Abstract
Background: Although many epidemiologic studies have investigated the CYP1A1 MspI gene polymorphisms and their associations with lung cancer (LC), definite conclusions cannot be drawn. Objective: To clarify the effects of CYP1A1 MspI polymorphisms on the risk of LC, an update meta-analysis was performed in only Chinese population. Methods: Related studies were identified from PubMed, Springer Link, Ovid, Chinese Wanfang Data Knowledge Service Platform, Chinese National Knowledge Infrastructure (CNKI), and Chinese Biology Medicine (CBM) till October 2014. Pooled ORs and 95% CIs were used to assess the strength of the associations. Results: A total of 22 studies including 3016 LC cases and 3932 controls were involved in this meta-analysis. Overall, significant association was found between CYP1A1 MspI polymorphism and LC risk when all studies in the Chinese population pooled into this meta-analysis (CC vs. TT: OR = 1.42, 95% CI = 1.11-1.80; CT + CC vs. TT: OR = 1.26, 95% CI = 1.06–1.50; CC vs. CT + TT: OR = 1.30, 95% CI = 1.04-1.61; C vs. T: OR = 1.21, 95% CI = 1.07-1.37). In subgroup analyses stratified by ethnicity and source of controls, significantly increased risk was found in Chinese Han people and in population-based studies. Conclusions: This meta-analysis provides the evidence that CYP1A1 MspI polymorphism may contribute to the LC development in the Chinese population and studies with large sample size and wider spectrum of population are warranted to verify this finding.
Keywords: Meta-analysis, CYP1A1 MspI, polymorphism, lung cancer, Chinese
Introduction
Lung cancer is the most commonly diagnosed cancer as well as the leading cause of cancer death in males globally, with 1.6 million newly confirmed cases and 1.4 million deaths from lung cancer annually [1]. Its incidence has been increasing in many parts of world, particularly in China, which has become a major public health challenge all the world [1]. In China, It was estimated that 605946 lung cancer cases were diagnosed in 2010, with a crude incidence rate of 46.08/100000, and 486 555 patients died from lung cancer, with a crude mortality rate of 37.00/100000 [2]. The mechanisms of lung carcinogenesis have not been fully illustrated. Although epidemiological evidence suggests that exposure to tobacco-associated carcinogens is clearly implicated in its etiology [3], only 20% of smokers develop lung cancer, suggesting that genetic variations and other environmental factors also play important roles in determining individual differences in lung cancer susceptibility [4,5]. In recent years, several common low-penetrance genes have been identified as potential lung cancer susceptibility genes. An important one is cytochrome P450 1A1 (CYP1A1), which plays an essential role in the metabolic activation of major classes of tobacco procarcinogen such as aromatic amines and polycyclic aromatic hydrocarbons (PAHs). So it may affect the metabolism of the environmental carcinogens and alter susceptibility to lung cancer.
The MspI polymorphism of CYP1A1 is one of the most extensively studied genes in lung cancer susceptibility over the last two decades. A point mutation (thymine (T) to cytosine (C)) of CYP1A1 at an MspI site in the 3’-untranslated region resulted in an MspI restriction site (m2 allele). There are three genotypes of CYP1A1 MspI polymorphisms: (i) homozygous m1/m1 allele without the MspI site (TT), (ii) heterozygote m1/m2 allele (TC), and (iii) homozygous m2/m2 allele (CC). The first research of the association between CYP1A1 MspI polymorphism and lung cancer was reported by Kawajiri and co-workers in 1990 among the Japan population [6], after which many studies analyzed the influence of CYP1A1 MspI polymorphism on lung cancer risk; no clear consensus, however, was reached. Meta-analyses of studies on the gene in other ethnic groups have been reported elsewhere and produced conflicting results [7-11]. In order to lessen the impact of different genetic background, we performed this update meta-analysis to assess the relationship of CYP1A1 MspI polymorphism with risk of lung cancer in only Chinese population.
Materials and methods
Materials
We searched for studies in the PubMed, Springer Link, Ovid, Chinese Wanfang Data Knowledge Service Platform, Chinese National Knowledge Infrastructure (CNKI), and Chinese Biology Medicine (CBM) to include in this meta-analysis, using the following Mesh terms: (“Lung Neoplasms” [MeSH] or “lung cancer” or “lung tumor” or “lung carcinoma” or “carcinoma of lung”) and (“P4501A1” or “CYP1A1”) and (China or Chinese or Taiwan). An upper date limit of 9 October 2014 was applied and no lower date limit was used. The search was performed without any restrictions on language and focused on studies conducted in humans. Concurrently, the reference lists of reviews and retrieved articles were searched manually.
