Abstract
Background: The cluster of differentiation antigen 14 (CD14) gene-159C/T polymorphism has been implied to be associated with coronary artery disease (CAD) susceptibility. However, the separate studies results are still conflicting between each other. Objective and methods: To investigate the relationship between CD14 gene-159C/T polymorphism and CAD, a meta-analysis including 4467 subjects from 7 individual studies was performed. The random or fixed effect models were used to evaluate the pooled odds ratios (ORs) and their corresponding 95% confidence intervals. Results: There was a significant association between CD14 gene -159C/T polymorphism and CAD in the whole population under allelic (OR: 1.280, 95% CI: 1.000-1.630, P=0.05), recessive (OR: 1.760, 95% CI: 1.120-2.750, P=0.01), homozygous (OR: 1.693, 95% CI: 1.008-2.843, P=0.046), and additive genetic models (OR: 1.278, 95% CI: 1.000-1.633, P=0.050). No significant association was found between them under dominant (OR: 0.580, 95% CI: 0.310-1.110, P=0.10) and heterozygous genetic models (OR: 1.334, 95% CI: 0.870-2.045, P=0.186). In the subgroup analysis, a significant association was detected in Chinese population (P<0.05), while there was no significant association in the Caucasian subgroup (P>0.05). Conclusions: CD14 gene -159C/T polymorphism was significantly associated with CAD susceptibility, particularly in the Chinese population. The person with T allele of CD14 gene -159C/T polymorphism might predispose to CAD. There was no distinct association between them in the Caucasian subgroup.
Keywords: CD14, -159C/T, polymorphism, coronary artery disease
Introduction
Coronary artery disease (CAD) is one of the main diseases threatening human health and contributing to human death. Atherosclerosis is the pathological foundation of CAD. Many hazards as inflammation, infection, and autoimmunity could lead to CAD. Hence, CAD is considered as a kind of inflammation disease. The inflammation reaction in the coronary artery atherosclerosis plaque leads to the intima damage, plaque rupture, and acute cardiac ischemia. The clinical practice has also shown that some inflammation markers could serve as the cardiovascular events predisposing predictors. Meanwhile, CAD has the heredity tendency and the heredity variation of the inflammation system might lead to the increased CAD risk [1]. The cluster of differentiation antigen 14 (CD14) is a multiple function inflammation cytokine which is mainly produced by mature mononuclear macrophage. CD14 plays a key role in the process of inflammation, immunity and atherosclerosis which is closely associated with cardiovascular disease [2].
CD14 acts as the receptor of lipopolysaccharide (LPS) which is the cytoderm ingredient of gram-negative bacteria. CD14 is one member of the glycoprotein family of the cell surface. With the help of LPS binding protein, CD14 could combine with LPS which lead to the cell activation, the pro-inflammatory cytokines release and the coagulator synthesis. The atherosclerosis formation was thus accelerated [3]. CD14 gene, located in 5q23-31, spans 3.9 kb which encodes the 55 KD glycoprotein with 375 amino acids. In CD14 gene -159C/T polymorphism, the -159th base cytosine (C) in the upstream of CD14 gene promoter region is replaced by thymine (T). The position -159 is close to a DNA element binding to nuclear protein which is involved in promoter activation. Hence, CD14 gene -159C/T polymorphism might influence the transcription and expression level of CD14 gene. In 1999, Baldini et al reported that in an Arizona population, the TT homozygotes of CD14 gene -159C/T polymorphism had significantly higher soluble CD14 (sCD14) levels than did both CC and CT genotypes carriers (P=0.01). They concluded that CD14 gene -159C/T polymorphism played a significant role in regulating circulating sCD14 levels [4].
Although many researches on the relationship between CD14 gene-159C/T polymorphism and CAD have been conducted so far, the individual studies results were still inconsistent. In 2002, Koch et al found no significant difference between CD14 gene -159C/T genotype distributions of control and CAD patients and they concluded that the CD14 gene -159C/T polymorphism was not related to CAD in an American population [5]. However, in 2005, Li et al found that the T allele of the CD14 gene -159C/T polymorphism might be a risk factor for CAD in a Chinese population [6]. Analogously, in 2010, Liu et al also obtained the same conclusion in another Chinese population [7].
In the present study, a meta-analysis involving 2798 CAD patients and 1669 controls from 7 separate studies was performed to estimate the relationship of CD14 gene -159C/T polymorphism and CAD.
Materials and methods
Publication search and inclusion criteria
The electronic databases including China National Knowledge Infrastructure, China Biological Medicine Database, Embase, PubMed, and Web of Science were retrieved by using the terms as “CD14”, “-159C/T”, “polymorphism” and “coronary artery disease”. The last research was updated on April 22, 2015 with the publication years ranging from 2005 to 2010.
