Abstract
Atherosclerosis is a chronic immune inflammatory disease. Atherosclerosis and relevant disease are threatening human life and health. Oxygenized low density lipoprotein (oxLDL) is a molecular basis in the pathogenesis of atherosclerosis and able to induce inflammation, stimulate immune system and interfere with lipid metabolism in the occurrence and development of atherosclerosis. Antibody against oxLDL has been an important molecule in the immune related pathogenesis of atherosclerosis. In available studies on atherosclerosis, antibody against oxLDL has been a focus, but how oxLDL acts to affect the atherosclerosis and relevant diseases, whether oxLDL is protective or detrimental, and whether oxLDL acts in different ways at different stages of atherosclerosis are still unclear. This paper focuses on the role of antibody against oxLDL in the atherosclerosis and relevant diseases, and summarizes the advances in this field, aiming to provide new clue and new methods for the therapy of atherosclerosis.
Keywords: Oxygenized low density lipoprotein, antibody against oxygenized low density lipoprotein, atherosclerosis, new detection method
Introduction
Atherosclerosis is a chronic inflammatory autoimmune disease and has been a leading cause of morbidity and mortality [1]. Atherosclerosis is characterized by intimal deposition of lipid in the arteries and its progression into atherosclerotic plaques. The progression of atherosclerosis may cause acute myocardial infarction, stroke and peripheral vascular diseases [2,3]. Human lipoprotein B-100 (ApoB-100) refers to the low-density lipoprotein in the circulation and it is also the carrier of low-density lipoprotein and main component involved in the pathogenesis of atherosclerosis [2-4]. Oxygenized low density lipoprotein (oxLDL) is a modified product of low-density lipoprotein after oxidation. OxLDL, different from low-density lipoprotein, is an exogenous antigen that should be recognized and cleared by the immune system. OxLDL is involved in some pathological processes of atherosclerosis (such as inflammation, immune reaction and lipid metabolic disorder) and has been a molecular basis in the formation of fatty streaks and atheromatous plaques, and plays important roles in the occurrence and development of atherosclerosis [1-5]. Macrophages as the first line of defense in the immune system are responsible for the phagocytosis and clearance of oxLDL [6]. They may phagocytize oxLDL unlimitedly and excessively via their surface scavenger receptor A and CD36, and then they become foamy cells which are the cellular basis in the pathogenesis of atherosclerosis [7]. However, the immune activity does not become disappeared, and B cells in the immune system may produce antibodies against different epitopes of oxLDL (anti-oxLDL). Anti-oxLDL has been studied for more than 30 years, but the roles (protective or detrimental) of anti-oxLDL in the occurrence and development of atherosclerosis and relevant diseases are still poorly understood and controversial [8-14]. Undeniably, anti-oxLDL is important in the occurrence and development of atherosclerosis and great progress has been achieved in the anti-oxLDL in recent years. This paper summarized the recent studies on anti-oxLDL and their conclusions, which may provide evidence for further explorations of roles of anti-oxLDL in the atherosclerosis and relevant diseases (Figure 1).
Figure 1.
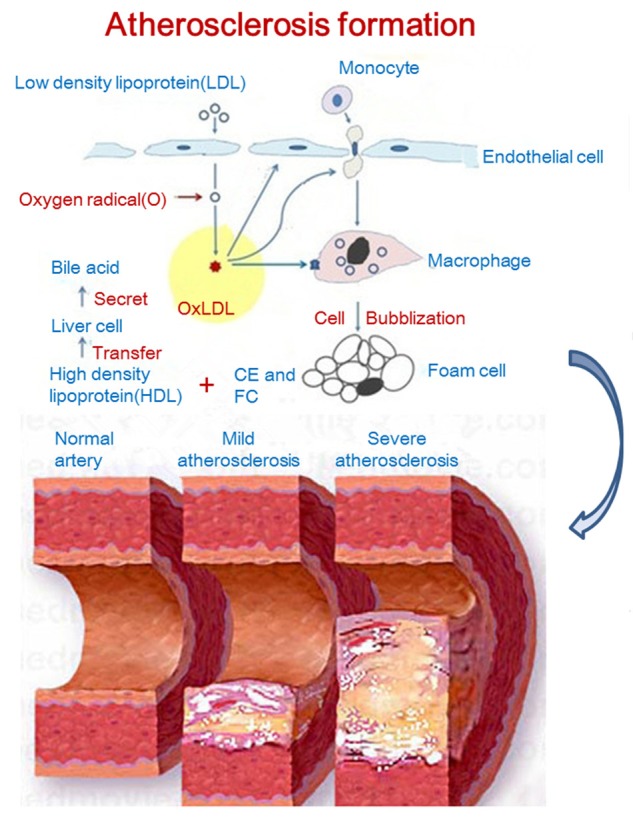
Process of oxygenized low density lipoprotein formation and its role in atherosclerosis progress.
