Abstract
Objective: Large-scale clinical studies have shown that ulcerative colities were related with colorectal cancer. In this study, animal model was established by AOM/DSS method to explore the activation of IL-6-STAT3-SOCS3 signaling pathway and the expression of pathway-related proteins in ulcerative colitis carcinogenesis, in order to lay a foundation for exploring the regulation mechanism of IL-6/STAT3/SOCS3 signaling pathway in ulcerative colitis carcinogenesis. Method: AOM/DSS modeling method was used to establish animal models of ulcerative colitis carcinogenesis; colonic mucosa specimens were collected at different time points for pathological examination. Immunohistochemical method and western blot were used to detect the expression of IL6, STAT3 and SOCS3 protein in the control group, UC model + empty vector group and UC model + STAT3 knockout group. Results: In UC model + empty vector group, IL6 and STAT3 expression was increased as lesion degree increased (P < 0.05). The expression of SOCS3 was weakened and the degree of activation decreased (P < 0.05). IL6 expression increased in UC model + STAT3 knockout group (P < 0.05) while the expression of SOCS3 decreased; compared with the UC model + empty vector group, there was a significant difference (P < 0.05). Conclusion: The expression and activation of IL6 and STAT3 expression were enhanced in ulcerative colitis carcinogenesis, and their expression increased with the lesion degree increased, reflecting the disease progression to a certain extent. The expression and activation of SOCS3 expression decreased. STAT3 had a certain effect on the expression of SOCS3, playing a certain regulatory role in ulcerative colitis carcinogenesis.
Keywords: Ulcerative colitis, signaling pathways, IL-6, STAT3, SOCS3
Introduction
Ulcerative colitis is an unexplained chronic and non-specific inflammatory bowel disease. It is easy to seizure and is a well-recognized disease difficult to treat. At the same time, the disease has a trend of canceration in long-term unhealing patients [1]. Clinical studies confirmed that ulcerative colitis had some relevance with colorectal cancer incidence. The occurrence of ulcerative colitis-related colorectal cancer experienced the pathological process of “inflammation-dysplasia-cancer” [2]. Recent studies have found that, JAK-STAT signaling pathway plays an important role in the inflammation-induced tumors. Nguyen et al [3] had found that STAT3 colon played an important role in inflammation-induced malignant tumors, primarily by Treg cell infiltration. Chakilam et al [4] found that the TNF-IL-6-STAT3 signaling pathway played an important role in UC-associated tumors. Compared to normal UC, death-associated protein kinase (DAPK) level was higher and phosphorylated STAT3 level was lower in cancerated UC epithelial cells. STAT3 combined with the anti-inflammatory promoter region of DAPK to inhibit DAPK expression, and induced IL-6 expression to strengthen self activation. SOCS3 activation has STAT3 dependent pathway and STAT3-independent pathway; in the development of UC to CRC, IL-6-STAT3-SOCS3 signaling pathway has different levels of activation, with different effects on the system and local immune function [5]. In this study, ICR male mice and STAT knockout mice were treated with AOM/DSS modeling method to obtain target animal models; colonic mucosa specimens were collected at different time points for pathological and protein expression detection, in order to explore the regulatory role of IL-6/STAT3/SOCS3 signaling pathway in ulcerative colitis carcinogenesis.
Material and methods
Material
ICR male mice and STAT3 knockout mice were purchased from the Western Biotechnology, Chongqing, China. According to the previous protocol [6], the establishment of UC animal models with pathological process of “Inflammation-dysplasia-cancer” was performed. The mice were divided into control group, UC model + empty vector group and UC model + STAT3 knockout group; meanwhile, 5 mice in each group were sacrificed respectively at the endof 2nd, 4th, 6th, 8th, 10th and 12th week and colorectal tissues were collected for pathological observation. Peripheral blood samples were collected. The colon tissue was cut longitudinally, spreaded and observed; ulcers, inflammation, mass and tumor were recorded. The sites with the most serious ulcer, atypical hyperplasia and tumors were separately intercepted, and tissue samples were collected and fixed in 4% formalin for paraffin embedding and sectioning.
