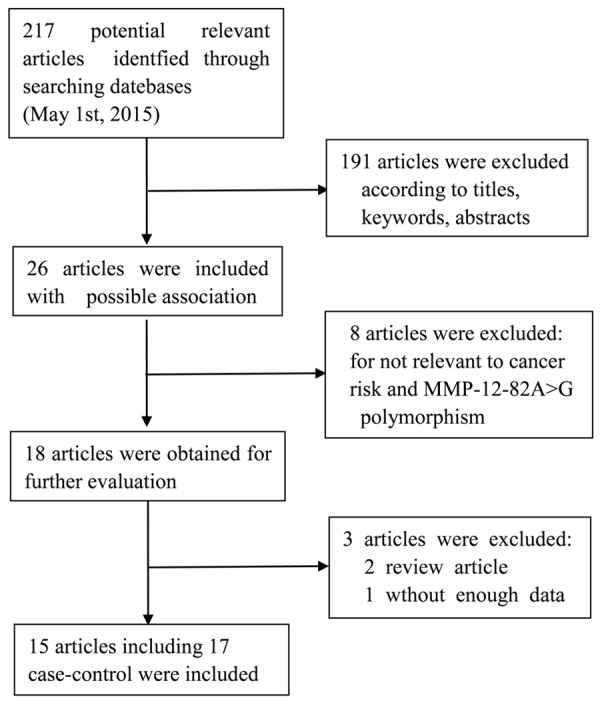
Search strategy of the studies included in this meta-analysis.
Table 1.
Characteristics of included studies in the meta-analysis
| First author [Ref.] | Year | Ethnicity | Cases/Controls | Cancer type | Source of control | Genotyping method | HWE |
|---|---|---|---|---|---|---|---|
| Shin [20] | 2005 | Asian | 1118/1223 | Breast cancer | PB | Taqman | 0.98 |
| Kader [21] | 2006 | European | 557/557 | Hepatocellular cancer | HB | Taqman | 0.06 |
| Su [22] | 2006 | European | 2014/1323 | Lung cancer | HB | Taqman | 0.32 |
| Woo [23] | 2007 | Asian | 185/304 | Colorectal cancer | PB | PCR-RFLP | 0.79 |
| Zhai [24] | 2007 | Asian | 433/480 | Hepatocellular cancer | HB | AS-PCR | 0.51 |
| Zhang-a [25] | 2008 | Asian | 316/609 | Esophageal cancer | PB | PCR-RFLP | 0.45 |
| Zhang-b [25] | 2008 | Asian | 243/609 | Gastric cancer | PB | PCR-RFLP | 0.45 |
| Li [26] | 2009 | Asian | 256/329 | Ovarian cancer | HB | PCR-RFLP | 0.76 |
| Li-a [28] | 2010 | Asian | 257/624 | Gastric cancer | HB | PCR-RFLP | 0.46 |
| Li-b [28] | 2010 | Asian | 335/624 | Esophageal cancer | HB | PCR-RFLP | 0.46 |
| Jia l [29] | 2010 | Asian | 300/300 | Ovarian cancer | HB | PCR-RFLP | 0.75 |
| Cheung [30] | 2012 | European | 309/279 | Esophageal cancer | HB | PCR | 0.90 |
| VAN [31] | 2013 | European | 385/619 | Colorectal cancer | HB | Taqman | 0.48 |
| Grudny [32] | 2013 | European | 53/54 | Lung cancer | HB | PCR-RFLP | 0.57 |
| Wieczorek [34] | 2013 | European | 241/199 | Bladder cancer | HB | PCR-RFLP | 0.35 |
| Wang [35] | 2013 | Asian | 300/300 | Lung cancer | HB | PCR-RFLP | 0.70 |
| Yang [36] | 2014 | Asian | 148/148 | Laryngeal carcinoma | HB | PCR-LDR | 0.87 |
Quantitative data synthesis
The results of the meta-analysis were listed in detail in Table 2. By pooling all eligible studies, MMP-12-82A>G polymorphism was not associated with increased cancer risk under all the five genetic models (allele model: OR=1.09, 95% CI=0.93-1.28, P=0.30; homozygous model: OR=1.14, 95% CI=0.78-1.67, P=0.48; heterozygous model: OR=1.06, 95% CI=0.89-1.26, P=0.54; recessive model: OR=1.15, 95% CI=0.79-1.68, P=0.46; dominant model: OR=1.08, 95% CI=0.91-1.28, P=0.41) (Figure 2). Even after stratified analyses based on ethnicity, we could not find significant relationship between MMP-12-82A>G polymorphism and