OxLDL is a molecular basis of atherosclerosis
What is oxLDL?
Parthasarathy et al summarized previous findings [15,16] and concluded that oxLDL is the negatively charged LDL after oxidative modification by oxidants (such as aldehydes) which results in the changes in the components and structure of LDL and makes it easy to be recognized and phagocytized by macrophages. In brief, oxLDL refers to the low density lipoprotein (LDL) after oxidative modification. The completeness of oxidation may result in different degrees of oxidation of LDL. OxLDL is not a single simple product, but refers to an entity of a group of products [4].
How does oxLDL form?
Parthasarathy et al [17] summarized that the formation of oxLDL from LDL has involvement of 2 steps: (1) mild oxidation: LDL is only oxidized, which may not cause change in the molecular structure of ApoB-100; (2) advanced oxidation: LDL is further oxidized in which the amino acids of ApoB-100 undergo oxidation, pro-teolysis and cross-linking [18,19]. Thus, the LDL can be oxidized at different levels. LDL with a low oxidation may not be recognized by the immune system and still acts as an endogenous substance which can be recognized by LDL receptor; LDL with saturated oxidation or peroxidation may be recognized as an exogenous substance and then cleared via the scavenger receptors.
OxLDL-molecular basis of atherosclerosis
OxLDL induces inflammation
OxLDL may induce inflammation, promote the synthesis and release of multiple pro-inflammatory factors by endothelial cells and facilitate the phagocytosis by macrophages to form foamy cells, which increase the endothelial injury and result in subendothelial deposition of oxLDL. Under this condition, the deposition of oxLDL granules and foamy cells leads to the formation of atherosclerotic plaques [20]. Trpkovic et al [21] used oxLDL antibodies from mouse (4E6, DLH3 and E06) to detect the circulating oxLDL of patients with cardiovascular diseases. They proposed that oxLDL was a molecular basis of atherosclerosis and played important roles in the different stages of atherosclerosis. OxLDL reduces the stability of atherosclerotic plaques and promotes the rupture of atherosclerotic plaques, resulting in cardiovascular events (such as thromboembolism); oxLDL is also involved in the pathogenesis of atherosclerosis related diseases (such as diabetes, obesity and metabolic syndrome) [21]. Trpkovic et al [21] also summarized that lipoprotein a (LP-a) was the most important carrier of oxidized phospholipids in human plasma.
OxLDL activates immune reaction
OxLDL is a molecular basis of atherosclerosis [13,22]. OxLDL is not only involved in the inflammation of atherosclerosis, but plays an important role in the immune reaction to promote the development of atherosclerosis [20]. OxLDL and its residues have a high immunogenicity and may stimulate B cells to produce antibodies against different epitopes of lipoprotein after oxidative modification [23]. Some antibodies against oxLDL and antigen-antibody complexes have been detected in the serum of atherosclerotic animals and patients [23], and these antibodies are found to be associated with diseases. However, there is no consensus on the role (beneficial or detrimental) of oxLDL in the occurrence and development of atherosclerosis [8-13,22].
Anti-oxLDL and its relationship with atherosclerosis related diseases
What is antibody against oxLDL?
Investigators [24] have summarized the concept of antibody against oxLDL although this concept is not comprehensive and reflects the views of some investigators: antibody against oxLDL refers to the complete human monoclonal immunoglobulin G1 against oxidized ApoB-100, which can inhibit the activation of inflammatory cells, interfere with the occurrence and development of inflammation cascade and then stabilize the plaques. The stabilized plaques are not easy to rupture, which reduces the formation of blood clots and the possibility of myocardial infarction due to embolism. In vitro and in vivo studies have revealed anti-oxLDL may inhibit the chemotaxis and pro-inflammation of macrophages to reduce the formation of atherosclerotic plaques [2-4,25,26]. Thus, some clinicians speculate that anti-oxLDL may be used for the secondary prevention of potential major cardiovascular events [24].