Methods
Pathological examination
HE staining of mice colorectal tissues obtained at different time points was performed; and histopathological scoring was condcuted according to the criteria shown in Table 1.
Table 1.
Colorectal histological score
| Characteristics of the colon in light microscopy | Score |
|---|---|
| Normal mucosa without damage | 0 |
| Crypt glands lost 1/3 | 1 |
| Crypt glands lost 2/3 | 2 |
| Crypt glands were all lost. Monolayer epithelial which was covered with lamina propria were existed and accompanied with inflammatory cell infiltration | 3 |
| Epithelial erosion and destruction were existed and accompanied with significant inflammatory cell infiltration | 4 |
Immunoassay
ELISA assay was used to detect the expression of IL-6, STAT3 and SOCS3. ELISA kit was provided by Beijing Mebo biotechnology company; detection was performed strictly in accordance with the operating instructions; measuring instrument was the ELISA analyzer provided by Beijing Shengda medical equipment company.
Western blot
Western blot for all biological samples was performed by Beijing Han Heng Biotechnology Co., LTD.
Statistical analysis
Spss20.0 software was used for statistical analysis; descriptive analysis, normality test and homogeneity of variance test were performed; in the descriptive analysis, normally distributed data were expressed as Mean ± SD; normally distributed data were analyzed by homogeneity of variance test before independent sample t-test; Spear-man method was used for correlation analysis; P < 0.05 indicated a statistically significant difference.
Results
Pathological changes (HE staining)
Control group: intestinal epithelial integrity, regular gland arrangement, normal crypt; no congestion and edema, erosion, ulceration, and inflammatory cell infiltration (Figure 1A).
Figure 1.
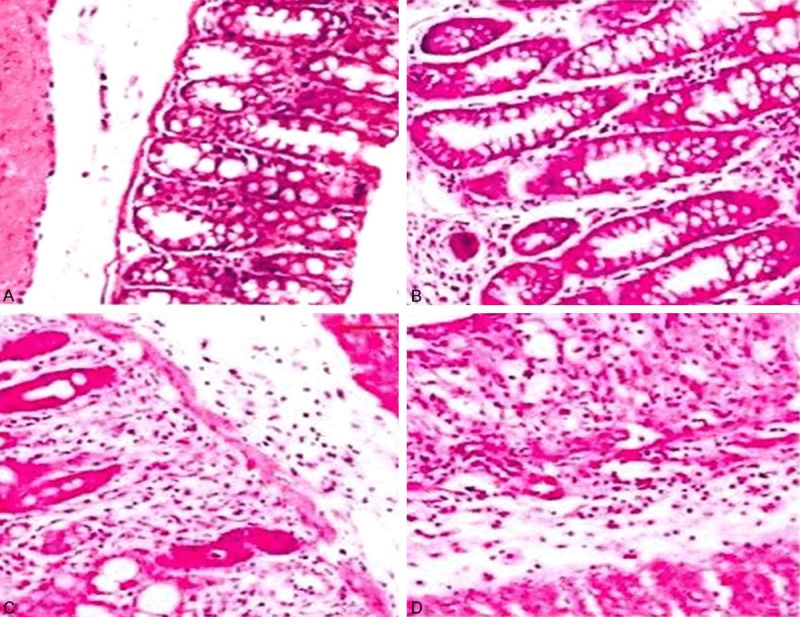
The image of pathological inflammation in mice. A: Normal mucosa; B-D: Mucosal pathological images at different times.
UC model group + empty vector: After two weeks, the mice had shown significant inflammations, epithelial cell injury and shedding, varying degrees of disappearence of glandular structures; with time prolonging, there were a large number of inflammatory cell infiltrations (Figure 1B-D), submucosal lymphocyte proliferation accompanied by the generation of lymphoid bubble, irregular gland arrangement, loss of polarity, deeply stained nuclei, increased nuclear cytoplasm ratio, and different degrees of dysplasia; and the evolution of “inflammation-dysplasia-cancer” was observed in the same colorectal cancer sample (Figure 2A-C).