increased cancer risk among Asian populations (allele model: OR=1.15, 95% CI=0.85-1.56, P=0.38; homozygous model: OR=1.98, 95% CI=0.32-12.09, P=0.46; heterozygous model: OR=1.15, 95% CI=0.83-1.60, P=0.40; recessive model: OR=1.99, 95% CI=0.33-12.16, P=0.46; dominant model: OR=1.15, 95% CI=0.84-1.59, P=0.38) and European populations (allele model: OR=1.00, 95% CI=0.90-1.11, P=0.98; homozygous model: OR=1.12, 95% CI=0.76-1.64, P=0.58; heterozygous model: OR=0.97, 95% CI=0.87-1.10, P=0.66; recessive model: OR=1.12, 95% CI=0.76-1.65, P=0.55; dominant model: OR=0.99, 95% CI=0.88-1.11, P=0.81) (Table 2). Furthermore, in the subgroup analyses based on source of control, we found no significant association between MMP-12-82A>G polymorphism and increased cancer risk in hospital-based (allele model: OR=1.16, 95% CI=0.96-1.41, P=0.13; homozygous model: OR=1.10, 95% CI=0.75-1.61, P=0.63; heterozygous model: OR=1.13, 95% CI=0.92-1.40, P=0.24; recessive model: OR=1.11, 95% CI=0.75-1.62, P=0.61; dominant model: OR=1.16, 95% CI=0.94-1.42, P=0.17) or population-based studies (allele model: OR=0.89, 95% CI=0.68-1.17, P=0.40; homozygous model: OR=4.34, 95% CI=0.48-38.88, P=0.19; heterozygous model: OR=0.83, 95% CI=0.63-1.10, P=0.19; recessive model: OR=4.39, 95% CI=0.49-39.32, P=0.19; dominant model: OR=0.86, 95% CI=0.65-1.13, P=0.28) (Table 2). However, when we stratified analysis by cancer type, strong association was observed in ovarian cancer (allele model: OR=2.44, 95% CI=1.45-4.08, P<0.01; heterozygous model: OR=2.50, 95% CI=1.48-4.22, P<0.01; dominant model: OR=2.50, 95% CI=1.48-4.22, P<0.01), but not in lung cancer, colorectal cancer, esophageal cancer, gastric cancer, hepatocellular carcinoma and other types of cancer (all P>0.05) (Table 2).
Table 2.
Stratified analysis of MMP-12-82A/G polymorphism and cancer risk
| G vs. A | GG vs. AA | GA vs. AA | GG vs. GA+AA | GG+GA vs. AA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| MMP-12-82A/G | OR (95% CI) | P a | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Overall | 1.09 (0.93-1.28) | 0.30 | 1.14 (0.78-1.67) | 0.48 | 1.06 (0.89-1.26) | 0.54 | 1.15 (0.79-1.68) | 0.46 | 1.08 (0.91-1.28) | 0.41 |
| Ethnicity | ||||||||||
| Asian | 1.15 (0.85-1.56) | 0.38 | 1.98 (0.32-12.09) | 0.46 | 1.15 (0.83-1.60) | 0.40 | 1.99 (0.33-12.16) | 0.46 | 1.15 (0.84-1.59) | 0.38 |