The included studies should follow such major criteria as the follows: a) Assessment of the association of CD14 gene -159C/T polymorphism and CAD. b) CAD was diagnosed as at least one coronary artery stenosis was no less than 50% by coronary angiography. The control group was the healthy population in the corresponding period without coronary artery stenosis through coronary angiography. c) The studies should be case-control or cohort studies published officially. d) The genotype member of the control group in the individual studies should follow the Hardy-Weinberg equilibrium (HWE).
Data extraction
The studies data was extracted based on a standard protocol. Three investigators performed the meta-analysis; two of whom searched out the individual studies duplicately, and the third investigator served as the arbitrator to settle the disagreement between the two investigators and reach an agreement finally. The present meta-analysis excluded the studies that did not meet the major selection criteria, that were repeated publications, or that supplied inadequate data. If similar data appeared in different papers by the same authors, the data was only used once. The following items as publication year, the first author’s name, ethnicity, country, matching criteria, number of genotypes, and total number of cases and controls should be displayed in the data.
Statistical analyses
There were six genetic models as the allelic (T allele distribution frequency), recessive (TT vs. CC+CT), dominant (CC vs. TT+CT), homozygous (TT vs. CC), heterozygous (CT vs. CC), and additive (T vs. C) genetic models in the current meta-analysis. The association of CD14 gene -159C/T polymorphism and CAD was compared by the odds ratios (ORs) and their corresponding 95% confidence intervals (CIs). The Chi-square-based Q-tests was adopted to calculate the heterogeneity among the studies with significance set at P<0.05 level [8]. If there was heterogeneity among the individual studies, the random-effect model (DerSimonian and Laird method) would be adopted [9]. Or else, the fixed-effect model was used (the Mantel-Haenszel method) [10]. The pooled OR was estimated by Z test and significance was set at P<0.05 level.
Fisher’s exact test was used to evaluate the HWE and significance was set at P<0.05 level. The funnel plot was adopted to assess the potential publication bias. Egger’s linear regression test on the natural logarithm scale of the OR was used to evaluate the funnel plot symmetry with significance set at P<0.05 level [11]. The Stata 12.0 software was used to perform the statistical analyses (StataCorp, College Station, TX, USA).
Results
Studies and populations
Fifteen manuscripts were searched out by the retrieval process, among which seven papers followed the inclusion criteria. Among the eight excluded studies, five papers were reviews, and three manuscripts were not involved with CD14 gene -159C/T polymorphism or CAD. No study was rejected for deviating from the HWE. All of information was extracted from 2798 CAD cases and 1669 controls (Table 1) [5-7,12-15]. Two countries were involved in the current meta-analysis as China and German. These populations belong to Chinese and Caucasian subgroups respectively. The Chinese subgroup includes 5 individual studies and the Caucasian subgroup consists of 2 individual studies.
Table 1.
Characteristics of the investigated studies of the association between the CD14 gene -159C/T polymorphism and coronary artery disease (CAD)
| Author | Year | Country | Ethnicity | CAD | Control | Matching criteria | Sample size (CAD/control) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| CC | CT | TT | CC | CT | TT | ||||||
| Li Y [6] | 2005 | China | Asian | 24 | 75 | 63 | 54 | 89 | 53 | Age, sex, ethnicity | 162/196 |
| Chen F [12] | 2006 | China | Asian | 10 | 52 | 46 | 23 | 61 | 34 | Age, sex, BMI, ethnicity | 108/118 |
| Wei YS [13] | 2006 | China | Asian | 47 | 128 | 71 | 39 | 139 | 80 | Age, sex, ethnicity | 246/258 |
| Li L [14] | 2007 | China | Asian | 29 | 95 | 69 | 47 | 124 | 54 | Age, sex, ethnicity | 193/225 |
| Liu XQ [7] | 2010 | China | Asian | 10 | 65 | 25 | 103 | 133 | 44 | Age, sex, ethnicity | 100/280 |
| Koch W [5] | 2002 | German | Caucasian | 505 | 888 | 398 | 88 | 177 | 75 | Age, sex, ethnicity | 1791/340 |
| Haberbosch W [15] | 2009 | German | Caucasian | 65 | 92 | 41 | 71 | 131 | 50 | Age, sex, ethnicity | 198/252 |
Abbreviations: CAD, coronary artery disease; BMI, body mass index; Polymerase chain reaction-restriction fragment length polymorphism genotyping method and Case-control study design were adopted in the above studies.