Figure 2.
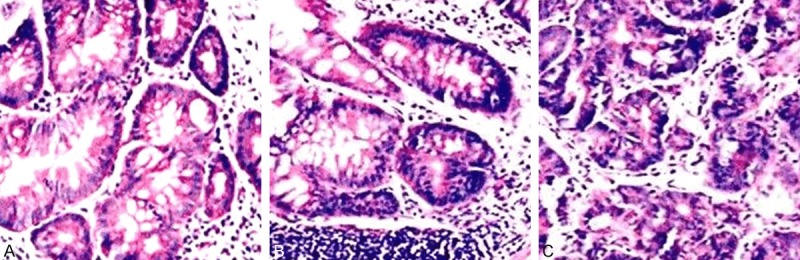
Inflammation-dysplasia-cancer carcinogenesis pathological process. A: Mild dysplasia; B: The severe dysplasia; C: Invasive cancer.
UC model + STAT3 knockout group: All mice had shown a significant inflammatory response which were similar with the results that shown in (Figure 1B-D), but over time, only few mice showed the same “Inflammation-dysplasia-cancer” sequence evolution with the UC model group + empty vector group (Figure 2A-C); while the vast majority of mice only showed significant inflammatory response.
Immunohistochemical results
ELISA to detect differences in IL6 level
At different time points, IL-6 levels in UC model + empty vector and UC model + STAT3 knockout groups were significantly higher than that in the control group (P < 0.01), and there was no significant difference between UC model + empty vector group and UC model + STAT3 knockout group in IL-6 level (P > 0.05, Figure 3).
Figure 3.
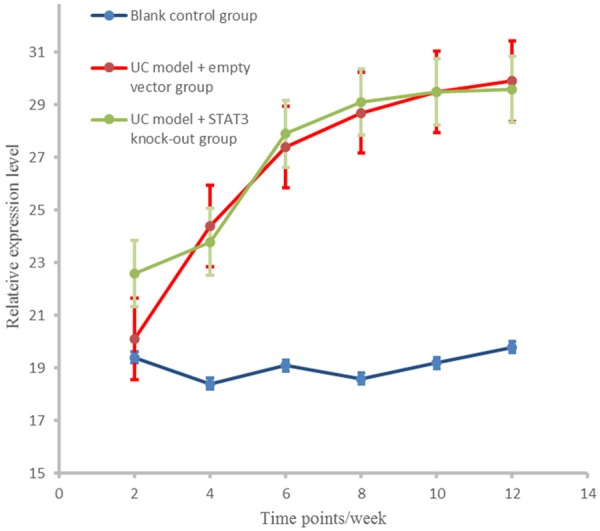
IL-6 expression level in different groups at different stages.
ELISA to detect differences in STAT3 level
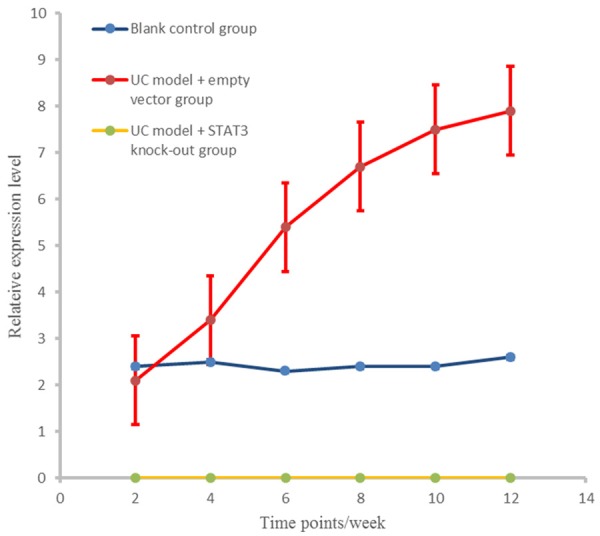
At different time points, STAT3 level in UC model + empty vector group was significantly higher than that in the control group (P < 0.01); no STAT3 had been found in UC model + STAT3 knockout group as shown in Figure 4.