History of studies on oxLDL
OxLDL has been studied for a long time, and studies on oxLDL are related to atherosclerosis and other diseases promoting atherosclerosis. Since Boyd et al [8] investigated the relationship between anti-oxLDL and atherosclerosis in WHHL rabbits in 1989, numerous studies have been conducted to explored the relationship between anti-oxLDL and atherosclerosis related diseases (some promote atherosclerosis development, and specific mechanisms are not addressed here) in patients with SLE [27], type 2 diabetes [28,29], or end stage renal disease [30], and in the elderly [31] and healthy subjects [32]. The roles of anti-oxLDL in the pathogenesis of some diseases and of the atherosclerosis in the presence of some diseases are extensively studied at different levels from distinct views, great progress has been achieved from these studies. Of note, the roles of anti-oxLDL in the atherosclerosis and relevant diseases are still controversial.
Advances in the roles of oxLDL in atherosclerosis relevant diseases
Current status
In recent years, great progress has been achieved in studies on anti-oxLDL. Although the controversy in the specific role of anti-oxLDL lasts for near 30 years, recent studies still fail to resolve this controversy (Table 1). Recent findings on the roles of anti-OxLDL in atherosclerosis and relevant diseases may be classified as three categories.
Table 1.
Recent findings on the roles of anti-OxLDL in atherosclerosis and relevant diseases
| Studies | Antibodies | Objective | Conclusion |
|---|---|---|---|
| Beger [5] | Anti-oxLDL/β2GPI | Arterial & venous disease [H] | Pathogenic |
| Babakr [18] | Anti-oxLDL | Impaired glucose tolerance and type 2 diabetes mellitus [H] | Pathogenic |
| Nowak [33] | Anti-oxLDL & anti-oxLDL/β2GPI (IgG and IgM) | Systemic lupus erythematosus [H] | Pathogenic |
| Tsiantoulas [38] | Anti-oxLDL (IgM) | ST-segment elevation myocardial infarction (STE-MI) [H] | Protective |
| Gironi [34] | Anti-oxLDL | Multiple sclerosis (MS) [H] | Protective |
| Izar [13] | Anti-oxLDL | Metabolic syndrome (MetS) with Acute coronary syndrome (ACS) [H] | Protective |
| Hosseini [35] | Anti-oxLDL (IgM) | Atherosclerosis [M] | Protective |
| Orbom [36] | Anti-oxLDL | Atherosclerosis [M] | Protective |
| Suthers [37] | Anti-oxLDL | Pneumococcal vaccination people [H] | Protective |
| Moohehati [39] | Anti-oxLDL (IgG) | Coronary artery disease (CAD) [H] | None |
| Sevince oK [40] | Anti-oxLDL | Hemodialysis patients [H] | None |
Footnotes: [H]: Human; [M]: Mice; None: nether pathogenic nor protective.
Anti-oxLDL is a pathogenic factor in atherosclerosis and relevant diseases
Beger et al [5] employed ELISA to detect anti-oxLDL IgG and anti-oxLDL/β-2GPI, aiming to confirm the detrimental effects of oxLDL. Babakr et al [18] also used ELISA to investigate anti-oxLDL and found anti-oxLDL (unspecified type) increased in patients with type 2 diabetes or impaired glucose tolerance and was positively related to the severity of obesity and BMI. Nowak et al [33] investigated the serum contents of anti-oxLDL and anti-oxLDL-β2GPI in SLE patients, and immunoassay showed serum anti-oxLDL increased in SLE patients, anti-oxLDL -β2GPI IgG and anti-oxLDL-β2GPI IgM in SLE patients were significantly higher than those in controls, and anti-oxLDL was positively related to LDL. These findings indicate that anti-oxLDL, anti-oxLDL-β2GPI IgM and anti-oxLDL-β2GPI IgG may increase the risk for cardiovascular diseases in SLE patients [33].