Pooled analyses
There was a significant association between CD14 gene -159C/T polymorphism and CAD in the whole population under allelic (OR: 1.280, 95% CI: 1.000-1.630, P=0.05), recessive (OR: 1.760, 95% CI: 1.120-2.750, P=0.01), homozygous (OR: 1.693, 95% CI: 1.008-2.843, P=0.046), and additive genetic models (OR: 1.278, 95% CI: 1.000-1.633, P=0.050). No significant association was found between them under dominant (OR: 0.580, 95% CI: 0.310-1.110, P=0.10) or heterozygous genetic model (OR: 1.334, 95% CI: 0.870-2.045, P=0.186).
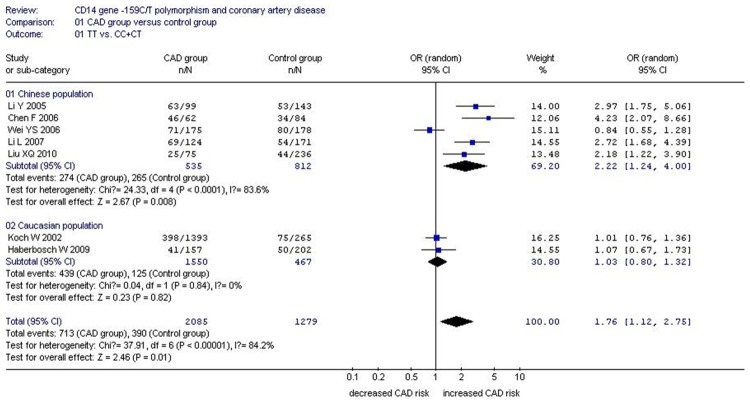
In the subgroup analysis, a significant association between CD14 gene -159C/T polymorphism and CAD was detected in the Chinese population under allelic (OR: 1.470, 95% CI: 1.080-1.990, P=0.01), recessive (OR: 2.220, 95% CI: 1.240-4.000, P=0.008), dominant (OR: 0.400, 95% CI: 0.170-0.970, P=0.04), homozygous (OR: 2.276, 95% CI: 1.138-4.552, P=0.020), and additive genetic models (OR: 1.466, 95% CI: 1.079-1.991, P=0.014). However, there was no significant association between CD14 gene -159C/T polymorphism and CAD under heterozygous genetic model (OR: 1.717, 95% CI: 0.936-3.152, P=0.081).
In the Caucasian subgroup, no significant association was found between CD14 gene -159C/T polymorphism and CAD under allelic (OR: 0.950, 95% CI: 0.830-1.090, P=0.46), recessive (OR: 1.030, 95% CI: 0.800-1.320, P=0.230), homozygous (OR: 0.916, 95% CI: 0.690-1.217, P=0.600), heterozygous (OR: 0.841, 95% CI: 0.666-1.062, P=0.146), and additive genetic models (OR: 0.949, 95% CI: 0.825-1.091, P=0.462). However, a significant association was only detected under a dominant genetic model (OR: 1.280, 95% CI: 1.000-1.620, P=0.046) (Table 2; Figures 1, 2, 3, 4, 5 and 6).
Table 2.
Summary of meta-analysis of association between the CD14 gene -159C/T polymorphism and coronary artery disease (CAD)
| Genetic model | Pooled OR (95% CI) | Z value | P value | Study number | CAD size | Control size | Pheterogeneity (I2%) |
|---|---|---|---|---|---|---|---|
| Allelic genetic model | 1.280 (1.000-1.630) | 1.96 | 0.05* | 7 | 2798 | 1669 | <0.00001* (83.2%) |
| Chinese subgroup | 1.470 (1.080-1.990) | 2.45 | 0.01* | 5 | 809 | 1077 | 0.0004 (80.6%) |
| Caucasian subgroup | 0.950 (0.830-1.090) | 0.74 | 0.46 | 2 | 1989 | 592 | 0.83 (0%) |
| Recessive genetic model | 1.760 (1.120-2.750) | 2.46 | 0.01* | 7 | 2798 | 1669 | <0.00001* (84.2%) |