Figure 4.
SATA3 expression levels in different groups at different stages.
ELISA to detect differences in SOCS3 level
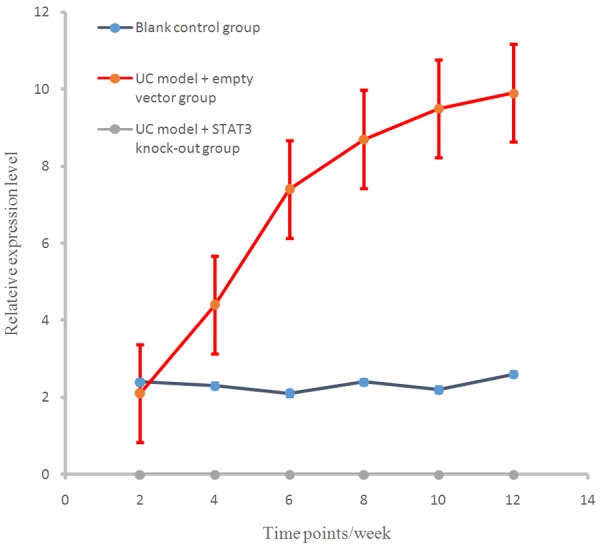
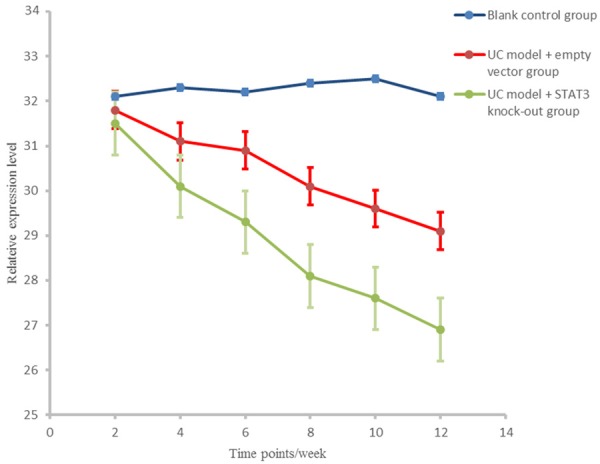
At different time points, SOCS3 levels in the control group and the UC model + STAT3 knockout group were significantly higher than that in UC model + empty vector group (P < 0.01), and there were significant differences between UC model + empty vector group and UC model + STAT3 knockout group in SOCS3 level (P < 0.01, Figure 5).
Figure 5.
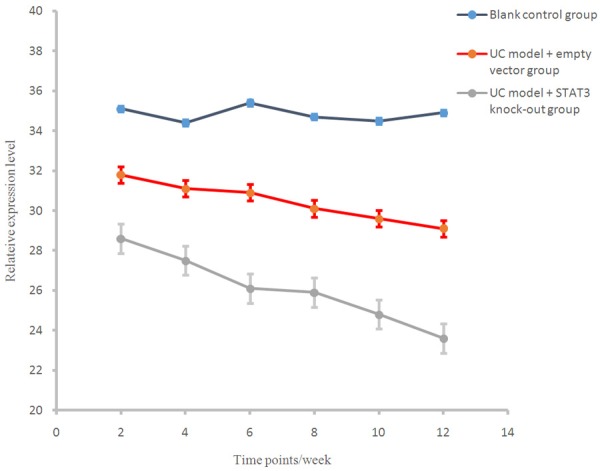
SOCS3 expression levels in different groups at different stages.