Inclusion/exclusion criteria
Studies included in this meta-analysis have to meet the following criteria: (1) case-control study or cohort study studying on association between the CYP1A1 MspI polymorphisms and lung cancer susceptibility; (2) all patients with the diagnosis of lung cancer confirmed by pathological or histological examination; (3) sufficient published data about sample size, odds ratio (OR), and their 95% confidence interval (CI); (4) all participants were Chinese; (5) the distribution of the genotypes in control groups was in the Hardy-Weinberg equilibrium. Studies were excluded when they were: (1) not case-control study or cohort study; (2) duplicate of previous publication; (3) based on incomplete data; (4) meta-analyses, letters, reviews, or editorial articles.
Data extraction
Information was extracted carefully from all eligible publications independently by two authors, based on the inclusion criteria above. Disagreements were resolved by discussion and if consensus was not achieved the decision was made by all the reviewers. The title and abstract of all potentially relevant articles were screened to determine their relevance. Full articles were also scrutinized if the title and abstract were ambiguous. The following information was collected from each study: first author’s surname, year of publication, geographical location, ethnicity of subjects, source of controls, total numbers of cases and controls, and the numbers of cases and controls who harbored the MspI genotypes. If data from any category were not reported in the primary study, the items were designated ‘not stated’. We did not contact the author of the primary study to request the information. Ethnicities were categorized as Han and other ethnic Chinese. Studies with different ethnic groups were considered as individual studies for our analyses.
Statistical analysis
Statistical analysis was conducted by using STATA statistical package (version 10, STATA, College Station, TX). The distributions of genotypes in controls were tested by Hardy-Weinberg equilibrium (HWE) using the Chi-square test. The association of CYP1A1 MspI polymorphisms and lung cancer risk was estimated by odds ratio (ORs) with 95% confidence intervals (CIs). The heterogeneity was tested by the Q-statistics with P-values < 0.1. Dependent on the results of heterogeneity test among individual studies, the fixed effect model (Mantel-Haenszel) or random effect model (DerSimonian and Laird) was selected to summarize the combined OR and their 95% CI. The significance of the pooled OR was determined by the z test. The sensitivity analysis was performed using different statistic models. Publication bias was investigated with the funnel plot, in which the Standard Error (SE) of log OR of each study was plotted against its OR. Funnel-plot asymmetry was further assessed by the method of Egger’s linear regression test. All the P values were two sided. P value less than 0.05 was considered statistically significant. In addition, subgroup analysis stratified by ethnicity, source of controls and geographical location was also performed.
Results
Eligible studies
According to the inclusion criteria, 22 studies [12-33] were included and 165 articles were excluded. The publication year of involved studies ranged from 1999 to 2014. The flow chart of study selection is shown in Figure 1. In total, 3016 lung cancer cases and 3932 controls were involved in this meta-analysis, which evaluated the relationship between CYP1A1 MspI and lung cancer risk. The source of controls was mainly based on a healthy population. Ten of these studies conducted for Chinese Han population, twelve studies not states ethnicity. The characteristics of the included studies are summarized in Table 1.
Figure 1.
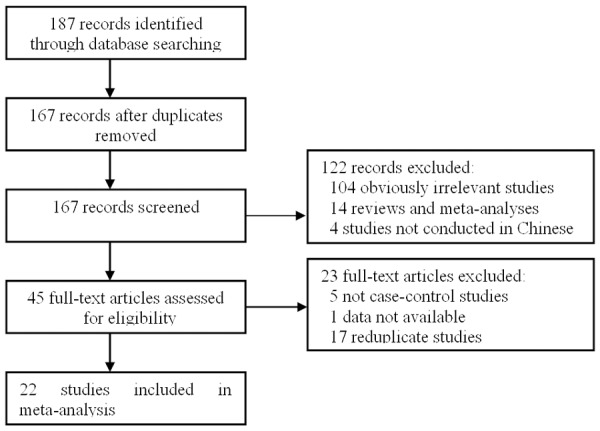
Flow diagram of the literature search.
Table 1.
