Abstract
Pulmonary hypertension is characterized by extensive vascular remodelling, leading to increased pulmonary vascular resistance and eventual death due to right heart failure. The pathogenesis of pulmonary hypertension involves vascular endothelial dysfunction and disordered vascular smooth muscle cell (VSMC) proliferation and migration, but the exact processes remain unknown. Sphingosine 1-phosphate (S1P) is a bioactive lysophospholipid involved in a wide spectrum of biological processes. S1P has been shown to regulate VSMC proliferation and migration and vascular tension via a family of five S1P G-protein-coupled receptors (S1P1-SIP5). S1P has been shown to have both a vasoconstrictive and vasodilating effect. The S1P receptors S1P1 and S1P3 promote, while S1P2 inhibits VSMC proliferation and migration in vitro in response to S1P. Moreover, it has been reported recently that sphingosine kinase 1 and S1P2 inhibitors might be useful therapeutic agents in the treatment of empirical pulmonary hypertension. The sphingosine kinase 1/S1P signalling pathways may play a role in the pathogenesis of pulmonary hypertension. Modulation of this pathway may offer novel therapeutic strategies.
Keywords: Pulmonary hypertension, sphingosine 1-phosphate, endothelial dysfunction, vascular smooth muscle cell, pulmonary vascular remodelling
Introduction
Pulmonary hypertension is characterized by abnormal remodelling of small pulmonary arteries, which leads to increased pulmonary vascular resistance and right heart failure [1,2]. Endothelial dysfunction is believed to be one of the early steps in the pulmonary hypertensive process, involving a decrease in the production of vasorelaxants, such as nitric oxide and prostacyclin, and an increase in the production of vasoconstrictors, such as endothelin [1-3]. This dysfunction leads to an increase in vascular smooth muscle cell (VSMC) proliferation, extracellular matrix deposition and hypercontractility.
Sphingosine 1-phosphate (S1P) is an important modulator of cell signalling that exerts its effects by binding to specific cell surface G-protein-coupled receptors. S1P is involved in a wide variety of physiological processes including angiogenesis, cell proliferation and migration, inflammatory cell trafficking, cytokine production, cytoskeletal reorganization, endothelial barrier regulation and the control of vasomotor tone [4-8]. These effects of S1P can regulate the vascular tone and the proliferation and migration of VSMCs in the systemic circulation [9-12].
It is important to establish the role of the S1P signalling pathway in the pathogenesis of pulmonary hypertension to understand how modulation of this pathway may offer novel therapeutic strategies. The exact processes involved in the pathogenesis of the disease remain unknown. The aim of this review article is to present an overview of the current understanding of the S1P signalling pathway in the pathogenesis of pulmonary hypertension.
S1P biosynthesis and signalling
Sphingolipids are ubiquitous components of the lipid bilayer of eukaryotic cells. As in the case of glycolipids, sphingolipid metabolism is regulated by numerous agonists that generate signalling molecules, including ceramide (N-acyl sphingosine), sphingosine, and S1P [13]. Ceramide, the backbone of sphingolipids, is produced by de novo synthesis and turnover of sphingolipids. After removal of the sphingolipid head groups during catabolism, deacylation of ceramide by ceramidases yields sphingosine [14]. Sphingosine is phosphorylated by type 1 and type 2 sphingosine kinases (SphK1 and SphK2) to form S1P. S1P can undergo degradation by one of two pathways: it may be converted to sphingosine by reversible dephosphorylation mediated by a variety of phosphohydrolases; or it may form ethanolamine phosphate and hexadecanol after undergoing irreversible cleavage mediated by S1P lyase [13].
Sphingosine 1-phosphate is a bioactive lysophospholipid that mediates many important cellular processes, including proliferation, migration, differentiation, cytoskeletal rearrangements, motility, angiogenesis, calcium mobilization, lymphocyte trafficking, and immune function [5-8]. Most cells have the enzymatic machinery to synthesize S1P. In serum and plasma, the S1P concentrations range about between 200 and 900 nM, but these values are likely to change under different pathological conditions. Sources of S1P in plasma include red blood cells [8], platelets [15], and endothelial cells [16]. S1P levels are reported to be 8-fold greater in the lungs than elsewhere [17].