Anti-oxLDL is a protective factor of atherosclerosis and relevant diseases
Gironi et al [34] investigated serum contents of coenzyme Q10 (CoQ10) and anti-oxLDL in patients with MS, and found natural anti-oxLDL was protective on MS and able to maintain the integrity of blood brain barrier. Izar et al [13] detected the plasma titer of anti-oxLDL in patients with early metabolic syndrome (MetS) secondary to acute coronary syndrome (ACS) and found the reduction in anti-oxLDL was positively associated with the severity of MetS. In a recent study, Hosseini et al [35] feed ApoE-/- mice with high lipid diet which were intraperitoneally injected with apoptotic cells or phosphatidylserine (PSL) simultaneously. Their results showed the atherosclerotic plaques in mice treated with PSL or apoptotic cells were significantly smaller than those in controls; B1a cells released IgM to reduce pro-inflammatory cytokines in the plaques (anti-oxLDL reduces ox-LDL and anti-leukocyte, anti-CD3 and anti-CD4 reduce T cells), exerting protective effect on atherosclerosis; after splenectomy, B1a cells were not produced and the protective effect was absent. Örbom1 et al [36] employed multi-radionuclide autoradiography and immunohistochemistry to detect anti-ox-LDL conjugated with 2-deoxy-2-[18F]fluoro-D-glucose (18F-FDG) or 131I/125I and un-conjugated antibodies in the aorta of mice. In different groups, the distribution of anti-ox-LDL was comparable. However, injection of antibody 73 h before treatment displayed a better protective effect on plaques as compared to that 18 h before treatment, implying that anti-oxLDL may exert anti-atherosclerotic effect. On the basis of the hypothesis that 23-valent pneumococcal vaccine may induce the production of protective factors such as anti-oxLDL’ Suthers et al [37] inoculated 116 old subjects with pneumococcal vaccine and preliminarily confirmed that anti-oxLDL possessed cardiovascular protective activities. Tsiantoulas et al [38] extracted circulating microparticles (MPs) from healthy subjects and patients with ST-segment elevation myocardial infarction (STE-MI), and the surface oxidation specific epitopes (OSE) on MPs were detected by flow cytometry. They found that OSE could specifically bind to anti-oxLDL IgM to reduce the incidence of cardiovascular events. This uncovers a new mechanism underlying the protective effect of anti-oxLDL IgM on the cardiovascular diseases (CVD).
Anti-oxLDL is not associated with atherosclerosis and relevant diseases
Moohebati et al [39] found that serum anti-oxLDL IgG was not associated with CAD and its severity as well as risk factors of cardiovascular diseases. Sevince et al [40] conducted a prospective study to investigate the relationship of oxLDL and anti-oxLDL with atherosclerosis in patients receiving dialysis, and their results confirmed only oxLDL was related to pre-existing atherosclerosis, anti-oxLDL was not associated with the occurrence of atherosclerosis, and both oxLDL and anti-oxLDL were not related to the development of atherosclerosis and mortality.
Reasons for discrepancies among studies
We speculate that following reasons may explain the discrepancies among available studies: (1) There is no consensus on the concept of oxLDL. To date, the chemical structure, molecular formula, binding part and reaction pattern of oxLDL have not been elucidated; (2) the classification is unclear. As shown in Table 1, distinct investigators employed antibodies of different types in studies. In several studies, authors addressed anti-oxLDL [13,33,34,36-40], but the types of these antibodies (IgG/IgM) were not described. This may cause 3 consequences or even more consequence if IgG is divided into IgG1 and IgG2. (3) The investigations are not comprehensive. As shown in Table 1, some investigators used IgG [39], and IgM was used in other studies [35,38]. These studies only explained the activities of antibodies of a specific type, and could not elucidate the actions of all the antibodies. (4) The investigations are not precise. We speculate that investigation with an antibody targeting a specific epitope (such as a peptide segment or a specific conformation) is easy to differentiate antibodies against different epitopes and different types of antibodies, which is also helpful for the detailed classification and accurate conclusion. (5) The differences in the sample selection as well as methods and time points of detection may also cause discrepancies among studies. We should learn lessons from available studies to smoothly achieve more achievements from our studies.
New methods for the detection of oxLDL and its antibody
There are many methods used for the detection of serum anti-oxLDL. Yamaguchi et al [41] summarized these methods which have been used since the introduction of ELISA. In recent years, some investigators employed positron emission tomography (PET) for the evaluation of pharmacokinetic characteristics of oxLDL and its distribution in tissues [24]. PET showed oxLDL was mainly distributed in blood pool and may be found in the heart, trunk and major vessels of limbs, and scattered signals were also observed in the liver, kidney, spleen and bone marrow [24]. Örbom et al [36] performed multi-radionuclide autoradiography to detect anti-oxLDL conjugated to 2-deoxy-2-[18F]fluoro-D-glucose (18F-FDG) or 131I/125I, aiming to investigate the anti-oxLDL distribution in atherosclerotic mice. Montano et al [42] used non-radionuclide assay to detect oxLDL. They added biotinylated oxLDL (Bt-OxLDL) into 96-well plates containing macrophages, and solid-phase chromatographic immunoassay was performed to detect the binding of Bt-OxLDL to macrophages after different treatments. This method is simple and has no radiation. Above explorations in the detection of anti-oxLDL and oxLDL are successful, and we believe that more simple, convenient and accurate methods will be developed with the progression of science and technology.