Characteristics of studies included in the meta-analysis
| Reference | Source of controls | Area | Ethnicity | Case no. | Control no. | Case | Control | HWE | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||
| TT | CT | CC | TT | CT | CC | X2 | P | ||||||
| Hu 1999 [12] | PB+HB | Guangdong | Han | 59 | 132 | 22 | 22 | 15 | 34 | 76 | 22 | 3.43 | 0.064 |
| Persson 1999 [13] | PB | Beijing | Not stated | 76 | 90 | 33 | 34 | 9 | 36 | 44 | 10 | 0.40 | 0.526 |
| Song 1999 [14] | PB | Beijing | Not stated | 150 | 391 | 35 | 93 | 22 | 162 | 173 | 56 | 0.79 | 0.375 |
| Song 2001 [15] | PB | Beijing | Not stated | 217 | 404 | 60 | 129 | 28 | 173 | 175 | 56 | 1.19 | 0.275 |
| Yin 2001 [16] | HB | Jiangsu | Not stated | 84 | 84 | 36 | 35 | 13 | 28 | 38 | 18 | 0.57 | 0.451 |
| Zhou 2002 [17] | HB | Hubei | Not stated | 92 | 98 | 34 | 43 | 15 | 51 | 34 | 13 | 3.29 | 0.070 |
| Wang 2003 [18] | PB | Beijing + Tianjin | Han | 162 | 181 | 64 | 76 | 22 | 65 | 78 | 38 | 2.54 | 0.111 |
| Dong 2004 [19] | HB | Sichuan | Not stated | 82 | 91 | 35 | 36 | 11 | 46 | 35 | 10 | 0.71 | 0.401 |
| Liang 2004 [20] | HB | Jiangsu | Han | 152 | 152 | 50 | 82 | 20 | 70 | 71 | 11 | 1.52 | 0.218 |
| Li 2004 [21] | HB | Beijing | Not stated | 217 | 200 | 93 | 88 | 36 | 85 | 89 | 26 | 0.13 | 0.722 |
| Li 2005 [22] | PB | Henan | Han | 103 | 138 | 27 | 64 | 12 | 73 | 54 | 11 | 0.05 | 0.819 |
| Ng 2005 [23] | HB | Singapore | Not stated | 124 | 162 | 22 | 61 | 41 | 19 | 87 | 56 | 2.87 | 0.090 |
| Qian 2006 [24] | PB | Tianjin | Han | 108 | 108 | 45 | 33 | 30 | 44 | 52 | 12 | 0.33 | 0.563 |
| Tao 2007 [25] | Not stated | Anhui | Not stated | 47 | 94 | 24 | 19 | 4 | 43 | 37 | 14 | 1.59 | 0.208 |
| Xia 2008 [26] | HB | Gansu | Han | 58 | 116 | 17 | 36 | 5 | 40 | 58 | 18 | 0.16 | 0.688 |
| Zhu 2010 [27] | PB | Hunan | Not stated | 160 | 160 | 55 | 68 | 37 | 68 | 66 | 26 | 2.08 | 0.149 |
| Tuerxun 2011 [28] | PB | Xinjiang | Han | 59 | 84 | 19 | 27 | 13 | 33 | 45 | 6 | 3.19 | 0.074 |
| Wang 2012 [29] | PB | Henan | Han | 209 | 256 | 61 | 106 | 42 | 87 | 120 | 49 | 0.44 | 0.508 |
| Li 2012 [30] | PB | Beijing | Not stated | 217 | 198 | 93 | 88 | 36 | 83 | 89 | 26 | 0.08 | 0.781 |
| Huang 2013 [31] | PB | Hunan | Han | 168 | 201 | 51 | 83 | 34 | 61 | 93 | 47 | 0.99 | 0.320 |
| Jiang 2014 [32] | PB | Inner Mongolia | Mongolian | 142 | 190 | 28 | 59 | 55 | 64 | 95 | 31 | 0.18 | 0.668 |
| Jiang 2014 [32] | PB | Inner Mongolia | Han | 180 | 266 | 37 | 71 | 72 | 87 | 126 | 53 | 0.36 | 0.547 |
| Yang 2014 [33] | PB | Hebei | Not stated | 150 | 136 | 60 | 64 | 26 | 62 | 56 | 18 | 0.87 | 0.350 |
PB: Population-based, HB: hospital-based.
Meta-analysis results
Table 2 lists the primary results. Overall, a significantly elevated risk of lung cancer was associated with three variants of CYP1A1 MspI (for CC vs. TT: OR = 1.42, 95% CI = 1.11-1.80, P = 0.005 for heterogeneity; for CT and CC combined vs. TT: OR = 1.26, 95% CI = 1.06-1.50, P = 0.000 for heterogeneity; for CC vs. CT and TT: OR = 1.30, 95% CI = 1.04-1.61, P = 0.000 for heterogeneity). For the allele C versus allele T, the pooled OR was 1.21 (95% CI = 1.07-1.37; P = 0.000 for heterogeneity) (Figure 2).
Table 2.