Many actions of S1P are mediated via five S1P G-protein-coupled receptor subtypes (S1P1-S1P5) [13,18,19]. Although S1P receptors are expressed in almost every cell type, S1P1, S1P2 and S1P3 are predominant in the vascular system [20]. Reverse transcription-polymerase chain reaction analysis showed that S1P1 and S1P3 messenger RNA (mRNA) were present in both pulmonary artery endothelial cells and pulmonary artery VSMCs, while S1P2 mRNA was confined to pulmonary artery VSMCs [21].
S1P in endothelial dysfunction
Pulmonary vasoconstriction is believed to be an early step in the pulmonary hypertensive process. Excessive vasoconstriction is related to endothelial dysfunction [3], and endothelial dysfunction is characterized by decreased levels of nitric oxide (NO) [22] and prostacyclin [23], which occur concomitantly with increased endothelin-1 levels [24]. NO is a potent pulmonary arterial vasodilator and a direct inhibitor of platelet activation and VSMC proliferation. The reduced NO bioavailability in pulmonary hypertension may be due to decreased endothelial NO synthase (eNOS) expression, inhibition of eNOS enzymatic activity or inactivation of NO by superoxide anion. Prostacyclin acts synergistically with NO to induce VSMC relaxation, inhibit platelet activation and prevent VSMC migration and proliferation.
S1P has been shown to inhibit inducible NOS expression and interleukin-1β-induced NO production in rat VSMCs [25]. In contrast, others have found that NO and prostaglandin I2 synthesis were stimulated by S1P in vascular endothelial cells and VSMCs in vitro [26-29]. A study by Morales-Ruiz et al. found that S1P activates eNOS downstream of S1P1 in bovine microvascular endothelial cells in vitro, suggesting that S1P drives pulmonary vasorelaxation [26].
It is important to note that S1P has been found to induce vasoconstriction instead of vasodilatation in some experimental systems. Studies have suggested that S1P exerts a vasoconstrictive response in the pulmonary vasculature through increasing the tension in isolated conduit pulmonary arterial segments [30,31]. S1P induced dose-dependent pulmonary vasoconstriction in the mouse via S1P2 and a Rho-kinase-mediated signal transduction pathway [32]. In addition, a study assessing vasoconstriction in normoxic and hypoxic isolated rat lungs suggested a significant role for S1P4 in S1P-induced vasoconstriction [33]. S1P may therefore play a role in the increased pulmonary vascular resistance associated with pulmonary hypertension. Further research is needed to determine the detailed mechanisms underlying the S1P-mediated regulation of vascular tone.
SphK1/S1P pathway in pulmonary vascular remodelling
Vascular remodelling is characterized by medial hypertrophy due to enhanced VSMC proliferation and migration, attenuated apoptosis and over proliferation of endothelial cells, all of which can result in lumen obstruction and pulmonary hypertension. The proliferation and migration of VSMCs induces an increase in the deposition of VSMCs on the vascular matrix [34]. An increase in extracellular matrix components and myofibroblast formation are also involved in the remodelling process [35,36].
The significantly increased expression of S1P in VSMCs subjected to hypoxia contributes to the proliferation and migration of these cells [37]. In addition, the growth of hypoxia-induced VSMCs is significantly inhibited by the competitive SphK inhibitor, D-erythro-N,N-dimethyl-sphingosine (DMS), indicating that intracellular S1P may play a regulatory role in key signalling in response to hypoxia [38]. DMS inhibits VSMC growth by inhibition of both extracellular signal-regulated kinase-1/2 (ERK-1/2) and phosphorylated protein kinase B (Akt) signalling, which are involved in regulating cell growth and survival [39]. Chronic hypoxia increases the phosphorylation of ERK-1/2 in response to S1P [40]. The enhancing effect of chronic hypoxia on the responsiveness of ERK1/2 to S1P might be related to the regulation of hypoxia-inducible factors, which are activated by ERK1/2 and control the expression of growth factors such as vascular endothelial growth factor. These growth factors can in turn promote vascular remodelling. Hypoxia was also shown to induce increased levels of SphK1 in human pulmonary artery VSMCs, but has no effect on S1P1 or S1P3 mRNA levels [40]. Further research is required to elucidate S1P-induced VSMC proliferation in response to hypoxia.