| European | 1.00 (0.90-1.11) | 0.98 | 1.12 (0.76-1.64) | 0.58 | 0.97 (0.87-1.10) | 0.66 | 1.12 (0.76-1.65) | 0.55 | 0.99 (0.88-1.11) | 0.81 |
| Source of control | ||||||||||
| HB | 1.16 (0.96-1.41) | 0.13 | 1.10 (0.75-1.61) | 0.63 | 1.13 (0.92-1.40) | 0.24 | 1.11 (0.75-1.62) | 0.61 | 1.16 (0.94-1.42) | 0.17 |
| PB | 0.89 (0.68-1.17) | 0.40 | 4.34 (0.48-38.88) | 0.19 | 0.83 (0.63-1.10) | 0.19 | 4.39 (0.49-39.32) | 0.19 | 0.86 (0.65-1.13) | 0.28 |
| Cancer type | ||||||||||
| Lung cancer | 1.21 (0.64-2.27) | 0.56 | 1.64 (0.17-15.48) | 0.66 | 1.03 (0.88-1.21) | 0.71 | 1.47 (0.19-11.32) | 0.72 | 1.16 (0.66-2.02) | 0.61 |
| Colorectal cancer | 0.90 (0.71-1.15) | 0.41 | 1.18 (0.51-2.74) | 0.69 | 0.83 (0.63-1.11) | 0.21 | 1.24 (0.54-2.86) | 0.61 | 0.86 (0.65-1.13) | 0.28 |
| Esophageal cancer | 0.87 (0.53-1.43) | 0.58 | 1.90 (0.47-7.69) | 0.37 | 0.85 (0.53-1.37) | 0.51 | 1.82 (0.45-7.35) | 0.40 | 0.86 (0.52-1.42) | 0.55 |
| Gastric cancer | 1.10 (0.72-1.68) | 0.66 | - | - | 1.10 (0.72-1.70) | 0.66 | - | - | 1.10 (0.72-1.70) | 0.66 |
| Ovarian cancer | 2.44 (1.45-4.08) | <0.01 | - | - | 2.50 (1.48-4.22) | <0.01 | - | - | 2.50 (1.48-4.22) | <0.01 |
| Hepatocellular carcinoma | 0.92 (0.73-1.15) | 0.46 | 1.75 (0.57-5.30) | 0.33 | 0.85 (0.66-1.10) | 0.20 | 1.83 (0.60-5.54) | 0.29 | 0.88 (0.68-1.12) | 0.29 |
| other cancer | 1.38 (0.75-2.53) | 0.30 | 3.08 (0.84-11.29) | 0.09 | 1.34 (0.66-2.73) | 0.42 | 3.06 (0.83-11.20) | 0.09 | 1.38 (0.70-2.72) | 0.35 |
OR odds ratio, CI confidence interval;
Random-effect model was used when P-value of Q-test for heterogeneity <0.05, otherwise fixed-effect model was used.
Figure 2.
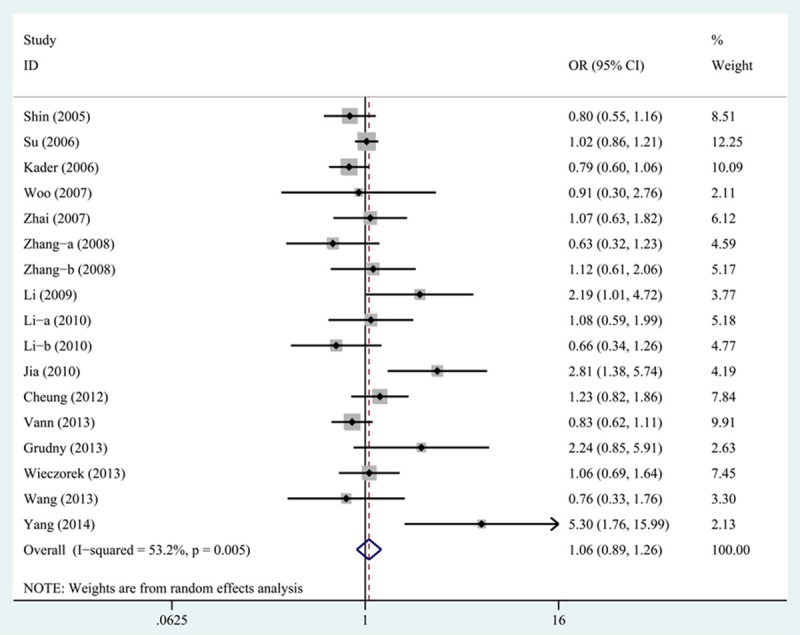
Forest plot of ORs for the association of MMP-12-82A>G polymorphism with cancer risk under heterozygous model (GA vs. AA).
Sensitivity analysis
The sensitivity analysis indicated that no individual study influenced the OR value of MMP-12-82A>G polymorphism. Thus, the results of our meta-analysis are statistically robust.
Publication bias
Begg’s funnel plot was performed to assess the publication bias of the selected articles. For MMP-12-82A>G polymorphism, the results of Begg’s funnel plot did not reveal any evidence of obvious asymmetry (Figure 3). Egger’s test also showed no significant evidence of publication bias (homozygous model: P=0.132).
Figure 3.