New risk factors of atherosclerosis
The traditional risk factors of atherosclerosis include advanced age, hypertension, dyslipidemia, diabetes, myocardial ischemia, menopause, obesity, smoking, cerebrovascular disease and metabolic syndrome [43]. Classic immune related diseases (such as rheumatoid arthritis [RA], systemic lupus erythematosus [SLE], spondyloarthropathy [SpA], systemic sclerosis [SSc], mixed connective tissue disease [MCTD]) are also related to the occurrence of atherosclerosis. Investigators have identified antiphospholipid antibody in the patients with antiphospholipid antibody syndrome (APS), SLE, RA and MCTD, which has been regarded as a risk factor [44]. Cytokines (such as tumor necrosis factor-α [TNF-α] and interleukin-6 [IL-6]), immune complexes and auto-antibodies (such as anti-oxLDL, anti-cardiolipin and anti-β2GPI) are found to be risk factors of atherosclerosis.
In recent years, 2 new findings are identified in studies on the risk factors of atherosclerosis: 1) Hyperhomocysteinemia: In as early as 1960s, McCully et al [45] for the first time reported that atherosclerosis and thromboembolism were related to hyperhomocysteinemia. However, until 1991, Clarke et al [46] found that hyperhomocysteinemia was an independent risk factor of cardiovascular disease and thus they proposed the hypothesis of “homocysteine-cardiovascular disease”, which has been paid attention to by some clinicians. Recently, Joseph and Tsunenobu [47] proposed a new hypothesis of “homocysteine-ion iron-cardiovascular disease”. 2) HIV: Yilmaz et al [48] detected the serum titer of anti-oxLDL in patients infected by human immunodeficiency virus (HIV) and results showed anti-oxLDl IgG was higher and anti-oxLDl IgM was lower in HIV patients than those in healthy subjects. Thus, high serum anti-oxLDl IgG and low serum anti-oxLDl IgM may be risk factors of cardiovascular diseases in HIV patients.
Prospective
The effect (protective or detrimental) of oxLDL on the atherosclerosis is still controversial. There is a probability to achieve a consensus on the role of oxLDL in the atherosclerosis, but more studies are required. New methods introduced in the present study [24,36,42] may provide evidence for our future investigations. Studies on MPs [25] bring us a new field for the investigations of oxLDL, and more new fields are required to identify in future studies. Whether the hypothesis of “homocysteine-ion iron-cardiovascular disease” becomes true is still needed in-depth studies. With the development of science and technology, more information on the role of oxLDL in the cardiovascular diseases at different stages will be understood. Under this condition, clinicians may take individualized measures to treat patients according to a specific stage of diseases, which may significantly improve the therapeutic efficacy.
Disclosure of conflict of interest
None.