Activation of S1P2 antagonizes S1P-induced VSMC proliferation and migration via a Rho-dependent pathway, whereas S1P1/S1P3 activation promotes VSMC proliferation and in vitro phenotypic modulation (Figure 1) [11,41-43]. S1P1, S1P2 and S1P3 are coupled to different and opposing signalling cascades. S1P1 couples exclusively with members of the Giα family, and S1P2 and S1P3 couple to multiple G proteins including Gqα and G12/13α [44]. S1P stimulates activation of phosphatidylinositol 3-kinase/Akt and ERK via S1P1, and RhoA via S1P2 [45,46]. S1P also induces the release of calcium from intracellular stores via S1P3 [45,46].
Figure 1.
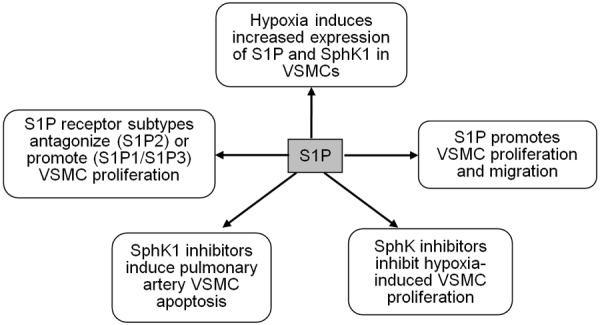
Roles of the sphingosine-1-phosphate (S1P) signalling pathway in pulmonary artery vascular smooth muscle cells (VSMCs). SphK1; sphingosine kinase type 1.
Basic fibroblast growth factor is involved in the physiological activities of VSMCs, including protection from apoptosis, promotion of proliferation and migration. In addition, basic fibroblast growth factor upregulates S1P1 in human pulmonary artery VSMCs [47], which may contribute to pulmonary vascular remodelling.
Studies have examined the effects of the S1P signalling pathway on pulmonary artery cells, and found that S1P increased Rho kinase activity in a time-dependent manner in pulmonary artery VSMCs [32]. Rho kinase has been shown to play an important role in the pathogenesis of pulmonary hypertension [21,48,49]. Research has also highlighted the role of SphK1 in the immunological pathogenesis of pulmonary arterial hypertension. Reduction of SphK1 activity increased pulmonary vascular hyper-responsiveness and contributed to the development of inflammation-associated pulmonary hypertension [50], and inhibition of SphK1 induced apoptosis in pulmonary artery VSMCs [51].
Empirical studies in which SphK1 and S1P2 inhibitors attenuate PH
It has recently been suggested that SphK1 and S1P2 inhibitors might be useful therapeutic agents in the treatment of pulmonary hypertension [52]. SphK1 and S1P were significantly increased in the lungs of experimental hypoxia-induced pulmonary hypertension mice and from patients with pulmonary hypertension. SphK1 deficient (SphK1-/-) mice were protected from hypoxia-induced pulmonary hypertension [52]. SKI2, the inhibitor of both SphK1 and SphK2 prevented the development of hypoxia-induced pulmonary hypertension and inhibited pulmonary vascular remodeling [52]. Moreover, JTE013, the S1P2 inhibitor prevented and reversed the development of hypoxia-induced pulmonary hypertension and pulmonary vascular remodeling [52]. Thus, these data suggested that Sphk1/S1P signalling pathway played a critical role in the development of hypoxia-induced pulmonary hypertension.
Conclusions
This review summarizes the roles of the S1P signalling pathway in the pathogenesis of pulmonary hypertension and suggests a new approach for the treatment of the disease. Evidence from in vivo and clinical studies is limited, and further studies are necessary to fully examine the potential therapeutic implications of targeting S1P and its receptors in pulmonary hypertension. Modulating the S1P signalling pathway may provide novel therapeutic strategies for pharmacological intervention in pulmonary hypertension.