| Chinese subgroup | 2.220 (1.240-4.000) | 2.67 | 0.008* | 5 | 809 | 1077 | <0.0001* (83.6%) |
| Caucasian subgroup | 1.030 (0.800-1.320) | 0.23 | 0.82 | 2 | 1989 | 592 | 0.84 (0%) |
| Dominant genetic model | 0.580 (0.310-1.110) | 1.65 | 0.10 | 7 | 2798 | 1669 | <0.00001* (91.5%) |
| Chinese subgroup | 0.400 (0.170-0.970) | 2.03 | 0.04* | 5 | 809 | 1077 | <0.00001* (90.8%) |
| Caucasian subgroup | 1.280 (1.000-1.620) | 2.00 | 0.046* | 2 | 1989 | 592 | 0.45 (0%) |
| Homozygous genetic model | 1.693 (1.008-2.843) | 1.99 | 0.046* | 7 | 2798 | 1669 | <0.00001* (82.9%) |
| Chinese subgroup | 2.276 (1.138-4.552) | 2.33 | 0.020* | 5 | 809 | 1077 | <0.00001* (81.9%) |
| Caucasian subgroup | 0.916 (0.690-1.217) | 0.60 | 0.546 | 2 | 1989 | 592 | 0.921 (0%) |
| Heterozygous genetic model | 1.334 (0.870-2.045) | 1.32 | 0.186 | 7 | 2798 | 1669 | <0.00001* (80.3%) |
| Chinese subgroup | 1.717 (0.936-3.152) | 1.75 | 0.081 | 5 | 809 | 1077 | <0.00001* (79.9%) |
| Caucasian subgroup | 0.841 (0.666-1.062) | 1.45 | 0.146 | 2 | 1989 | 592 | 0.616 (0%) |
| Additive genetic model | 1.278 (1.000-1.633) | 1.96 | 0.050* | 7 | 2798 | 1669 | <0.00001* (83.2%) |
| Chinese subgroup | 1.466 (1.079-1.991) | 2.45 | 0.014 | 5 | 809 | 1077 | <0.00001* (80.6%) |
| Caucasian subgroup | 0.949 (0.825-1.091) | 0.74 | 0.462 | 2 | 1989 | 592 | 0.833 (0%) |
P≤0.05.
Abbreviations: CAD, coronary artery disease; CI, confidence interval; OR, odds ratio; CAD size, the total number of CAD cases; control size, the total number of control group; Allelic genetic model, T allele distribution frequency; recessive genetic model, TT vs. CC+CT; Dominant genetic model, CC vs. TT+CT; Homozygous genetic mode, TT vs. CC; Heterozygous genetic model, CT vs. CC; Additive genetic model, total T allele vs. total C allele.
Figure 1.
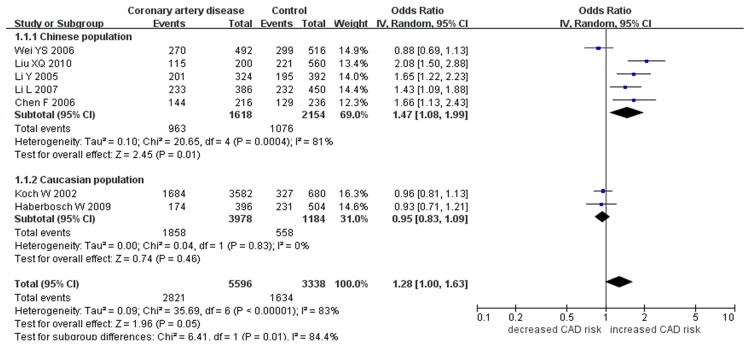
Forest plot of CAD associated with CD14 gene -159C/T polymorphism under an allelic genetic model (distribution of T allelic frequency of CD14 gene -159C/T polymorphism).
Figure 2.
Forest plot of CAD associated with CD14 gene -159C/T polymorphism under a recessive genetic model (TT vs. CC+CT).
Figure 3.
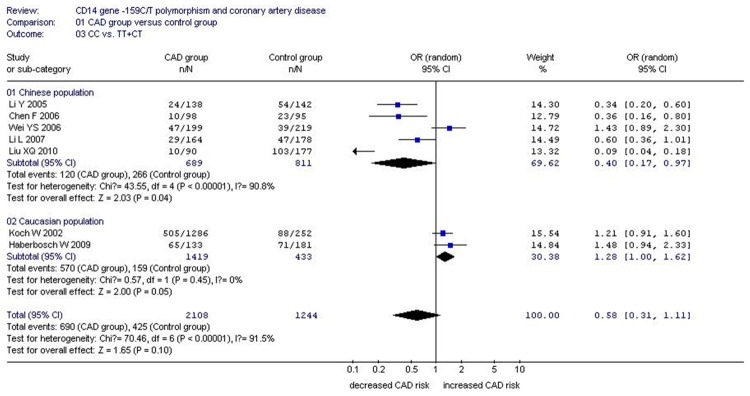
Forest plot of CAD associated with CD14 gene -159C/T polymorphism under a dominant genetic model (CC vs. TT+CT).
Figure 4.