References
- 1.Steinberg D, Witztum JL. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:2311–2316. doi: 10.1161/ATVBAHA.108.179697. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 3.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson J, Nordin Fredrikson G, Schiopu A, Shah PK, Jansson B, Carlsson R. Oxidized LDL antibodies in treatment and risk assessment of atherosclerosis and associated cardiovascular disease. Curr Pharm Des. 2007;13:1021–1030. doi: 10.2174/138161207780487557. [DOI] [PubMed] [Google Scholar]
- 5.Berger JS, Rockman CB, Guyer KE, Lopez LR. Proatherogenic oxidized low-density lipoprotein/beta2-glycoprotein I complexes in arterial and venous disease. J Immunol Res. 2014;2014:234316. doi: 10.1155/2014/234316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kzhyshkowska J, Neyen C, Gordon S. Role of macrophage scavenger receptors in atherosclerosis. Immunobiology. 2012;217:492–502. doi: 10.1016/j.imbio.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 8.Boyd HC, Gown AM, Wolfbauer G, Chait A. Direct evidence for a protein recognized by a monoclonal antibody against oxidatively modified LDL in atherosclerotic lesions from a Watanabe heritable hyperlipidemic rabbit. Am J Pathol. 1989;135:815–825. [PMC free article] [PubMed] [Google Scholar]
- 9.Nilsson J, Hansson GK. Autoimmunity in atherosclerosis: a protective response losing control? J Intern Med. 2008;263:464–478. doi: 10.1111/j.1365-2796.2008.01945.x. [DOI] [PubMed] [Google Scholar]
- 10.Virella G, Lopes-Virella MF. Lipoprotein autoantibodies: measurement and significance. Clin Diagn Lab Immunol. 2003;10:499–505. doi: 10.1128/CDLI.10.4.499-505.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shoenfeld Y, Wu R, Dearing LD, Matsuura E. Are anti-oxidized low-density lipoprotein antibodies pathogenic or protective? Circulation. 2004;110:2552–2558. doi: 10.1161/01.CIR.0000143225.07377.EA. [DOI] [PubMed] [Google Scholar]
- 12.Zampieri S, Iaccarino L, Ghirardello A, Tarricone E, Arienti S, Sarzi-Puttini P, Gambari P, Doria A. Systemic lupus erythematosus, atherosclerosis, and autoantibodies. Ann N Y Acad Sci. 2005;1051:351–361. doi: 10.1196/annals.1361.077. [DOI] [PubMed] [Google Scholar]
- 13.Izar MC, Fonseca HA, Pinheiro LF, Monteiro CM, Povoa RM, Monteiro AM, Figueiredo-Neto AM, Gidlund MA, Fonseca FA. Adaptive immunity is related to coronary artery disease severity after acute coronary syndrome in subjects with metabolic syndrome. Diab Vasc Dis Res. 2013;10:32–39. doi: 10.1177/1479164112443374. [DOI] [PubMed] [Google Scholar]
- 14.Shimoni S, Bar I, Zilberman L, George J. Autoantibodies to oxidized low-density lipoprotein in patients with aortic regurgitation: association with aortic diameter size. Cardiology. 2014;128:54–61. doi: 10.1159/000357835. [DOI] [PubMed] [Google Scholar]
- 15.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 16.Parthasarathy S. Modified Lipoproteins in the Pathogenesis of Atherosclerosis. Austin, Tex: RG Landes Co; 1994. [Google Scholar]
- 17.Parthasarathy S, Raghavamenon A, Garelnabi MO, Santanam N. Oxidized low-density lipoprotein. Methods Mol Biol. 2010;610:403–417. doi: 10.1007/978-1-60327-029-8_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Babakr AT, Elsheikh OM, Almarzouki AA, Assiri AM, Abdalla BE, Zaki HY, Fatani SH, NourEldin EM. Relationship between oxidized low-density lipoprotein antibodies and obesity in different glycemic situations. Diabetes Metab Syndr Obes. 2014;7:513–520. doi: 10.2147/DMSO.S70904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuura E, Atzeni F, Sarzi-Puttini P, Turiel M, Lopez LR, Nurmohamed MT. Is atherosclerosis an autoimmune disease? BMC Med. 2014;12:47. doi: 10.1186/1741-7015-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grundy SM. Oxidized LDL and atherogenesis: relation to risk factors for coronary heart disease. Clin Cardiol. 1993;16:I3–5. doi: 10.1002/clc.4960161303. [DOI] [PubMed] [Google Scholar]