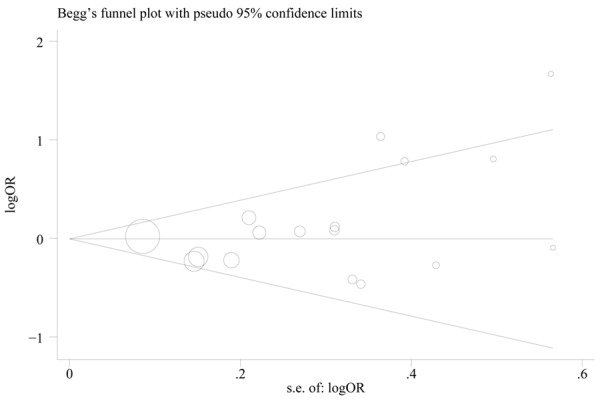
Begg’s funnel plot of the association between MMP-12-82A>G polymorphism and cancer risk under heterozygous model (GA vs. AA). Circles represent the weight of studies.
Discussion
In this meta-analysis, we retrieved seventeen case-control studies with 7,450 cases and 7,348 controls and systematically evaluated the association between -82A>G polymorphism in promoter region of MMP-12 and the risk of cancer. Overall, we observed that MMP-12-82A>G polymorphism was not significantly associated with increased risk of cancer under all genetic models. In addition, no matter when we stratified analysis by ethnicity or source of control, there was no strong relationship in any genetic models. However, when we stratified analysis by cancer type, significant association was identified in ovarian cancer, not in other types of cancer. Collectively, these data suggest that MMP-12-82A>G polymorphism is a genetic risk factor for developing ovarian cancer.
MMP-12 is a member of MMP family that is mainly produced by macrophages and inflammatory cells and is involved in tissue regeneration, wound repair, and the regulation of immune surveillance [35-37]. MMP-12 could cleave plasminogen and collagen XVIII, resulting in the generation of angiostatin and endostatin that exert angiostatic effects [38,39]. On the other side, MMP-12 could promote angiogenesis through cleaving diverse components of the extracellular matrix including collagen type IV and fibrin [40]. It has been suggested that MMP-12 is implicated in the processes of pro-tumorigenesis by inhibiting cancer cells apoptosis and promoting cancer cells invasion and migration [41,42]. Considering that the SNP of MMP-12-82A>G could affect the expression of MMP-12 and increase the risk of cancer, the association between MMP-12 promoter gene polymorphism and the risk of cancer has been a focus of recent studies.
Shin et al. reported no association between MMP-12-82A>G polymorphism and breast cancer risk [20]. Similarly, no association between MMP-12-82A>G polymorphism and cancer risk was reported in other types of cancer [21-24]. Nevertheless, Li et al. demonstrated that MMP-12-82A>G polymorphism was related to the risk of ovarian cancer [26]. Therefore, the relationship between MMP-12-82A>G polymorphism and cancer risk remains controversial. To the best of our knowledge, this is the first meta-analysis that comprehensively evaluated the effect of MMP-12-82A>G polymorphism on cancer risk. Our results showed that there was no significant association between MMP-12-82A>G polymorphism and cancer risk whether by stratified analysis based on ethnicity or the source of control, or by general analysis under all genetic models. Interestingly, in the stratified analysis based on cancer type, MMP-12-82A>G polymorphism might contribute to an increased susceptibility to ovarian cancer but not lung cancer, colorectal cancer, esophageal cancer, gastric cancer, hepatocellular carcinoma and other types of cancer. To a large extent, this discrepancy may be explained by the fact that different types of cancer have diverse mechanism of carcinogenesis. Another reason may be that the pathways of carcinogen metabolism are complicated and can be affected by a variety of lifestyle-related factors and environmental factors. Additionally, the size of cancer types is small, thus we are unable to detect a significant association in other types of cancer. Therefore, further studies with larger sample sizes in diverse cancers are needed to evaluate the association between MMP-12-82A>G polymorphism and cancer risk based on cancer types.
In our meta-analysis, no significant evidence of publication bias was observed, suggesting that our results are reliable. However, several limitations should be considered. First of all, the available data about MMP-12-82A>G consist of twenty case-control studies involving 7,450 cases and 7,348 controls, which may not provide sufficient power to explore the exact correlation. Hence, studies with larger sample sizes and representative population are warranted to validate the current findings. Second, our results were based on single-factor estimates, thus the association of MMP-12-82A>G polymorphism with cancer risk might be affected by other factors, such as the age, gender, family history and environmental factors. Third, only the published studies were included in this meta-analysis, the possible effect of unpublished studies should be considered.