Summary ORs and 95% CI of CYP1A1 MspI polymorphism and lung cancer risk
| Analysis model | Study groups | N | OR 95% CI (random effect) | OR 95% CI (fixed effect) | Pa |
|---|---|---|---|---|---|
| C vs. T | Total | 23 | 1.21 (1.07-1.37) | 1.23 (1.15-1.32) | 0.000 |
| Chinese Han | 10 | 1.25 (1.02-1.55) | 1.25 (1.12-1.39) | 0.000 | |
| Population-based | 14 | 1.32 (1.13-1.53) | 1.31 (1.21-1.42) | 0.000 | |
| Hospital-based | 7 | 1.10 (0.89-1.34) | 1.10 (0.96-1.26) | 0.064 | |
| CC vs. TT | Total | 23 | 1.42 (1.11-1.80) | 1.44 (1.25-1.67) | 0.005 |
| Chinese Han | 10 | 1.55 (1.00-2.38) | 1.49 (1.20-1.85) | 0.000 | |
| Population-based | 14 | 1.66 (1.23-2.24) | 1.62 (1.37-1.92) | 0.000 | |
| Hospital-based | 7 | 1.11 (0.73-1.70) | 1.13 (0.83-1.52) | 0.092 | |
| CC + CT vs. TT | Total | 23 | 1.26 (1.06-1.50) | 1.31 (1.18-1.45) | 0.000 |
| Chinese Han | 10 | 1.28 (0.97-1.68) | 1.29 (1.11-1.51) | 0.002 | |
| Population-based | 14 | 1.41 (1.14-1.73) | 1.42 (1.26-1.61) | 0.001 | |
| Hospital-based | 7 | 1.15 (0.84-1.56) | 1.16 (0.95-1.42) | 0.044 | |
| CC vs. TT + CT | Total | 23 | 1.30 (1.04-1.61) | 1.31 (1.16-1.49) | 0.000 |
| Chinese Han | 10 | 1.43 (0.95-2.16) | 1.40 (1.15-1.69) | 0.000 | |
| Population-based | 14 | 1.43 (1.07-1.92) | 1.41 (1.21-1.63) | 0.000 | |
| Hospital-based | 7 | 1.09 (0.82-1.44) | 1.08 (0.83-1.40) | 0.349 |
P value for heterogeneity.
Figure 2.
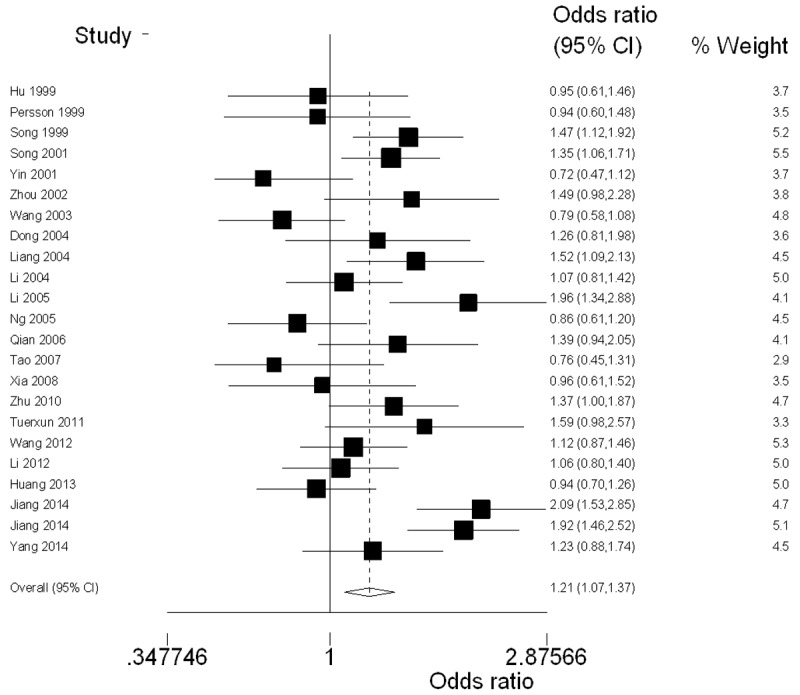
Forest plot (random-effects model) of lung cancer risk associated with CYP1A1 MspI polymorphism using the allele genetic model.
In the stratified analysis by ethnicity, significantly increased risks were observed among Chinese Han people under Allele model (OR = 1.25, 95% CI = 1.02-1.55; P = 0.000 for heterogeneity) and Homozygous model (OR = 1.55, 95% CI = 1.00-2.38; P = 0.000 for heterogeneity). In the subgroup analysis by source of controls, significantly increased association was found in population-based studies under all genetic models (for C vs. T: OR = 1.32, 95% CI = 1.13-1.53, P = 0.000 for heterogeneity; for CC vs. TT: OR = 1.66, 95% CI = 1.23-2.24, P = 0.000 for heterogeneity; for CT and CC combined vs. TT: OR = 1.41, 95% CI = 1.14-1.73, P = 0.001 for heterogeneity; for CC vs. CT and TT: OR = 1.43, 95% CI = 1.07-1.92, P = 0.000 for heterogeneity) whereas not found in hospital-based studies.