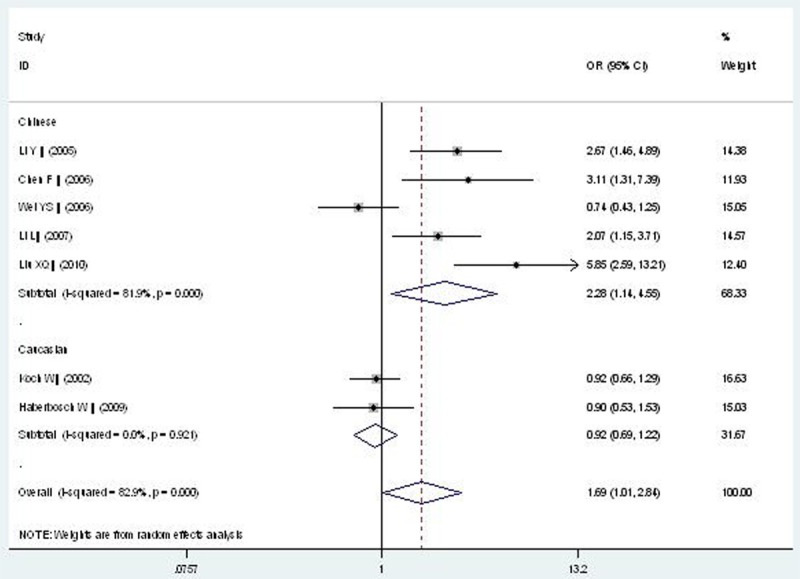
Forest plot of CAD associated with CD14 gene -159C/T polymorphism under a homozygous genetic model (TT vs. CC).
Figure 5.
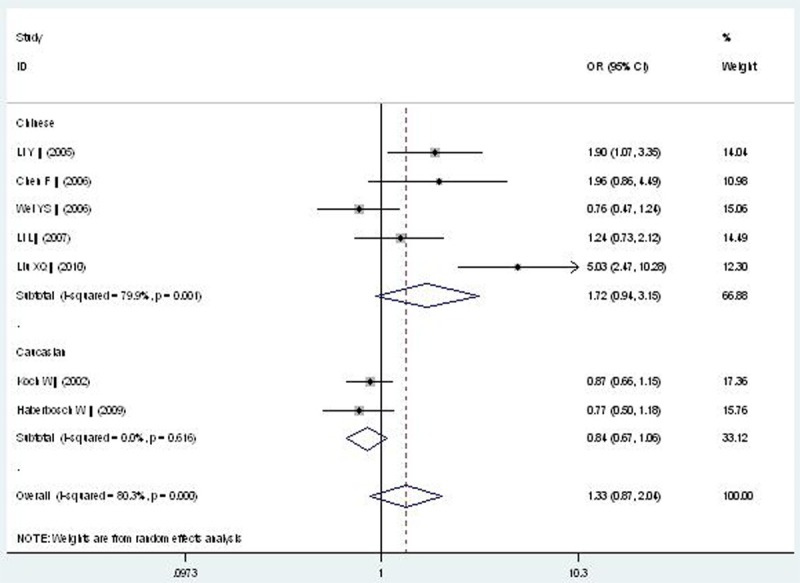
Forest plot of CAD associated with CD14 gene -159C/T polymorphism under a heterozygous genetic model (CT vs. CC).
Figure 6.
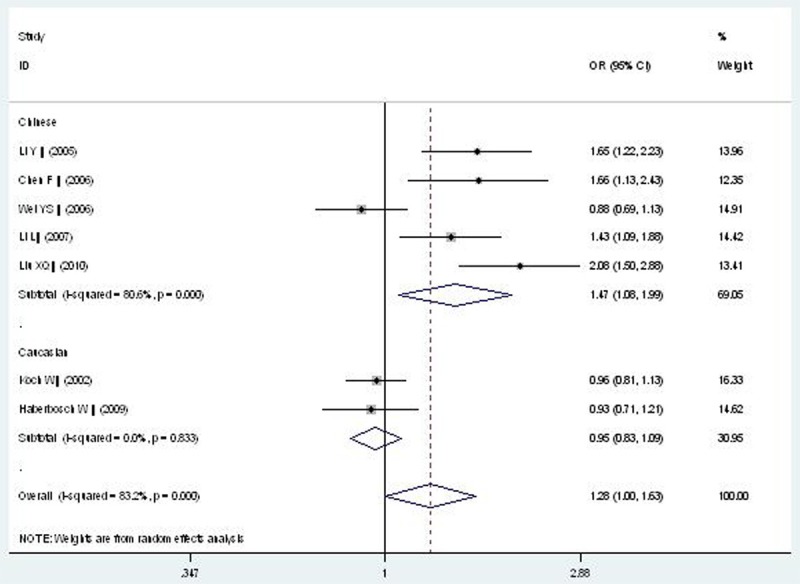
Forest plot of CAD associated with CD14 gene -159C/T polymorphism under an additive genetic model (T vs. C).