In conclusion, the current meta-analysis suggests that MMP-12-82A>G polymorphism may not alter the risk of overall cancer, but contribute to an increased risk of ovarian cancer. However, comprehensive studies with larger sample sizes, especially involving many types of cancer, are necessary to confirm our findings.
Acknowledgements
This study was supported in part by the National Nature Science Foundation of China (Grant No. 81272466), Natural Science Foundation of Heilongjiang Province (Grant No. QC2011C037), and Research Fund of The First Affiliated Hospital of Harbin Medical University (Grant No. 2012B011).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.He YQ, Zhu JH, Huang SY, Cui Z, He J, Jia WH. The association between the polymorphisms of TNF-α and non-Hodgkin lymphoma: a meta-analysis. Tumour Biol. 2015;35:12509–17. doi: 10.1007/s13277-014-2569-6. [DOI] [PubMed] [Google Scholar]
- 3.Ding Q, Fan B, Fan Z, Ding L, Li F, Tu W, Jin X, Shi Y, Wang J. Interleukin-10-819C>T polymorphism contributed to cancer risk: evidence from 29 studies. Cytokine. 2013;61:139–45. doi: 10.1016/j.cyto.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Xu J, Yin Z, Cao S, Gao W, Liu L, Yin Y, Liu P, Shu Y. Systematic review and meta-analysis on the association between IL-1B polymorphisms and cancer risk. PLoS One. 2013;8:e63654. doi: 10.1371/journal.pone.0063654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–4. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 6.Muller D, Wolf C, Abecassis J, Millon R, Engelmann A, Bronner G, Rouyer N, Rio MC, Eber M, Methlin G, Chambon P, Basset P. Increased stromelysin 3 gene expression is associated with increased local invasiveness in head and neck squamous cell carcinomas. Cancer Res. 1993;53:165–9. [PubMed] [Google Scholar]
- 7.Ikebe T, Shinohara M, Takeuchi H, Beppu M, Kurahara S, Nakamura S, Shirasuna K. Gelatinolytic activity of matrix metalloproteinase in tumor tissues correlates with the invasiveness of oral cancer. Clin Exp Metastasis. 1999;17:315–23. doi: 10.1023/a:1006642428826. [DOI] [PubMed] [Google Scholar]
- 8.Imanishi Y, Fujii M, Tokumaru Y, Tomita T, Kanke M, Kanzaki J, Kameyama K, Otani Y, Sato H. Clinical significance of expression of membrane type 1 matrix metalloproteinase and matrix metalloproteinase-2 in human head and neck squamous cell carcinoma. Hum Pathol. 2000;31:895–904. doi: 10.1053/hupa.2000.9756. [DOI] [PubMed] [Google Scholar]
- 9.Nabha SM, dos Santos EB, Yamamoto HA, Belizi A, Dong Z, Meng H, Saliganan A, Sabbota A, Bonfil RD, Cher ML. Bone marrow stromal cells enhance prostate cancer cell invasion through type I collagen in an MMP-12 dependent manner. Int J Cancer. 2008;122:2482–90. doi: 10.1002/ijc.23431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng KT, Qi X, Kong KL, Cheung BY, Lo CM, Poon RT, Fan ST, Man K. Overexpression of matrix metalloproteinase-12 (MMP-12) correlates with poor prognosis of hepatocellular carcinoma. Eur J Cancer. 2011;47:2299–305. doi: 10.1016/j.ejca.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 11.Kim JM, Kim HJ, Koo BS, Rha KS, Yoon YH. Expression of matrix metalloproteinase-12 is correlated with extracapsular spread of tumor from nodes with metastasis in head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2013;270:1137–42. doi: 10.1007/s00405-012-2161-x. [DOI] [PubMed] [Google Scholar]
- 12.Zheng J, Chu D, Wang D, Zhu Y, Zhang X, Ji G, Zhao H, Wu G, Du J, Zhao Q. Matrix metalloproteinase-12 is associated with overall survival in Chinese patients with gastric cancer. J Surg Oncol. 2013;107:746–51. doi: 10.1002/jso.23302. [DOI] [PubMed] [Google Scholar]