Western blot results
Differences in IL6 protein expression
Western blot assay showed that there were no significant differences in the IL6 expression level in early (2 weeks) sacrificed mice among different groups (P > 0.05). In the mice sacrificed after four or more weeks, the expression levels of IL-6 in UC model + empty vector and UC model + STAT3 knockout groups were significantly higher than that in the control group (P < 0.01); while there was no significant difference between the UC model + empty vector group and UC model + STAT3 knockout group (P > 0.05, Figures 6, 7).
Figure 6.
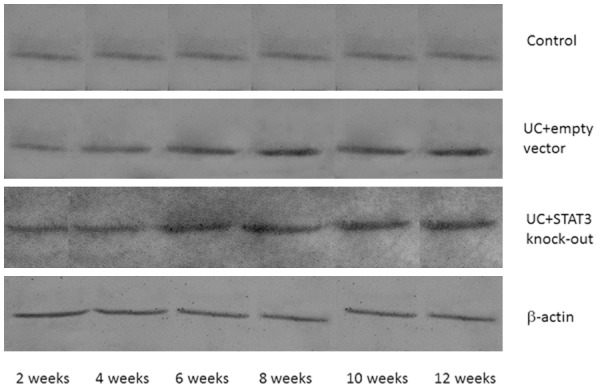
IL-6 protein expression in different time points in each group.
Figure 7.
IL-6 protein expression level in different group at different stages.
Differences in STAT3 protein expression
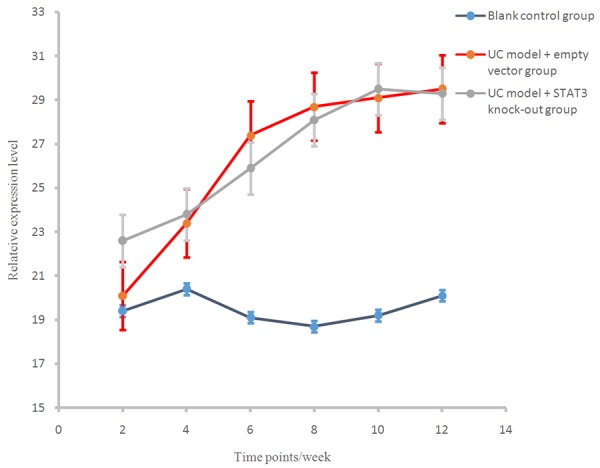
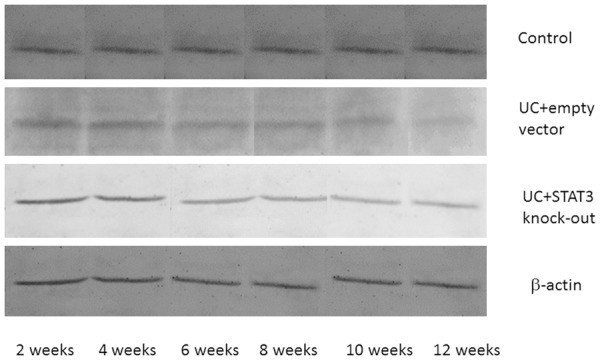
Western blot assay showed that there were no significant differences in the STAT3 expression level in early (2 weeks) sacrificed mice among different groups (P > 0.05). In the mice sacrificed after four or more weeks, the expression level of STAT3 in UC model + empty vector group was significantly higher than that in the control group (P < 0.01, Figures 8, 9). No STAT3 expression was found in UC model + STAT3 knock out group.
Figure 8.
IL-6 protein expression in different time points in each group.
Figure 9.
SATA3 protein expression level in different group at different stages.