There was significant heterogeneity in the whole population under all of the genetic models. In the subgroup analysis stratified by ethnicity, there was no heterogeneity in the Caucasian subgroup under all of the genetic models (Pheterogeneity>0.05, I2=0%). However, the heterogeneity was reduced a little but still significant in the Chinese population under all of the genetic models (Pheterogeneity<0.0001, I2: 79.9%-90.8%).
Bias diagnostics
The funnel plot and Egger’s test were adopted to evaluate the publication bias among the individual studies. No visual publication bias was found in the Begg’s funnel plot under the additive genetic model (Figure 7). In addition, no significant difference in the Egger’s test yet, which indicated that publication bias did not exist in the present meta-analysis by using additive genetic model (T=2.48, P=0.056).
Figure 7.
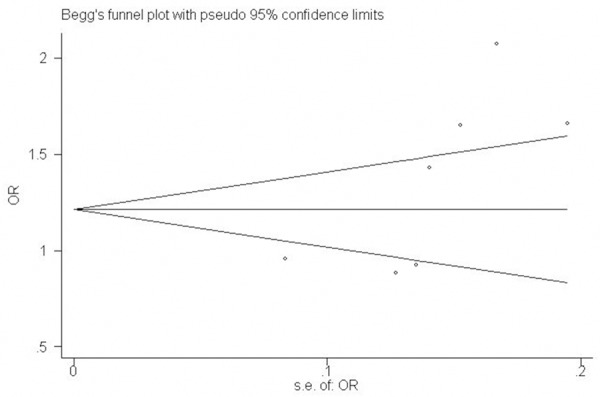
Begg’s funnel plot for studies of the association of CAD associated with CD14 gene -159C/T polymorphism under an additive genetic model (T vs. C). The horizontal and vertical axis correspond to the OR and confidence limits. OR: odds ratio; SE: standard error.
Discussion
In the present meta-analysis, a significant association was found in the whole population between CD14 gene -159C/T polymorphism and CAD under allelic (OR: 1.28), recessive (OR: 1.76), homozygous (OR: 1.693), and additive genetic models (OR: 1.278). No significant association was found under a dominant (OR: 0.58) or heterozygous genetic model (OR: 1.334). In the subgroup analysis stratified by ethnicity, there was a more significant association in Asian subgroup than that in the whole population under allelic (OR: 1.47), recessive (OR: 2.22), dominant (OR: 0.40), homozygous (OR: 2.276), and additive genetic models (OR: 1.466). However, no significant association was detected in the Caucasian subgroup (P>0.05). In conclusion, it was indicated that the carriers with T allele of CD14 gene -159C/T polymorphism might predispose to CAD, except in the Caucasian population. The negative conclusion in the Caucasian subgroup was possibly not only associated with the different race, but also with the few manuscripts, because only two researches were included in this subgroup. Hence, the current conclusion needs to be further confirmed by more researches in the Caucasian subgroup in the future.
There was significant heterogeneity in the whole population under all of the genetic models. In the subgroup analysis stratified by ethnicity, the heterogeneity did not exist any longer in the Caucasian subgroup (P>0.05). However, the heterogeneity still existed in the Chinese population (P<0.05).
CD14 receptor is the membrane glycoprotein with 365 amino acids. As the major LPS receptor, CD14 has the function of identifying initial immune system molecules. CD14 participates in the monocytes activation, the interaction of leukocyte and endothelial cells (ECs), apoptosis regulation of monocytes and ECs. Thus, CD14 plays a key role in the process of atherosclerosis and complications. CD14 could specially combine with LPS, then transfer the activation signal to the downstream pathway through the Toll like receptor 4 and bone marrow differentiation protein-2. By this way, the monocytes-macrophage system was launched and many pro-inflammation cytokines as tumor necrosisα, interleukin-1, interleukin-6 and so on were released. These cytokines have multi-functions in the process of mediating initial immune response and inflammation reaction which cause the histocyte damage, disturbance of immunologic function and vascular smooth muscle cells proliferation [16-18]. In addition, the combination of CD14 and LPS can induce the transcription of endothelial leukocyte adhesion molecule-1 and vascular cell adhesion molecule-1 which prompt the cell adhesion and signal transmission. Additionally, the CD14 and LPS could induce macrophagocyte and ECs to release the tissue factor with procoagulant activity and protease, thus facilitate the thrombus formation. Hence, CD14 is the key molecule in the systemic inflammatory response and atherosclerosis [19]. T allele of CD14 gene -159C/T polymorphism can increase the CD14 level and prompt CAD progress by influencing CD14 gene transcription and expression [20].