- 13.Kahlert C, Pecqueux M, Halama N, Dienemann H, Muley T, Pfannschmidt J, Lasitschka F, Klupp F, Schmidt T, Rahbari N, Reissfelder C, Kunz C, Benner A, Falk C, Weitz J, Koch M. Tumour-site-dependent expression profile of angiogenic factors in tumour-associated stroma of primary colorectal cancer and metastases. Br J Cancer. 2014;110:441–9. doi: 10.1038/bjc.2013.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung IC, Chen LC, Chung AK, Chao M, Huang HY, Hsueh C, Tsang NM, Chang KP, Liang Y, Li HP, Chang YS. Matrix metalloproteinase 12 is induced by heterogeneous nuclear ribonucleoprotein K and promotes migration and invasion in nasopharyngeal carcinoma. BMC Cancer. 2014;14:348. doi: 10.1186/1471-2407-14-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lv FZ, Wang JL, Wu Y, Chen HF, Shen XY. Knockdown of MMP12 inhibits the growth and invasion of lung adenocarcinoma cells. Int J Immunopathol Pharmacol. 2015;28:77–84. doi: 10.1177/0394632015572557. [DOI] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 17.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 18.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin A, Cai Q, Shu XO, Gao YT, Zheng W. Genetic polymorphisms in the matrix metalloproteinase 12 gene (MMP12) and breast cancer risk and survival: the Shanghai breast cancer study. Breast Cancer Res. 2005;7:R506–12. doi: 10.1186/bcr1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kader AK, Shao L, Dinney CP, Schabath MB, Wang Y, Liu J, Gu J, Grossman HB, Wu X. Matrix metalloproteinase polymorphisms and bladder cancer risk. Cancer Res. 2006;66:11644–8. doi: 10.1158/0008-5472.CAN-06-1212. [DOI] [PubMed] [Google Scholar]
- 22.Su L, Zhou W, Asomaning K, Lin X, Wain JC, Lynch TJ, Liu G, Christiani DC. Genotypes and haplotypes of matrix metalloproteinase 1, 3 and 12 genes and the risk of lung cancer. Carcinogenesis. 2006;27:1024–9. doi: 10.1093/carcin/bgi283. [DOI] [PubMed] [Google Scholar]
- 23.Woo M, Park K, Nam J, Kim JC. Clinical implications of matrix metalloproteinase-1, -3, -7, -9, -12, and plasminogen activator inhibitor-1 gene polymorphisms in colorectal cancer. J Gastroenterol Hepatol. 2007;22:1064–70. doi: 10.1111/j.1440-1746.2006.04424.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhai Y, Qiu W, Dong XJ, Zhang XM, Xie WM, Zhang HX, Yuan XY, Zhou GQ, He FC. Functional polymorphisms in the promoters of MMP-1, MMP-2, MMP-3, MMP-9, MMP-12 and MMP-13 are not associated with hepatocellular carcinoma risk. Gut. 2007;56:445–7. doi: 10.1136/gut.2006.112706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang XJ, Wang N, Zhou RM, Dong XJ, Li Y. Correlation of MMP -12 polymorphism to risk of squamous cell carcinoma and gastric cardiac adenocarcinoma. Cancer Res Prev Treat. 2008;35:740–3. [Google Scholar]
- 26.Li Y, Jia JH, Kang S, Zhang XJ, Zhao J, Wang N, Zhou RM, Sun DL, Duan YN, Wang DJ. The functional polymorphisms on promoter region of matrix metalloproteinase-12, -13 genes may alter the risk of epithelial ovarian carcinoma in Chinese. Int J Gynecol Cancer. 2009;19:129–33. doi: 10.1111/IGC.0b013e31819a1d8e. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Sun DL, Duan YN, Zhang XJ, Wang N, Zhou RM, Chen ZF, Wang SJ. Association of functional polymorphisms in MMPs genes with gastric cardia adenocarcinoma and esophageal squamous cell carcinoma in high incidence region of north china. Mol Biol Rep. 2010;37:197–205. doi: 10.1007/s11033-009-9593-4. [DOI] [PubMed] [Google Scholar]
