Abstract
Background: Objective: Aim to investigate the proportion of CD14+CD16+ monocytes and understand the pathogenesis of this monocyte subset in acute leukemia. Methods: Flow cytometry was utilized to study the phenotype expression of CD14+CD16+ monocytes and CD3+ T lymphocytes in peripheral blood derived from patients with acute leukemia. All the data were analyzed by SPSS 13.0 software. Results: The proportion of CD14+CD16+ monocytes including both intermediate and non-classical monocytes, increased significantly in patients with acute leukemia and changed negatively or positively according to the disease process. Meanwhile, the proportion of CD14+CD16+ monocytes was inversely correlated with absolute number of CD4+ T lymphocytes, ratio of CD4+/CD8+ T cells, and positively correlated with the proportion of neutrophil granulocytes. Conclusions: The proportion of CD14+CD16+ monocytes (especially the intermediate subpopulation) is related to the progression of acute leukemia, and the expansion of this monocyte subset could indicate the severity of the disease.
Keywords: Acute leukemia, inflammation, monocyte subset, T lymphocyte
Introduction
Monocytes are known as a heterogeneous group of cells originated from myelomonocytic precursors in bone marrow. They are released into blood circulation upon maturation and remain for a few days, then migrate to tissues and differentiate to macrophages or dendritic cells [1]. The major roles of monocytes are hosting defense against pathogens and maintaining normal tissue structures and functions [2].
Based on the differences in phenotype, function, and several genetic evidences, human peripheral blood monocytes have been initially subdivided into two distinct subsets: CD14+CD16- and CD14+CD16+ cells [3-6]. Ancuta et al. [7] reported that there was heterogeneity in CD14+CD16+ monocytes. Three major monocyte subsets were acknowledged as shown in Figure 1: classical (CD14++CD16-), intermediate (CD14++CD16+) and non-classical (CD14+CD16++) monocytes. Although the biological mechanisms of CD14+CD16- and CD14+CD16+ cells have not been well characterized, the differences between these two subsets have been highly focused by a considerable number of studies. The results of the studies significantly advanced that while CD14+CD16+ cells represent only about 10% of peripheral blood monocytes, they are definitely the major participants in immunological response [8]. The proportion of these CD14+CD16+ monocytes was increased in various disease conditions and was demonstrated associated with inflammation [9,10].
Figure 1.
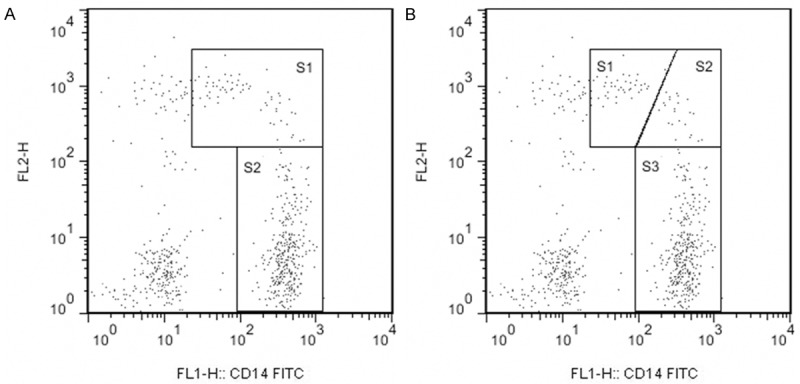
Gating strategy of the monocyte subsets based on relative expression of CD14 and CD16. A. Shows the traditional gating of CD14+CD16+ (S1) and CD14+CD16- (S2) monocyte subsets. B. Shows the new gating of non-classical (S1), intermediate (S2) and classical (S3) monocyte subsets.
Acute leukemia (AL) is a group of malignancies characterized by impaired hematopoiesis. Leukemia cells proliferate malignantly in bone marrow and other hematopoietic tissues, inhibit normal hematopoiesis and cause infection, bleeding or anemia to patients. In recent years, the incidence of acute leukemia is increasing, but the survival rate is still low. The reason may be associated with proliferating tumor cells and impairing anti-tumor immune function. This phenomenon indicates that it is important to understand the pathogenesis of AL. Coincidentally, pro-inflammatory activity of CD14+CD16+ monocytes points out a good direction for us.