There was no internationally relevant meta-analysis on the association of CAD with CD14 gene -159C/T polymorphism so far. However, there were some limitations in the current meta-analysis. The present meta-analysis still lack large-scale or prospective studies on the association of CD14 gene -159C/T polymorphism and CAD. The death cases were not recruited in each individual study which might lead to the research results power insufficiency and reduce the current meta-analysis demonstration intensity. The internal CD14 level was influenced not only by the CD14 gene -159C/T polymorphism, but also by other polymorphism as -260C/T gene polymorphism, and other environmental factors as hypertension, hyperlipemia, and diabetes mellitus [21]. CAD is a multiple gene inheritance disease and many micro-effect genes inevitably generate a general effect. Other genes polymorphisms as intercellular adhesion molecule-1 gene E469K polymorphism, methylenetetrahydrofolate reductase gene C677T polymorphism, plasminogen activator inhibitor-1 gene 4G/5G polymorphism, ATP-binding cassette transporter A1 gene R219K polymorphism, apo A5 gene -1131T/C, FgB -455G/A, -148C/T, and CETP gene TaqIB polymorphisms might confer the risk to CAD [22-25].
Finally, CD14 gene -159C/T polymorphism was significantly associated with CAD susceptibility, particularly in the Chinese population. The persons with the T allele of CD14 gene-159C/T polymorphism might predispose to CAD. This conclusion might help us formulate a new CAD individual therapy strategy. Considering the above limitations, more studies on the association of CD14 gene-159C/T polymorphism and CAD needed to be performed to further verify the ultimateness in the future.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (NSFC 81100073 to Dr Yan-yan Li), Excellent Young and Middle-Aged Teachers Assistance Program of Nanjing Medical University for Dr Yan-yan Li (2013-2015, JX2161015034), Jiangsu Overseas Research & Training Program for University Prominent Young & Middle-aged Teachers and Presidents (2014), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). Thank all our colleagues working in the Department of geriatrics, the First Affiliated Hospital of Nanjing Medical University.
Disclosure of conflict of interest
None.
References
- 1.Woods A, Brull DJ, Humphries SE, Montgomery HE. Genetics of inflammation and risk of coronary artery disease: the central role of interleukin-6. Eur Heart J. 2000;21:1574–1583. doi: 10.1053/euhj.1999.2207. [DOI] [PubMed] [Google Scholar]
- 2.Hohda S, Kimura A, Sasaoka T, Hayashi T, Ueda K, Yasunami M, Okabe M, Fukuta N, Kurosawa T, Izumi T. Association study of CD14 polymorphism with myocardial infarction in a Japanese population. Jpn Heart J. 2003;44:613–22. doi: 10.1536/jhj.44.613. [DOI] [PubMed] [Google Scholar]
- 3.Arroyo-Espliguero R, El-Sharnouby K, Vázquez-Rey E, Kalidas K, Jeffery S, Kaski JC. CD14 C(-260)T promoter polymorphism and prevalence of acute coronary syndromes. Int J Cardiol. 2005;98:307–12. doi: 10.1016/j.ijcard.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Baldini M, Lohman IC, Halonen M, Erickson RP, Holt PG, Martinez FD. A Polymorphism* in the 5’ flanking region of the CD14 gene is associated with circulating soluble CD14 levels and with total serum immunoglobulin E. Am J Respir Cell Mol Biol. 1999;20:976–83. doi: 10.1165/ajrcmb.20.5.3494. [DOI] [PubMed] [Google Scholar]
- 5.Koch W, Kastrati A, Mehilli J, von Beckerath N, Schömig A. CD14 gene -159C/T polymorphism is not associated with coronary artery disease and myocardial infarction. Am Heart J. 2002;143:971–6. doi: 10.1067/mhj.2002.122512. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Xiong XQ, Zhang PA, Ming KH. Association of C-159T polymorphism in promoter region of CD14 and coronary heart disease. Chinese Journal of Medical Genetics. 2005;22:687–90. [PubMed] [Google Scholar]
- 7.Liu XQ, Yang JY. The association between C-159T polymorphism in promoter region of CD14 polymorphism and coronary heart disease. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2010;20:427–9. [PubMed] [Google Scholar]
- 8.Cochran WG. The effectiveness of adjustment by subclassification in removing bias in observational studies. Biometrics. 1968;24:295–313. [PubMed] [Google Scholar]