Acknowledgements
This work was supported by National Natural Science Foundation of China (81100037, 81360049); Yunnan Applied Basic Research Projects (2011FB154, 2013FZ231, 2013FZ230); Yunnan Applied Basic Research Projects-Joint Special Project (2014FA018).
Disclosure of conflict of interest
None.
References
- 1.Zaiman A, Fijalkowska I, Hassoun PM, Tuder RM. One hundred years of research in the pathogenesis of pulmonary hypertension. Am J Respir Cell Mol Biol. 2005;33:425–431. doi: 10.1165/rcmb.F307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J, Harrington RA, Anderson JL, Bates ER, Bridges CR, Eisenberg MJ, Ferrari VA, Grines CL, Hlatky MA, Jacobs AK, Kaul S, Lichtenberg RC, Moliterno DJ, Mukherjee D, Pohost GM, Schofield RS, Shubrooks SJ, Stein JH, Tracy CM, Weitz HH, Wesley DJ. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc. , and the Pulmonary Hypertension Association. Circulation. 2009;119:2250–2294. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 3.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Dantas AP, Igarashi J, Michel T. Sphingosine 1-phosphate and control of vascular tone. Am J Physiol Heart Circ Physiol. 2003;284:H2045–2052. doi: 10.1152/ajpheart.01089.2002. [DOI] [PubMed] [Google Scholar]
- 5.Tao R, Zhang J, Vessey DA, Honbo N, Karliner JS. Deletion of the sphingosine kinase-1 gene influences cell fate during hypoxia and glucose deprivation in adult mouse cardiomyocytes. Cardiovasc Res. 2007;74:56–63. doi: 10.1016/j.cardiores.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Maceyka M, Payne SG, Milstien S, Spiegel S. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim Biophys Acta. 2002;1585:193–201. doi: 10.1016/s1388-1981(02)00341-4. [DOI] [PubMed] [Google Scholar]
- 7.Saba JD, Hla T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ Res. 2004;94:724–734. doi: 10.1161/01.RES.0000122383.60368.24. [DOI] [PubMed] [Google Scholar]
- 8.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 9.Salomone S, Yoshimura S, Reuter U, Foley M, Thomas SS, Moskowitz MA, Waeber C. S1P3 receptors mediate the potent constriction of cerebral arteries by sphingosine-1-phosphate. Eur J Pharmacol. 2003;469:125–134. doi: 10.1016/s0014-2999(03)01731-x. [DOI] [PubMed] [Google Scholar]
- 10.Coussin F, Scott RH, Wise A, Nixon GF. Comparison of sphingosine 1-phosphate-induced intracellular signaling pathways in vascular smooth muscles: differential role in vasoconstriction. Circ Res. 2002;91:151–157. doi: 10.1161/01.res.0000028150.51130.36. [DOI] [PubMed] [Google Scholar]
- 11.Wamhoff BR, Lynch KR, Macdonald TL, Owens GK. Sphingosine-1-phosphate receptor subtypes differentially regulate smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2008;28:1454–1461. doi: 10.1161/ATVBAHA.107.159392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoue S, Nakazawa T, Cho A, Dastvan F, Shilling D, Daum G, Reidy M. Regulation of arterial lesions in mice depends on differential smooth muscle cell migration: a role for sphingosine-1-phosphate receptors. J Vasc Surg. 2007;46:756–763. doi: 10.1016/j.jvs.2007.05.055. [DOI] [PubMed] [Google Scholar]
- 13.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 14.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 15.Tani M, Sano T, Ito M, Igarashi Y. Mechanisms of sphingosine and sphingosine 1-phosphate generation in human platelets. J Lipid Res. 2005;46:2458–2467. doi: 10.1194/jlr.M500268-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murata N, Sato K, Kon J, Tomura H, Okajima F. Quantitative measurement of sphingosine 1-phosphate by radioreceptor-binding assay. Anal Biochem. 2000;282:115–120. doi: 10.1006/abio.2000.4580. [DOI] [PubMed] [Google Scholar]