Sensitive analysis and bias diagnosis
In order to compare the difference and evaluate the sensitivity of the meta-analyses, we used both models (the fixed effect model and random effect model) to evaluate the stability of the meta-analysis. All the results were not materially altered (Table 2). Hence, results of the sensitivity analysis suggest that the data in this meta-analysis are relatively stable and credible.
The Begg’s funnel plot and Egger’s test were performed to assess the publication bias of literatures. The shape of the funnel plots did not reveal obvious asymmetry (Figure 3). Then, the Egger’s test was used to provide statistical evidence of funnel plot symmetry. The Egger’s test indicated that there were no obvious publication bias under the allele model in overall analyses (t = -0.96, P = 0.349).
Figure 3.
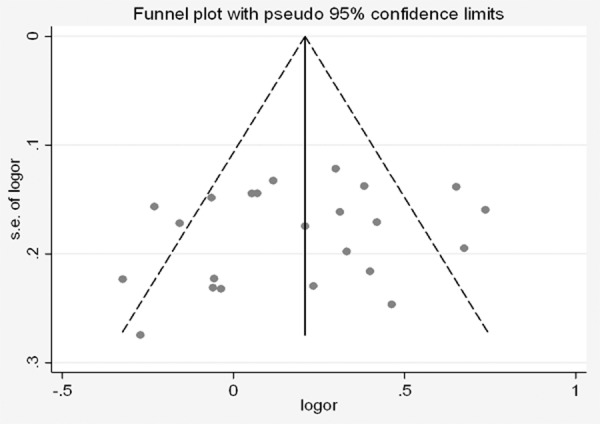
Begg’s funnel plot of CYP1A1 MspI polymorphism and lung cancer risk under the allele genetic model.
Discussion
CYP genes are large families of endoplasmic and cytosolic enzymes that catalyze the activation and detoxification, respectively, of reactive electrophilic compounds, including many environmental carcinogens (e.g., benzo [a] pyrene). CYP1A1 is a phase I enzyme that regulates the metabolic activation of major classes of tobacco procarcinogens, such as aromatic amines and PAHs [34]. Thus, CYP1A1 may affect the metabolism of environmental carcinogens and alter the susceptibility to lung cancer. Although many studies analyzing the research results about the association between CYP1A1 MspI polymorphism and lung cancer, definite conclusions cannot be drawn. Therefore, we did this updated meta-analysis to estimate the relationship between CYP1A1 MspI polymorphism and susceptibility to lung cancer among the Chinese population only, in order to lessen the impact of different genetic background. This meta-analysis involved 22 articles with 3016 lung cancer cases and 3932 controls. The results indicated a significant association between CYP1A1 MspI gene polymorphism and lung cancer risk in the Chinese population. The sensitivity analysis confirmed the reliability and stability of the meta-analysis and no publication bias was found among studies by Egger’s test. Therefore, the findings from our meta-analysis provide a strong evidence for the association between CYP1A1 MspI polymorphism and risk of lung cancer in the Chinese population, and the CC genotype contributes to increased risk of lung cancer in Chinese. Our results were consistent with a previously published meta-analysis in Chinese populations [35], which found that compared with the wild-type homozygous genotype (TT), lung cancer risk for the combined variant genotypes (CC and CT) was 1.34-fold (95% CI = 1.08-1.67) (Z = 2.64, P = 0.008). However, this previously published meta-analysis did not search some other databases in China (Wanfang Data Knowledge Service Platform and CBM), and included a smaller number of studies than ours did.
The effects of genetic polymorphisms on cancer risk seem to be affected by ethnicity background. Several studies have shown that the CYP1A1 MspI polymorphism is associated with an increased lung cancer risk in Asian population [7,35]. However, previous researches, including some pooled analyses suggest that there is not an established association between this polymorphism and increased lung cancer risk in Caucasian populations [36-38]. Therefore, we performed the subgroup analyses by ethnicity and source of controls. The results revealed that significant association with susceptibility for the development of lung cancer was found in Chinese Han people and in population-based studies whereas not in hospital-based studies. The hospital-based studies usually have some biases because such controls may just represent a sample of ill-defined reference population, and may not be representative of the general population.