However, the function of CD14+CD16+ monocytes proportion in AL was rarely reported. In the current work, we examined the proportion of CD14+CD16+ monocytes including intermediate and non-classical monocytes in patients with AL. We found pronounced expansions of these subsets in patients in period of onset compared with the patients in remission and normal controls. We further found that the proportion of CD14+CD16+ monocytes had relations with absolute number of CD4+ T lymphocytes and the ratio of CD4+/CD8+ T cells. These findings support the crucial role of CD14+CD16+ monocytes in immune regulation, and suggest the value of detecting this monocyte subset in clinical diagnosis and treatment.
Materials and methods
Sample collection
Blood samples from 68 patients with AL (37 males and 31 females, median age 37.04, range, 3-77 years) and 50 healthy individuals (19 males and 31 females, median age 44.62, range, 8-77 years) were collected with informed consent from Central Hospital of Taian. Cases related to acute or chronic infection, hepatic insufficiency, heart failure, diabetes and hypertension had not been taken into consideration while deciding a criticism for subject selection.
Flow cytometry detection
EDTA-anticoagulated blood cells were stained for 15 min with fluorochrome-conjugated (anti-CD14-FITC, anti-CD16-PE, or Tritest CD4-FITC/CD8-PE/CD3-PerCP, Becton Dickinson [BD] Biosciences). Peripheral blood mononuclear cells were isolated using FACS Lysing Solution (BD Biosciences) and then washed with phosphate buffer solution (PBS, Solarbio). Finally cells were processed in the FACSCalibur Flow Cytometer (BD Biosciences) and then analyzed with CellQuest, MultiSet software and FlowJo, as shown in Figure 2.
Figure 2.
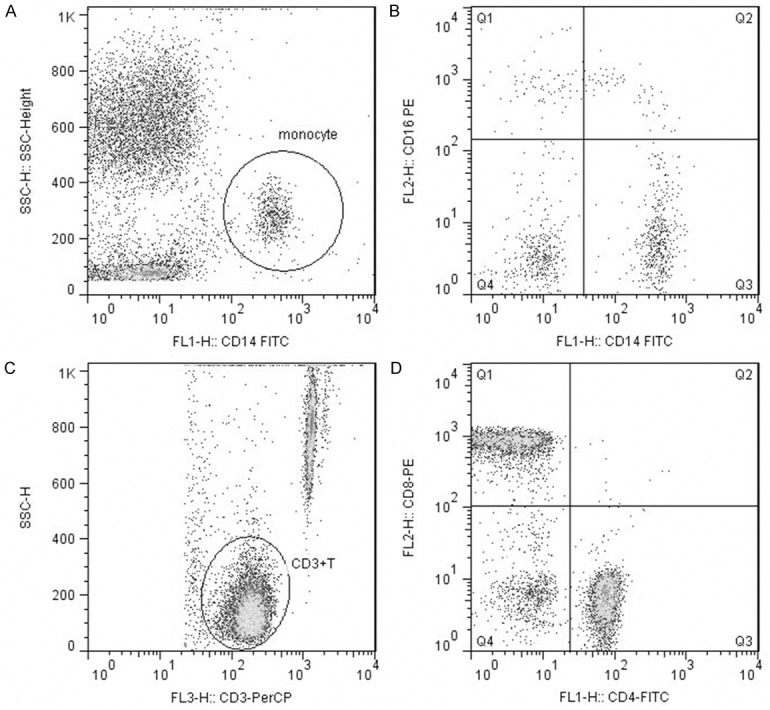
Representative flow cytometry analysis of monocyte subsets and lymphocyte subsets. Monocytes were assessed using a SSC-Height/CD14-FITC dot plot (A). Monocyte subsets were assessed using a CD16-PE/CD14-FITC dot plot (B). Mature Lymphocytes (CD3+ T cells) were assessed using a SSC-Height/CD3-PerCP dot plot (C). Lymphocyte subsets were assessed using a CD8-PE/CD4-FITC dot plot (D).
Statistical analysis
Results were presented as the mean ± SD. The proportions of CD14+CD16+ monocytes between groups were compared by one-way analysis of variance (ANOVA), while the correlation among the proportion of monocyte subsets, lymphocyte subsets and peripheral white blood cells were calculated with employing Pearson analysis. Statistical significance was considered by a p-value less than 0.05.