- 18.Hla T, Lee MJ, Ancellin N, Paik JH, Kluk MJ. Lysophospholipids--receptor revelations. Science. 2001;294:1875–1878. doi: 10.1126/science.1065323. [DOI] [PubMed] [Google Scholar]
- 19.Usui S, Sugimoto N, Takuwa N, Sakagami S, Takata S, Kaneko S, Takuwa Y. Blood lipid mediator sphingosine 1-phosphate potently stimulates platelet-derived growth factor-A and -B chain expression through S1P1-Gi-Ras-MAPK-dependent induction of Kruppel-like factor 5. J Biol Chem. 2004;279:12300–12311. doi: 10.1074/jbc.M305025200. [DOI] [PubMed] [Google Scholar]
- 20.Kono M, Mi Y, Liu Y, Sasaki T, Allende ML, Wu YP, Yamashita T, Proia RL. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem. 2004;279:29367–29373. doi: 10.1074/jbc.M403937200. [DOI] [PubMed] [Google Scholar]
- 21.Harvey KA, Welch Z, Sliva D, Siddiqui RA. Role of Rho kinase in sphingosine 1-phosphate-mediated endothelial and smooth muscle cell migration and differentiation. Mol Cell Biochem. 2010;342:7–19. doi: 10.1007/s11010-010-0461-2. [DOI] [PubMed] [Google Scholar]
- 22.Cella G, Bellotto F, Tona F, Sbarai A, Mazzaro G, Motta G, Fareed J. Plasma markers of endothelial dysfunction in pulmonary hypertension. Chest. 2001;120:1226–1230. doi: 10.1378/chest.120.4.1226. [DOI] [PubMed] [Google Scholar]
- 23.Tuder RM, Cool CD, Geraci MW, Wang J, Abman SH, Wright L, Badesch D, Voelkel NF. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999;159:1925–1932. doi: 10.1164/ajrccm.159.6.9804054. [DOI] [PubMed] [Google Scholar]
- 24.Frasch HF, Marshall C, Marshall BE. Endothelin-1 is elevated in monocrotaline pulmonary hypertension. Am J Physiol. 1999;276:L304–310. doi: 10.1152/ajplung.1999.276.2.L304. [DOI] [PubMed] [Google Scholar]
- 25.Machida T, Hamaya Y, Izumi S, Iizuka K, Igarashi Y, Minami M, Levi R, Hirafuji M. Sphingosine 1-phosphate inhibits nitric oxide production induced by interleukin-1beta in rat vascular smooth muscle cells. J Pharmacol Exp Ther. 2008;325:200–209. doi: 10.1124/jpet.107.127290. [DOI] [PubMed] [Google Scholar]
- 26.Morales-Ruiz M, Lee MJ, Zollner S, Gratton JP, Scotland R, Shiojima I, Walsh K, Hla T, Sessa WC. Sphingosine 1-phosphate activates Akt, nitric oxide production, and chemotaxis through a Gi protein/phosphoinositide 3-kinase pathway in endothelial cells. J Biol Chem. 2001;276:19672–19677. doi: 10.1074/jbc.M009993200. [DOI] [PubMed] [Google Scholar]
- 27.Kimura T, Sato K, Kuwabara A, Tomura H, Ishiwara M, Kobayashi I, Ui M, Okajima F. Sphingosine 1-phosphate may be a major component of plasma lipoproteins responsible for the cytoprotective actions in human umbilical vein endothelial cells. J Biol Chem. 2001;276:31780–31785. doi: 10.1074/jbc.M104353200. [DOI] [PubMed] [Google Scholar]
- 28.Damirin A, Tomura H, Komachi M, Tobo M, Sato K, Mogi C, Nochi H, Tamoto K, Okajima F. Sphingosine 1-phosphate receptors mediate the lipid-induced cAMP accumulation through cyclooxygenase-2/prostaglandin I2 pathway in human coronary artery smooth muscle cells. Mol Pharmacol. 2005;67:1177–1185. doi: 10.1124/mol.104.004317. [DOI] [PubMed] [Google Scholar]