- 21.Trpkovic A, Resanovic I, Stanimirovic J, Radak D, Mousa SA, Cenic-Milosevic D, Jevremovic D, Isenovic ER. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit Rev Clin Lab Sci. 2014:1–16. doi: 10.3109/10408363.2014.992063. [DOI] [PubMed] [Google Scholar]
- 22.Libby P. Vascular biology of atherosclerosis: overview and state of the art. Am J Cardiol. 2003;91:3A–6A. doi: 10.1016/s0002-9149(02)03143-0. [DOI] [PubMed] [Google Scholar]
- 23.Svensjo E, Boschcov P, Ketelhuth DF, Jancar S, Gidlund M. Increased microvascular permeability in the hamster cheek pouch induced by oxidized low density lipoprotein (oxLDL) and some fragmented apolipoprotein B proteins. Inflamm Res. 2003;52:215–220. doi: 10.1007/s000110300074. [DOI] [PubMed] [Google Scholar]
- 24.Kamath AV, Williams SP, Bullens S, Cowan KJ, Stenberg Y, Cherry SR, Rendig S, Kukis DL, Griesemer C, Damico-Beyer LA, Bunting S. Pharmacokinetics and biodistribution of a human monoclonal antibody to oxidized LDL in cynomolgus monkey using PET imaging. PLoS One. 2012;7:e45116. doi: 10.1371/journal.pone.0045116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holvoet P, Harris TB, Tracy RP, Verhamme P, Newman AB, Rubin SM, Simonsick EM, Colbert LH, Kritchevsky SB. Association of high coronary heart disease risk status with circulating oxidized LDL in the well-functioning elderly: findings from the Health, Aging, and Body Composition study. Arterioscler Thromb Vasc Biol. 2003;23:1444–1448. doi: 10.1161/01.ATV.0000080379.05071.22. [DOI] [PubMed] [Google Scholar]
- 26.Meisinger C, Baumert J, Khuseyinova N, Loewel H, Koenig W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation. 2005;112:651–657. doi: 10.1161/CIRCULATIONAHA.104.529297. [DOI] [PubMed] [Google Scholar]
- 27.Bassi N, Zampieri S, Ghirardello A, Tonon M, Zen M, Beggio S, Matsuura E, Doria A. oxLDL/beta2GPI complex and anti-oxLDL/beta2GPI in SLE: prevalence and correlates. Autoimmunity. 2009;42:289–291. doi: 10.1080/08916930902828247. [DOI] [PubMed] [Google Scholar]
- 28.Lopez LR, Hurley BL, Simpson DF, Matsuura E. Oxidized low-density lipoprotein/beta2-glycoprotein I complexes and autoantibodies in patients with type 2 diabetes mellitus. Ann N Y Acad Sci. 2005;1051:97–103. doi: 10.1196/annals.1361.050. [DOI] [PubMed] [Google Scholar]
- 29.Piarulli F, Lapolla A, Sartore G, Rossetti C, Bax G, Noale M, Minicuci N, Fiore C, Marchioro L, Manzato E, Fedele D. Autoantibodies against oxidized LDLs and atherosclerosis in type 2 diabetes. Diabetes Care. 2005;28:653–657. doi: 10.2337/diacare.28.3.653. [DOI] [PubMed] [Google Scholar]
- 30.Shoji T, Fukumoto M, Kimoto E, Shinohara K, Emoto M, Tahara H, Koyama H, Ishimura E, Nakatani T, Miki T, Tsujimoto Y, Tabata T, Nishizawa Y. Antibody to oxidized low-density lipoprotein and cardiovascular mortality in end-stage renal disease. Kidney Int. 2002;62:2230–2237. doi: 10.1046/j.1523-1755.2002.00692.x. [DOI] [PubMed] [Google Scholar]
- 31.Barud W, Palusinski R, Beltowski J, Wojcicka G. Inverse relationship between total testosterone and anti-oxidized low density lipoprotein antibody levels in ageing males. Atherosclerosis. 2002;164:283–288. doi: 10.1016/s0021-9150(02)00069-2. [DOI] [PubMed] [Google Scholar]
- 32.Fukumoto M, Shoji T, Emoto M, Kawagishi T, Okuno Y, Nishizawa Y. Antibodies against oxidized LDL and carotid artery intima-media thickness in a healthy population. Arterioscler Thromb Vasc Biol. 2000;20:703–707. doi: 10.1161/01.atv.20.3.703. [DOI] [PubMed] [Google Scholar]
- 33.Nowak B, Szmyrka-Kaczmarek M, Durazinska A, Plaksej R, Borysewicz K, Korman L, Wiland P. Anti-ox-LDL antibodies and anti-ox-LDL-B2GPI antibodies in patients with systemic lupus erythematosus. Adv Clin Exp Med. 2012;21:331–335. [PubMed] [Google Scholar]
- 34.Gironi M, Borgiani B, Mariani E, Cursano C, Mendozzi L, Cavarretta R, Saresella M, Clerici M, Comi G, Rovaris M, Furlan R. Oxidative stress is differentially present in multiple sclerosis courses, early evident, and unrelated to treatment. J Immunol Res. 2014;2014:961863. doi: 10.1155/2014/961863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosseini H, Li Y, Kanellakis P, Tay C, Cao A, Tipping P, Bobik A, Toh BH, Kyaw T. Phosphatidylserine liposomes mimic apoptotic cells to attenuate atherosclerosis by expanding polyreactive IgM producing B1a lymphocytes. Cardiovasc Res. 2015;106:443–52. doi: 10.1093/cvr/cvv037. [DOI] [PubMed] [Google Scholar]