Results
The proportion of CD14+CD16+ monocytes, non-classical monocytes and intermediate monocytes
The expression of CD14+CD16+ monocytes (including the non-classical and intermediate subsets) in AL patients and normal controls was established in Table 1.
Table 1.
The expression of CD14+CD16+ monocytes in AL patients and normal controls (x̅ ± s)
| AL patients in period of onset | AL patients in remission | Normal controls | |
|---|---|---|---|
| N | 68 | 68 | 50 |
| The proportion of CD14+CD16+ monocytes | 18.56 ± 6.89 | 7.73 ± 3.48 | 6.29 ± 1.91 |
| The proportion of non-classical monocytes | 6.34 ± 3.82 | 2.60 ± 1.92 | 2.75 ± 1.09 |
| The proportion of intermediate monocytes | 10.24 ± 5.49 | 4.51 ± 2.47 | 3.00 ± 1.37 |
As depicted in Figure 3, the proportion of CD14+CD16+ monocytes in patients in period of onset was significantly increased compared with both patients in remission and normal controls (P< 0.01). However, no significant difference between the data of patients in remission and normal controls was observed (P > 0.05).
Figure 3.
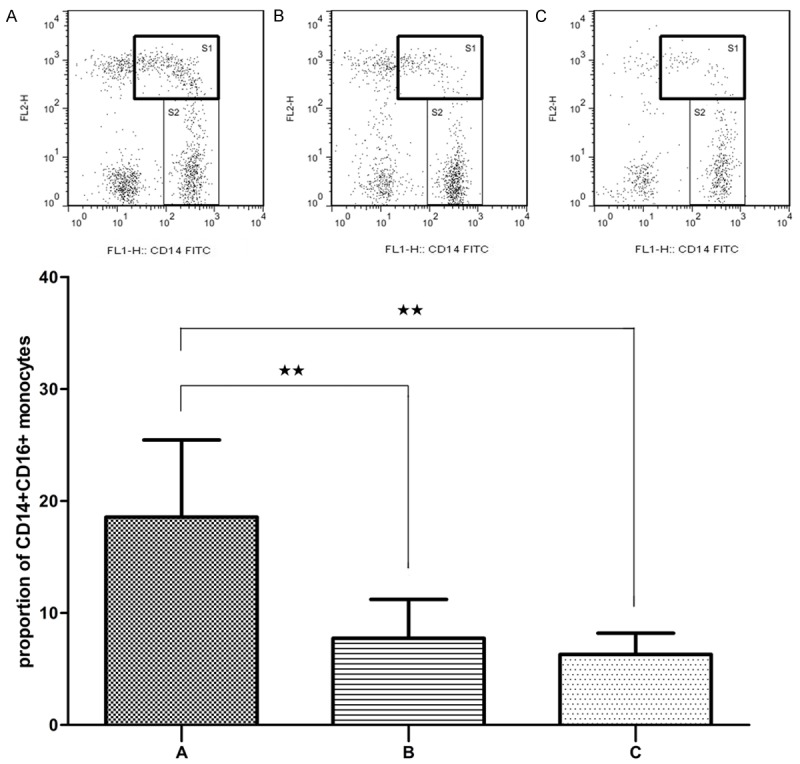
Flow cytometry analysis of CD14+CD16+ monocytes (S1) expression in AL patients in period of onset (A), patients in remission (B) and normal controls (C). Data are presented as the mean proportion of CD14+CD16+ monocytes with standard deviation. *P < 0.05, **P < 0.01.
As depicted in Figure 4, compared with patients in remission and normal controls, the proportion of non-classical monocytes in patients in period of onset significantly increased (P < 0.01). While the difference between the patients in remission and normal controls was not pronounced (P > 0.05).
Figure 4.