The pathways of carcinogen metabolism are complex, mediated by the activities of multiple genes. The effect of any single gene might have a limited impact on lung cancer risk than have so far been anticipated. Lung cancer has some known major environmental determinants other than tobacco smoke, and large studies with detailed exposure information are needed to evaluate reliably any moderate genetic effects. Otherwise, some limitations should be acknowledged. Firstly, we didn’t perform subgroup analysis on smoking status et al, because of the lack of sufficient data. Another potential limitation was that our results were based on unadjusted estimates. More precise analyses can be conducted if individual data were available, which would allow for the adjustment by other covariates including age, sex, location, race and other factors. Finally, heterogeneity can interfere with the interpretation of the results of a meta-analysis. Although we minimized this likelihood by performing a careful search of published studies and subgroup analyses, significant inter-study heterogeneity nevertheless existed in nearly every comparison. The presence of heterogeneity can result from differences in the selection of controls, age distribution and the prevalence of lifestyle factors. In spite of these limitations, our meta-analysis still had some advantages. We obeyed the inclusion and exclusion criteria strictly to reduce selection bias. A funnel plot and Egger’s linear regression test was used to assess publication bias. In addition, the impact of different genetic background was lessened by means of including the studies performed in the Chinese population only, and the test of the Hardy-Weinberg equilibrium for distribution of the genotypes in control groups suggested that there was no significantly different genetic background among the participants.
In conclusion, our meta-analysis supports that CYP1A1 MspI polymorphism might contribute to individual susceptibility to lung cancer in the Chinese population. Concerning lung cancer with multifactorial etiology, to further evaluate gene-gene and gene-environment interactions on CYP1A1 MspI polymorphism and lung cancer, larger studies in selected populations with different environmental background or other risk factors are required. Such studies taking these factors into account may eventually lead to have a better, comprehensive understanding of the association between the CYP1A1 MspI polymorphism and lung cancer risk.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Zheng R, Zeng H, Zhang S, Fan Y, Qiao Y, Zhou Q, Chen W. Lung cancer incidence and mortality in China, 2010. Thoracic Cancer. 2014;5:330–6. doi: 10.1111/1759-7714.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vineis P, Alavanja M, Buffler P, Fontham E, Franceschi S, Gao YT, Gupta PC, Hackshaw A, Matos E, Samet J, Sitas F, Smith J, Stayner L, Straif K, Thun MJ, Wichmann HE, Wu AH, Zaridze D, Peto R, Doll R. Tobacco and cancer: recent epidemiological evidence. J Natl Cancer Inst. 2004;96:99–106. doi: 10.1093/jnci/djh014. [DOI] [PubMed] [Google Scholar]
- 4.Osann KE. Epidemiology of lung cancer. Curr Opin Pulm Med. 1998;4:198–204. doi: 10.1097/00063198-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Hecht SS. Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention. Lancet Oncol. 2002;3:461–469. doi: 10.1016/s1470-2045(02)00815-x. [DOI] [PubMed] [Google Scholar]
- 6.Kawajiri K, Nakachi K, Imai K, Yoshii A, Shinoda N, Watanabe J. Identification of genetically high risk individuals to lung cancer by DNA polymorphisms of the cytochrome P450IA1 gene. FEBS. 1990;1:131–133. doi: 10.1016/0014-5793(90)80721-t. [DOI] [PubMed] [Google Scholar]