- 36.Orbom A, Jansson B, Schiopu A, Evans-Axelsson S, Nilsson J, Fredrikson GN, Strand SE. Multi-radionuclide digital autoradiography of the intra-aortic atherosclerotic plaques using a monoclonal antibody targeting oxidized low-density lipoprotein. Am J Nucl Med Mol Imaging. 2014;4:172–180. [PMC free article] [PubMed] [Google Scholar]
- 37.Suthers B, Hansbro P, Thambar S, McEvoy M, Peel R, Attia J. Pneumococcal vaccination may induce anti-oxidized low-density lipoprotein antibodies that have potentially protective effects against cardiovascular disease. Vaccine. 2012;30:3983–3985. doi: 10.1016/j.vaccine.2012.03.084. [DOI] [PubMed] [Google Scholar]
- 38.Tsiantoulas D, Perkmann T, Afonyushkin T, Mangold A, Prohaska TA, Papac-Milicevic N, Millischer V, Bartel C, Horkko S, Boulanger CM, Tsimikas S, Fischer MB, Witztum JL, Lang IM, Binder CJ. Circulating microparticles carry oxidation-specific epitopes and are recognized by natural IgM antibodies. J Lipid Res. 2015;56:440–448. doi: 10.1194/jlr.P054569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moohebati M, Kabirirad V, Ghayour-Mobarhan M, Esmaily H, Tavallaie S, Akhavan Rezayat A, Pourghadamyari H, Sahebkar A. Investigation of serum oxidized low-density lipoprotein IgG levels in patients with angiographically defined coronary artery disease. Int J Vasc Med. 2014;2014:845960. doi: 10.1155/2014/845960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sevinc Ok E, Kircelli F, Asci G, Altunel E, Ertilav M, Sipahi S, Bozkurt D, Duman S, Ozkahya M, Toz H, Ok E. Neither oxidized nor anti-oxidized low-density lipoprotein level is associated with atherosclerosis or mortality in hemodialysis patients. Hemodial Int. 2012;16:334–341. doi: 10.1111/j.1542-4758.2012.00683.x. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi Y, Kunitomo M, Haginaka J. Assay methods of modified lipoproteins in plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;781:313–330. doi: 10.1016/s1570-0232(02)00433-6. [DOI] [PubMed] [Google Scholar]
- 42.Montano EN, Boullier A, Almazan F, Binder CJ, Witztum JL, Hartvigsen K. Development and application of a nonradioactive binding assay of oxidized low-density lipoprotein to macrophage scavenger receptors. J Lipid Res. 2013;54:3206–3214. doi: 10.1194/jlr.D040923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lubrano V, Balzan S. Consolidated and emerging inflammatory markers in coronary artery disease. World J Exp Med. 2015;5:21–32. doi: 10.5493/wjem.v5.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soltesz P, Kerekes G, Der H, Szucs G, Szanto S, Kiss E, Bodolay E, Zeher M, Timar O, Szodoray P, Szegedi G, Szekanecz Z. Comparative assessment of vascular function in autoimmune rheumatic diseases: considerations of prevention and treatment. Autoimmun Rev. 2011;10:416–425. doi: 10.1016/j.autrev.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 45.McCully KS. Homocystinuria, arteriosclerosis, methylmalonic aciduria, and methyltransferase deficiency: a key case revisited. Nutr Rev. 1992;50:7–12. doi: 10.1111/j.1753-4887.1992.tb02454.x. [DOI] [PubMed] [Google Scholar]
- 46.Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B, Graham I. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324:1149–1155. doi: 10.1056/NEJM199104253241701. [DOI] [PubMed] [Google Scholar]
- 47.Baggott JE, Tamura T. Homocysteine, iron and cardiovascular disease: a hypothesis. Nutrients. 2015;7:1108–1118. doi: 10.3390/nu7021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yilmaz A, Jennbacken K, Fogelstrand L. Reduced IgM levels and elevated IgG levels against oxidized low-density lipoproteins in HIV-1 infection. BMC Infect Dis. 2014;14:143. doi: 10.1186/1471-2334-14-143. [DOI] [PMC free article] [PubMed] [Google Scholar]