- 28.Jia J, Kang S, Zhao J, Zhang X, Wang N, Zhou R, Li Y. Association of functional polymorphisms on mmp-12 and mmp-13 gene promoter region with epithelial ovarian carcinoma. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2010;27:209–13. doi: 10.3760/cma.j.issn.1003-9406.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 29.Cheung WY, Zhai R, Bradbury P, Hopkins J, Kulke MH, Heist RS, Asomaning K, Ma C, Xu W, Wang Z, Hooshmand S, Su L, Christiani DC, Liu G. Single nucleotide polymorphisms in the matrix metalloproteinase gene family and the frequency and duration of gastroesophageal reflux disease influence the risk of esophageal adenocarcinoma. Int J Cancer. 2012;131:2478–86. doi: 10.1002/ijc.27541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.VAN Nguyen S, Skarstedt M, Löfgren S, Zar N, Andersson RE, Lindh M, Matussek A, Dimberg J. Gene polymorphism of matrix metalloproteinase-12 and -13 and association with colorectal cancer in Swedish patients. Anticancer Res. 2013;33:3247–50. [PubMed] [Google Scholar]
- 31.Grudny J, Kołakowski J, Kruszewski M, Szopiński J, Sliwiński P, Wiatr E, Winek J, Załęska J, Zych J, Roszkowski-Śliż K. Association of genetic dependences between lung cancer and chronic obstructive pulmonary disease. Pneumonol Alergol Pol. 2013;81:308–18. [PubMed] [Google Scholar]
- 32.Wieczorek E, Reszka E, Jablonowski Z, Jablonska E, Beata Krol M, Grzegorczyk A, Gromadzinska J, Sosnowski M, Wasowicz W. Genetic polymorphisms in matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs), and bladder cancer susceptibility. BJU Int. 2013;112:1207–14. doi: 10.1111/bju.12230. [DOI] [PubMed] [Google Scholar]
- 33.Wang W, Zeng H, Wang R. Association between the matrix metalloproteinase -12 gene polymorphism and susceptibility of non-small cell lung cancer in northern China. Med J NDFNC. 2013;34:301–3. [Google Scholar]
- 34.Yang Fei, Zhao ZJ, Zhao Z, Zhang J, Fu K, He YT, Jing SH. Association between polymorphism on MMP-12 and MMP-13 genes promoter region and genetic susceptibility to laryngeal squamous cell carcinoma. Chin J Cancer Prev Treat. 2014;21:1219–22. [Google Scholar]
- 35.Zhang H, Chang M, Hansen CN, Basso DM, Noble-Haeusslein LJ. Role of matrix metalloproteinases and therapeutic benefits of their inhibition in spinal cord injury. Neurotherapeutic. 2011;8:206–20. doi: 10.1007/s13311-011-0038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bellayr IH, Mu X, Li Y. Biochemical insights into the role of matrix metalloproteinases in regeneration: challenges and recent developments. Future Med Chem. 2009;1:1095–111. doi: 10.4155/fmc.09.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qu P, Yan C, Du H. Matrix metalloproteinase 12 overexpression in myeloid lineage cells plays a key role in modulating myelopoiesis, immune suppression, and lung tumorigenesis. Blood. 2011;117:4476–89. doi: 10.1182/blood-2010-07-298380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong Z, Kumar R, Yang X, Fidler IJ. Macrophage-derived metalloelastase is responsible for the generation of angiostatin in Lewis lung carcinoma. Cell. 1997;88:801–10. doi: 10.1016/s0092-8674(00)81926-1. [DOI] [PubMed] [Google Scholar]
- 39.Cornelius LA, Nehring LC, Harding E, Bolanowski M, Welgus HG, Kobayashi DK, Pierce RA, Shapiro SD. Matrix metalloproteinases generate angiostatin: effects on neovascularization. J Immunol. 1998;161:6845–52. [PubMed] [Google Scholar]
- 40.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 41.Yang XS, Liu SA, Liu JW, Yan Q. Fucosyltransferase IV enhances expression of MMP-12 stimulated by EGF via the ERK1/2, p38 and NF-κB pathways in A431 cells. Asian Pac J Cancer Prev. 2012;13:1657–62. doi: 10.7314/apjcp.2012.13.4.1657. [DOI] [PubMed] [Google Scholar]
- 42.Zhan Y, Abi Saab WF, Modi N, Stewart AM, Liu J, Chadee DN. Mixed lineage kinase 3 is required for matrix metalloproteinase expression and invasion in ovarian cancer cells. Exp Cell Res. 2012;318:1641–8. doi: 10.1016/j.yexcr.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