- 7.Wu B, Liu K, Huang H, Yuan J, Yuan W, Wang S, Chen T, Zhao H, Yin C. MspI and Ile462Val polymorphisms in CYP1A1 and overall cancer risk: a meta-analysis. PLoS One. 2013;8:e85166. doi: 10.1371/journal.pone.0085166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji YN, Wang Q, Lin XQ, Suo LJ. CYP1A1 MspI polymorphisms and lung cancer risk: an updated meta-analysis involving 20,209 subjects. Cytokine. 2012;59:324–34. doi: 10.1016/j.cyto.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 9.Zhan P, Wang Q, Qian Q, Wei SZ, Yu LK. CYP1A1 MspI and exon7 gene polymorphisms and lung cancer risk: an updated meta-analysisand review. J Exp Clin Cancer Res. 2011;30:99. doi: 10.1186/1756-9966-30-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z, Li Z, Niu X, Ye X, Yu Y, Lu S, Chen Z. The effect of CYP1A1 polymorphisms on the risk of lung cancer: a global meta-analysis based on 71 case-control studies. Mutagenesis. 2011;26:437–46. doi: 10.1093/mutage/ger002. [DOI] [PubMed] [Google Scholar]
- 11.Houlston RS. CYP1A1 polymorphisms and lung cancer risk: a meta-analysis. Pharmacogenetics. 2000;10:105–14. doi: 10.1097/00008571-200003000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Hu Y, Zhang Q. Genetic polymorphisms of CYP1A1 and susceptibility of lung cancer. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 1999;16:26–28. [PubMed] [Google Scholar]
- 13.Persson I, Johansson I, Lou YC, Yue QY, Duan LS, Bertilsson L, Ingelman-Sundberg M. Genetic polymorphism of xenobiotic metabolizing enzymes among Chinese lung cancer patients. Int J Cancer. 1999;81:325–329. doi: 10.1002/(sici)1097-0215(19990505)81:3<325::aid-ijc2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 14.Song N, Tan W, Tang H, Lin D. Impact of cytochrome P450 1A1 gene mutations on the risk of development of lung cancer in a Chinese population. Ai Zheng. 1999;18:495–98. [Google Scholar]
- 15.Song N, Tan W, Xing D, Lin D. CYP1A1 polymorphism and risk of lung cancer in relation to tobacco smoking: a case-control study in China. Carcinogenesis. 2001;22:11–16. doi: 10.1093/carcin/22.1.11. [DOI] [PubMed] [Google Scholar]
- 16.Yin L, Pu Y, Liu TY, Tung YH, Chen KW, Lin P. Genetic polymorphisms of NAD(P)H quinine oxidoreductase, CYP1A1 and microsomal epoxide hydrolase and lung cancer risk in Nanjing, China. Lung Cancer. 2001;33:133–41. doi: 10.1016/s0169-5002(01)00182-9. [DOI] [PubMed] [Google Scholar]
- 17.Zhou XW, Shi Y, Zhou YK. The relationship between CYP1A1 genetic polymorphism and susceptibility to lung cancer. J Environ Occup Med. 2002;19:355–7, 387. [Google Scholar]
- 18.Wang JW, Deng YF, Li L, Kuriki K, Ding J, Pan X, Zhuge X, Jiang J, Luo C, Lin P, Tokudome S. Association of GSTM1, CYP1A1 and CYP2E1 genetic polymorphisms with susceptibility to lung adenocarcinoma: a case-control study in Chinese population. Cancer Sci. 2003;94:448–52. doi: 10.1111/j.1349-7006.2003.tb01463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong CT, Yang Q, Jiang B, Dong QN. Study on the relationship between polymorphisms of CYP1A1 gene and susceptibility of lung cancer in Sichuan population. Zhongguo Fei Ai Za Zhi. 2004;7:38–42. doi: 10.3779/j.issn.1009-3419.2004.01.10. [DOI] [PubMed] [Google Scholar]
- 20.Liang GY, Pu YP, Yin LH. Studies of the genes related to lung cancer susceptibility in Nanjing Han population, China. Hereditas (Beijing) 2004;26:584–8. [PubMed] [Google Scholar]
- 21.Li WY, Lai BT, Zhan XP. The relationship between genetic polymorphism of metabolizing enzymes and the susceptibility to lung cancer. Chin J Epidemiol. 2004;25:1042–5. [PubMed] [Google Scholar]
- 22.Li Y, Tang XY, Ma XT. Cytochrome P-450 1A1 polymorphisms and susceptibility to lung cancer: a case-control study on Chinese population in Henan. Henan Med Res. 2005;14:227–9. [Google Scholar]
- 23.Ng DP, Tan KW, Zhao B, Seow A. CYP1A1 polymorphisms and risk of lung cancer in non-smoking Chinese women: influence of environmental tobacco smoke exposure and GSTM1/T1 genetic variation. Cancer Causes Control. 2005;16:399–405. doi: 10.1007/s10552-004-5476-0. [DOI] [PubMed] [Google Scholar]
- 24.Qian BY, Han HW, Gu F, He M, Li HX, Song FJ. Case-control study genetic polymorphism in CYP1A1 and GSTM1 and smoking and susceptibility to lung cancer. Chin J Clin Oncol. 2006;33:500–2. [Google Scholar]