- 29.Nodai A, Machida T, Izumi S, Hamaya Y, Kohno T, Igarashi Y, Iizuka K, Minami M, Hirafuji M. Sphingosine 1-phosphate induces cyclooxygenase-2 via Ca2+-dependent, but MAPK-independent mechanism in rat vascular smooth muscle cells. Life Sci. 2007;80:1768–1776. doi: 10.1016/j.lfs.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Hsiao SH, Constable PD, Smith GW, Haschek WM. Effects of exogenous sphinganine, sphingosine, and sphingosine-1-phosphate on relaxation and contraction of porcine thoracic aortic and pulmonary arterial rings. Toxicol Sci. 2005;86:194–199. doi: 10.1093/toxsci/kfi167. [DOI] [PubMed] [Google Scholar]
- 31.Thomas GD, Snetkov VA, Patel R, Leach RM, Aaronson PI, Ward JP. Sphingosylphosphorylcholine-induced vasoconstriction of pulmonary artery: activation of non-store-operated Ca2+ entry. Cardiovasc Res. 2005;68:56–64. doi: 10.1016/j.cardiores.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 32.Szczepaniak WS, Pitt BR, McVerry BJ. S1P2 receptor-dependent Rho-kinase activation mediates vasoconstriction in the murine pulmonary circulation induced by sphingosine 1-phosphate. Am J Physiol Lung Cell Mol Physiol. 2010;299:L137–145. doi: 10.1152/ajplung.00233.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ota H, Beutz MA, Ito M, Abe K, Oka M, McMurtry IF. S1P(4) receptor mediates S1P-induced vasoconstriction in normotensive and hypertensive rat lungs. Pulm Circ. 2011;1:399–404. doi: 10.4103/2045-8932.87309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan SY, Loscalzo J. Pathogenic mechanisms of pulmonary arterial hypertension. J Mol Cell Cardiol. 2008;44:14–30. doi: 10.1016/j.yjmcc.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99:675–691. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 36.Jeffery TK, Wanstall JC. Pulmonary vascular remodeling: a target for therapeutic intervention in pulmonary hypertension. Pharmacol Ther. 2001;92:1–20. doi: 10.1016/s0163-7258(01)00157-7. [DOI] [PubMed] [Google Scholar]
- 37.Tanski W, Roztocil E, Davies MG. Sphingosine-1-phosphate induces G(alphai)-coupled, PI3K/ras-dependent smooth muscle cell migration. J Surg Res. 2002;108:98–106. doi: 10.1006/jsre.2002.6529. [DOI] [PubMed] [Google Scholar]
- 38.Yun JK, Kester M. Regulatory role of sphingomyelin metabolites in hypoxia-induced vascular smooth muscle cell proliferation. Arch Biochem Biophys. 2002;408:78–86. doi: 10.1016/s0003-9861(02)00526-x. [DOI] [PubMed] [Google Scholar]
- 39.McDonald RA, Pyne S, Pyne NJ, Grant A, Wainwright CL, Wadsworth RM. The sphingosine kinase inhibitor N,N-dimethylsphingosine inhibits neointimal hyperplasia. Br J Pharmacol. 2010;159:543–553. doi: 10.1111/j.1476-5381.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmad M, Long JS, Pyne NJ, Pyne S. The effect of hypoxia on lipid phosphate receptor and sphingosine kinase expression and mitogen-activated protein kinase signaling in human pulmonary smooth muscle cells. Prostaglandins Other Lipid Mediat. 2006;79:278–286. doi: 10.1016/j.prostaglandins.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Ryu Y, Takuwa N, Sugimoto N, Sakurada S, Usui S, Okamoto H, Matsui O, Takuwa Y. Sphingosine-1-phosphate, a platelet-derived lysophospholipid mediator, negatively regulates cellular Rac activity and cell migration in vascular smooth muscle cells. Circ Res. 2002;90:325–332. doi: 10.1161/hh0302.104455. [DOI] [PubMed] [Google Scholar]