- 9.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 10.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 11.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen F, Tang AG. Study on the association of C-159T polymorphism in promoter region of CD14 and coronary heart disease in Hunan Han population of China. Changsha: Zhongnan University Press; 2006. p. 9. [Google Scholar]
- 13.Wei YS, Liu YG, Tang RG, Lan JS. Association between CD14 gene promoter region polymorphisms and coronary heart disease. J Fourth Mil Med Univ. 2006;27:630–33. [Google Scholar]
- 14.Li L, Zhang K, Qiu F, Gu GY, Luo XY, et al. Association of C(-159)T polymorphism in the promoter of CD14 gene with coronary heart disease in Han population of Jiangsu Province. Journal of Clinical Rehabilitative Tissue Engineering Research. 2007;11:8749–52. [Google Scholar]
- 15.Haberbosch W, Unkelbach K, Schuster D, Gardemann A, Tillmanns H, Hölschermann H. CD14 promoter polymorphism (- 159C-->t) is not associated with myocardial infarction or coronary artery disease in patients with assumed high genetic risk. Thorac Cardiovasc Surg. 2009;57:386–90. doi: 10.1055/s-0029-1185876. [DOI] [PubMed] [Google Scholar]
- 16.Wierda RJ, Kuipers HF, van Eggermond MC, Benard A, van Leeuwen JC, Carluccio S, Geutskens SB, Jukema JW, Marquez VE, Quax PH, van den Elsen PJ. Epigenetic control of CCR5 transcript levels in immune cells and modulation by small molecules inhibitors. J Cell Mol Med. 2012;16:1866–77. doi: 10.1111/j.1582-4934.2011.01482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendel I, Feige E, Yacov N, Salem Y, Levi I, Propheta-Meiran O, Shoham A, Ishai E, George J, Harats D, Breitbart E. VB-201, an oxidized phospholipid small molecule, inhibits CD14- and Toll-like receptor-2-dependent innate cell activation and constrains atherosclerosis. Clin Exp Immunol. 2013;175:126–37. doi: 10.1111/cei.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Estruch M, Bancells C, Beloki L, Sanchez-Quesada JL, Ordóñez-Llanos J, Benitez S. CD14 and TLR4 mediate cytokine release promoted by electronegative LDL in monocytes. Atherosclerosis. 2013;229:356–62. doi: 10.1016/j.atherosclerosis.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Kane JP, Havel RJ. Polymorphism of the lipopolysaccharide receptor (CD14) and myocardial infarction. New evidence for a role of gram-negative bacterial infection? Circulation. 1999;99:3210–2. doi: 10.1161/01.cir.99.25.3210. [DOI] [PubMed] [Google Scholar]
- 20.Griga T, Klein W, Epplen JT, Hebler U, Stachon A, May B. CD14 expression on monocytes and soluble CD14 plasma levels in correlation to the promotor polymorphism of the endotoxin receptor CD14 gene in patients with inactive Crohn’s disease. Hepatogastroenterology. 2005;52:808–11. [PubMed] [Google Scholar]
- 21.Pu H, Yin J, Wu Y, Zhang D, Wang Y, Zhou R, Jiang L, Liu Y. The association between CD14 gene C-260T polymorphism and coronary heart disease risk: a meta-analysis. Mol Biol Rep. 2013;40:4001–8. doi: 10.1007/s11033-012-2478-y. [DOI] [PubMed] [Google Scholar]
- 22.Yanyan L. Intercellular adhesion molecule-1 E469K gene polymorphism and coronary artery disease in the Chinese population: a meta-analysis involving 3065 subjects. Clin Cardiol. 2012;35:55–60. doi: 10.1002/clc.20972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li YY. Methylenetetrahydrofolate reductase C677T gene polymorphism and coronary artery disease in a Chinese Han population: a meta-analysis. Metabolism. 2012;61:846–52. doi: 10.1016/j.metabol.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Li YY. Plasminogen activator inhibitor-1 4G/5G gene polymorphism and coronary artery disease in the Chinese Han population: a meta-analysis. PLoS One. 2012;7:e33511. doi: 10.1371/journal.pone.0033511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li YY, Zhang H, Qin XY, Lu XZ, Yang B, Chen ML. ATP-binding cassette transporter A1 R219K polymorphism and coronary artery disease in Chinese population: a meta-analysis of 5,388 participants. Mol Biol Rep. 2012;39:11031–9. doi: 10.1007/s11033-012-2006-0. [DOI] [PubMed] [Google Scholar]
- 26.Li YY, Wu XY, Xu J, Qian Y, Zhou CW, Wang B. Apo A5 -1131T/C, FgB -455G/A, -148C/T, and CETP TaqIB gene polymorphisms and coronary artery disease in the Chinese population: a meta-analysis of 15,055 subjects. Mol Biol Rep. 2013;40:1997–2014. doi: 10.1007/s11033-012-2257-9. [DOI] [PubMed] [Google Scholar]