Differences in SOCS3 protein expression
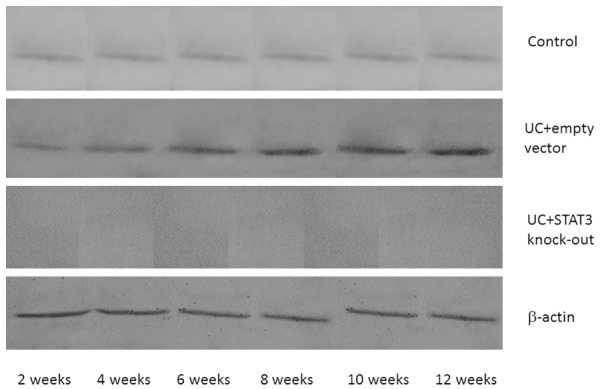
Western blot assay showed that there were no significant differences in the SOCS3 expression level in early (2 weeks) sacrificed mice among different groups (P > 0.05). In the mice sacrificed after four or more weeks, the expression levels of SOCS3 in control group and UC model + STAT3 knock-out group were significantly higher than that in UC model + empty vector group (P < 0.01); meanwhile, there were significant differences between the UC model + empty vector group and UC model + STAT3 knockout group (P < 0.05, Figures 10, 11).
Figure 10.
IL-6 protein expression in different time points in each group.
Figure 11.
SATA3 protein expression level in different group at different stages.
Correlation analysis on expression of IL-6, STAT3 and SOCS3
Spearman correlation analysis showed that in the UC model + empty vector group, IL6 protein expression level had positive correlation with STAT3 protein expression , which had negative correlation with SOCS3 protein expression (P < 0.01) with r values were 0.703 and -0.662, respectively. In the UC model + STAT3 knockout group, the three factors showed no correlation (P > 0.05, data not shown).
Discussion
In recent years, as people’s living standards improve, the incidence of UC has a significantly increasing trend [7]. With studies being carried out on the pathogenesis of UC, people have a certain understanding of the disease. Most scholars believe that cytokine imbalance is a core part of UC which induces non-specific intestinal inflammation [8].
IL-6/STAT3/SOCS3 signaling pathway participated in the inflammatory-atypical hyperplasia-cancer complex pathological processes broadly. Most people believed that STAT3 was closely related to body’s inflammatory response, immune response and apoptosis process. Many studies have been reported STAT3 played an important role in mice with UC model and humans with certain acute and chronic inflammation diseases [9]. Thus, more and more people concerned IL-6/STAT3/SOCS3 signaling pathway.
IL-6/STAT3 signaling pathway received stimulation of extracellular signals, involved in the regulation, development, apoptosis and other physiological processes of cell growth. Many cytokines and growth factors completed signal transduction through IL-6/STAT3 signaling pathway, including SOCS3, EGF, etc. In the DSS-induced colitis model, the activation of STAT3 was reduced in IL6 gene-deficient mice to some degree and the colitis progress was slow, so we consider that IL-6/STAT3 was an important pathways for the progress of inflammatory bowel disease [10].
Because IL-6/STAT3 pathway persistently presented full activation in the ulcerative colitis carcinogenesis, and the degree of activation showed a positive correlation with progress of the disease to a certain degree. Therefore detection of molecule and the corresponding target genes which played a key role on this path can be used as diagnostic indicators of UC induced cancer. It also can provide reliable reference for early treatment and prevention of this disease [11].
IL-6, STAT3, SOCS3 were different part of IL-6/STAT3/SOCS3 signaling pathway. We consider alleviating the condition by blocking this pathway. In our study, animal experiments showed that in UC mouse with STAT3 knock-out, the product of SOCS3 and its protein content changed significantly in volume, indicating that STAT3 played a certain role in the remission of disease, but it did not completely block the progress of the disease.
Conclusion
This study successfully established the animal model of UC “Inflammation-dysplasia-cancer” pathological processes. The study found that the expression and activation of IL6 and STAT3 increased during carcinogenesis in ulcerative colitis. To some extent, the expression of both two factors increased with the progress of the disease. SOCS3 expression weakened, and the degree of activation decreased. STAT3 had a certain effect on the expression of SOCS3, and had a certain regulation on ulcerative colitis carcinogenesis to a certain extent. With further researches on the mechanisms of UC disease, it is expected to provide a new way of thinking for treatment.