- 42.Sugimoto N, Takuwa N, Okamoto H, Sakurada S, Takuwa Y. Inhibitory and stimulatory regulation of Rac and cell motility by the G12/13-Rho and Gi pathways integrated downstream of a single G protein-coupled sphingosine-1-phosphate receptor isoform. Mol Cell Biol. 2003;23:1534–1545. doi: 10.1128/MCB.23.5.1534-1545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goparaju SK, Jolly PS, Watterson KR, Bektas M, Alvarez S, Sarkar S, Mel L, Ishii I, Chun J, Milstien S, Spiegel S. The S1P2 receptor negatively regulates platelet-derived growth factor-induced motility and proliferation. Mol Cell Biol. 2005;25:4237–4249. doi: 10.1128/MCB.25.10.4237-4249.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Windh RT, Lee MJ, Hla T, An S, Barr AJ, Manning DR. Differential coupling of the sphingosine 1-phosphate receptors Edg-1, Edg-3, and H218/Edg-5 to the G(i), G(q), and G(12) families of heterotrimeric G proteins. J Biol Chem. 1999;274:27351–27358. doi: 10.1074/jbc.274.39.27351. [DOI] [PubMed] [Google Scholar]
- 45.Ishii I, Ye X, Friedman B, Kawamura S, Contos JJ, Kingsbury MA, Yang AH, Zhang G, Brown JH, Chun J. Marked perinatal lethality and cellular signaling deficits in mice null for the two sphingosine 1-phosphate (S1P) receptors, S1P(2)/LP(B2)/EDG-5 and S1P(3)/LP(B3)/EDG-3. J Biol Chem. 2002;277:25152–25159. doi: 10.1074/jbc.M200137200. [DOI] [PubMed] [Google Scholar]
- 46.Watterson KR, Ratz PH, Spiegel S. The role of sphingosine-1-phosphate in smooth muscle contraction. Cell Signal. 2005;17:289–298. doi: 10.1016/j.cellsig.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 47.Birker-Robaczewska M, Studer R, Haenig B, Menyhart K, Hofmann S, Nayler O. bFGF induces S1P1 receptor expression and functionality in human pulmonary artery smooth muscle cells. J Cell Biochem. 2008;105:1139–1145. doi: 10.1002/jcb.21918. [DOI] [PubMed] [Google Scholar]
- 48.Xing XQ, Gan Y, Wu SJ, Chen P, Zhou R, Xiang XD. Rho-kinase as a potential therapeutic target for the treatment of pulmonary hypertension. Drug News Perspect. 2006;19:517–522. doi: 10.1358/dnp.2006.19.9.1050426. [DOI] [PubMed] [Google Scholar]
- 49.Xing XQ, Gan Y, Wu SJ, Chen P, Zhou R, Xiang XD. Statins may ameliorate pulmonary hypertension via RhoA/Rho-kinase signaling pathway. Med Hypotheses. 2007;68:1108–1113. doi: 10.1016/j.mehy.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 50.Haberberger RV, Tabeling C, Runciman S, Gutbier B, Konig P, Andratsch M, Schutte H, Suttorp N, Gibbins I, Witzenrath M. Role of sphingosine kinase 1 in allergen-induced pulmonary vascular remodeling and hyperresponsiveness. J Allergy Clin Immunol. 2009;124:933–941. e1–9. doi: 10.1016/j.jaci.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 51.Tonelli F, Lim KG, Loveridge C, Long J, Pitson SM, Tigyi G, Bittman R, Pyne S, Pyne NJ. FTY720 and (S)-FTY720 vinylphosphonate inhibit sphingosine kinase 1 and promote its proteasomal degradation in human pulmonary artery smooth muscle, breast cancer and androgen-independent prostate cancer cells. Cell Signal. 2010;22:1536–1542. doi: 10.1016/j.cellsig.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J, Tang H, Sysol JR, Moreno-Vinasco L, Shioura KM, Chen T, Gorshkova I, Wang L, Huang LS, Usatyuk PV, Sammani S, Zhou G, Raj JU, Garcia JG, Berdyshev E, Yuan JX, Natarajan V, Machado RF. The sphingosine kinase 1/sphingosine-1-phosphate pathway in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;190:1032–1043. doi: 10.1164/rccm.201401-0121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