- 25.Tao W, Jin Y, Yu Z. The effects of CYP1A1 gene polymorphism and p16 gene methylation on the risk of lung cancer. Acta Universitatis Medicinalis Anhui. 2007;42:62–66. [Google Scholar]
- 26.Xia Y, Sun QF, Shang B. P7olymorphisms of the cytochrome P450 and glutathion s-transferase genes associated with lung cancer susceptibility for the residents in high radon-exposed area. Chin J Radiol Med Prot. 2008;28:327–332. [Google Scholar]
- 27.Zhu XX, Hu CP, Gu QH. CYP1A1 polymorphisms, lack of glutathione S-transferase M1 (GSTM1), cooking oil fumes and lung cancer risk in nonsmoking women. Zhonghua Jie He He Hu Xi Za Zhi. 2010;33:817–22. [PubMed] [Google Scholar]
- 28.Maimaiti T, Matyusup A, Wupuer H, Ablimit A, Mamatyusupu D. Association Study of the Single Nucleotide Polymorphism of CYP1A1 Gene and Lung Cancer. Biotechnology. 2011;21:39–43. [Google Scholar]
- 29.Wang N, Wu Y, Zhou X, Wu Y. Association between genetic polymorphism of metabolizing enzymes and DNA repairing enzymes and the susceptibility of lung cancer in Henan population. J Hygiene Res. 2012;41:251–6. [PubMed] [Google Scholar]
- 30.Li W, Yue W, Zhang L, Zhao X, Ma L, Yang X, Zhang C, Wang Y, Gu M. Polymorphisms in GSTM1, CYP1A1, CYP2E1, and CYP2D6 are associated with susceptibility and chemotherapy response in non-small-cell lung cancer patients. Lung. 2012;190:91–98. doi: 10.1007/s00408-011-9338-8. [DOI] [PubMed] [Google Scholar]
- 31.Huang FM, Chen HC, Khan MA, Yang FL, Wan XX, Xu AH, Ou-yang FD, Zhang DZ. CYP2A6, CYP1A1, and CYP2D6 polymorphisms in lung cancer patients from Central South China. Med Oncol. 2013;30:521. doi: 10.1007/s12032-013-0521-z. [DOI] [PubMed] [Google Scholar]
- 32.Jiang XY, Chang FH, Bai TY, Lv XL, Wang MJ. Susceptibility of lung cancer with polymorphisms of CYP1A1, GSTM1, GSTM3, GSTT1 and GSTP1 genotypes in the population of Inner Mongolia region. Asian Pac J Cancer Prev. 2014;15:5207–14. doi: 10.7314/apjcp.2014.15.13.5207. [DOI] [PubMed] [Google Scholar]
- 33.Yang CJ, Liu F, Sun L, Liu JJ, Liu KY. Susceptibility of lung cancer with polymorphisms of CYP1A1 and smoking. Chin J Gerontology. 2014;34:616–618. [Google Scholar]
- 34.Guengerich FP, Shimada T. Activation of procarcinogens by human cytochrome P450 enzymes. Mutat Res. 1998;400:201–13. doi: 10.1016/s0027-5107(98)00037-2. [DOI] [PubMed] [Google Scholar]
- 35.Shi X, Zhou S, Wang Z, Zhou Z, Wang Z. CYP1A1 and GSTM1 polymorphisms and lung cancer risk in Chinese populations: a meta-analysis. Lung Cancer. 2008;59:155–63. doi: 10.1016/j.lungcan.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Vineis P, Veglia F, Anttila S, Benhamou S, Clapper ML, Dolzan V, Ryberg D, Hirvonen A, Kremers P, Le Marchand L, Pastorelli R, Rannug A, Romkes M, Schoket B, Strange RC, Garte S, Taioli E. CYP1A1, GSTM1 and GSTT1 polymorphisms and lung cancer: a pooled analysis of gene-gene interactions. Biomarkers. 2004;9:298–305. doi: 10.1080/13547500400011070. [DOI] [PubMed] [Google Scholar]
- 37.Raimondi S, Boffetta P, Anttila S, Bröckmoller J, Butkiewicz D, Cascorbi I, Clapper ML, Dragani TA, Garte S, Gsur A, Haidinger G, Hirvonen A, Ingelman-Sundberg M, Kalina I, Lan Q, Leoni VP, Le Marchand L, London SJ, Neri M, Povey AC, Rannug A, Reszka E, Ryberg D, Risch A, Romkes M, Ruano-Ravina A, Schoket B, Spinola M, Sugimura H, Wu X, Taioli E. Metabolic gene polymorphisms and lung cancer risk in non-smokers. An update of the GSEC study. Mutat Res. 2005;592:45–57. doi: 10.1016/j.mrfmmm.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Hung RJ, Boffetta P, Brockmoller J, Butkiewicz D, Cascorbi I, Clapper ML, Garte S, Haugen A, Hirvonen A, Anttila S, Kalina I, Le Marchand L, London SJ, Rannug A, Romkes M, Salagovic J, Schoket B, Gaspari L, Taioli E. CYP1A1 and GSTM1 genetic polymorphisms and lung cancer risk in Caucasian nonsmokers: a pooled analysis. Carcinogenesis. 2003;24:875–82. doi: 10.1093/carcin/bgg026. [DOI] [PubMed] [Google Scholar]