Disclosure of conflict of interest
None.
References
- 1.Shivakumar BM, Rotti H, Vasudevan TG, Balakrishnan A, Chakrabarty S, Bhat G, Rao L, Pai CG, Satyamoorthy K. Copy number variations are progressively associated with the pathogenesis of colorectal cancer in ulcerative colitis. World J Gastroenterol. 2015;21:616–22. doi: 10.3748/wjg.v21.i2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yashiro M. Ulcerative colitis-associated colorectal cancer. World J Gastroenterol. 2014;2:16389–97. doi: 10.3748/wjg.v20.i44.16389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen AV, Wu YY, Liu Q, Wang D, Nguyen S, Loh R, Pang J, Friedman K, Orlofsky A, Augenlicht L, Pollard JW, Lin EY. STAT3 in epithelial cells regulates inflammation and tumor progression to malignant state in colon. Neoplasia. 2013;15:998–1008. doi: 10.1593/neo.13952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakilam S, Gandesiri M, Rau TT, Agaimy A, Vijayalakshmi M, Ivanovska J, Wirtz RM, Schulze-Luehrmann J, Benderska N, Wittkopf N, Chellappan A, Ruemmele P, Vieth M, Rave-Fränk M, Christiansen H, Hartmann A, Neufert C, Atreya R, Becker C, Steinberg P, Schneider-Stock R. Death-associated protein kinase controls STAT3 activity in intestinal epithelial cells. Am J Pathol. 2013;182:1005–20. doi: 10.1016/j.ajpath.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Li Y, Tian Y, Zhu W, Gong J, Zhang W, Yu C, Gu L, Li N, Li J. Triptolide induces suppressor of cytokine signaling-3 expression and promotes lamina propria mononuclear cells apoptosis in Crohn’s colitis. Int Immunopharmacol. 2013;16:268–74. doi: 10.1016/j.intimp.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Deuring J, Peppelenbosch MP, Kuipers EJ, de Haar C, van der Woude CJ. STAT1, STAT6 and adenosine 3’,5’-cyclic monophosphate (cAMP) signaling drive SOCS3 expression in inactive ulcerative colitis. Mol Med. 2012;18:1412–9. doi: 10.2119/molmed.2012.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen R, Pan S, Lai K, Lai LA, Crispin DA, Bronner MP, Brentnall TA. Up-regulation of mitochondrial chaperone TRAP1 in ulcerative colitis associated colorectal cancer. World J Gastroenterol. 2014;20:17037–48. doi: 10.3748/wjg.v20.i45.17037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nowacki TM, Brückner M, Eveslage M, Tepasse P, Pott F, Thoennissen NH, Hengst K, Ross M, Bettenworth D. The risk of colorectal cancer in patients with ulcerative colitis. Dig Dis Sci. 2015;60:492–501. doi: 10.1007/s10620-014-3373-2. [DOI] [PubMed] [Google Scholar]
- 9.Xu AT, Li Y, Zhao D, Shen J, Xu XT, Qiao YQ, Zhu MM, Wang TR, Cui Y, Ai LY, Ran ZH. High Suppressor of Cytokine Signaling-3 Expression Impairs STAT3-dependent Protective Effects of Interleukin-22 in Ulcerative Colitis in Remission. Inflamm Bowel Dis. 2015;21:241–50. doi: 10.1097/MIB.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 10.Koukos G, Polytarchou C, Kaplan JL, Morley-Fletcher A, Gras-Miralles B, Kokkotou E, Baril-Dore M, Pothoulakis C, Winter HS, Iliopoulos D. MicroRNA-124 regulates STAT3 expression and is down-regulated in colon tissues of pediatric patients with ulcerative colitis. Gastroenterology. 2013;145:842–52. e2. doi: 10.1053/j.gastro.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26:54–74. doi: 10.1016/j.smim.2014.01.001. [DOI] [PubMed] [Google Scholar]