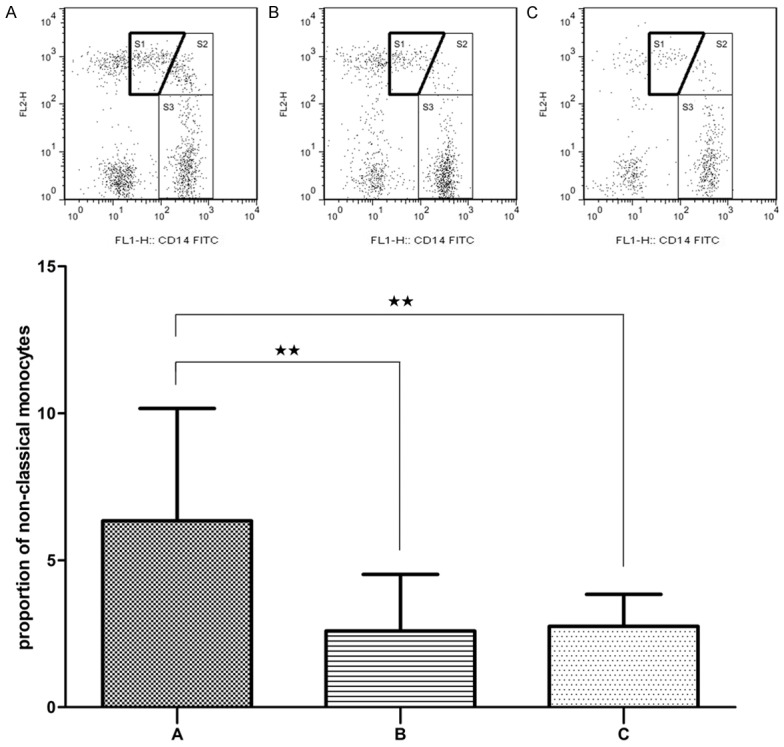
Flow cytometry analysis of non-classical monocytes (S1) expression in AL patients in period of onset (A), patients in remission (B) and normal controls (C). Data are presented as the mean proportion of CD14+CD16+ monocytes with standard deviation. *P < 0.05, **P < 0.01.
As showed in Figure 5, the proportion of intermediate monocytes in patients in period of onset was significantly higher than that of the patients in remission and normal controls (P < 0.01). While the difference between the patients in remission and normal controls was also significant (P < 0.05).
Figure 5.
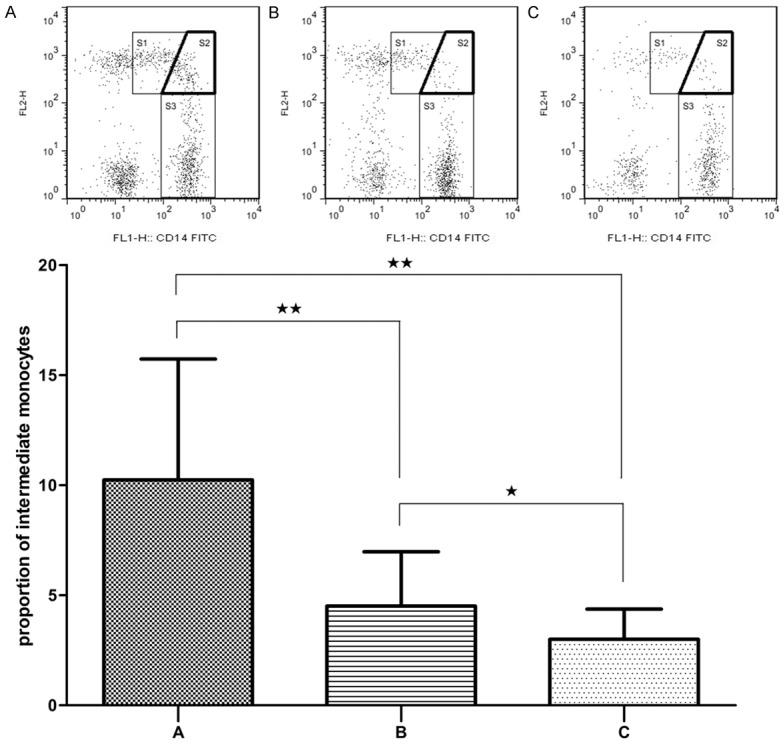
Flow cytometry analysis of intermediate monocytes (S2) expression in AL patients in period of onset (A), patients in remission (B) and normal controls (C). Data are presented as the mean proportion of CD14+CD16+ monocytes with standard deviation. *P < 0.05, **P < 0.01.
The correlation between CD14+CD16+ monocytes and other blood cells
The expression of CD14+CD16+ monocytes and other blood cells in AL patients was established in Table 2.
Table 2.
The expression of CD14+CD16+ monocytes and other blood cells in AL patients (x̅ ± s)
| AL patients | |
|---|---|
| N | 50 |
| The proportion of CD14+CD16+ monocytes | 19.14 ± 7.61 |
| Absolute number of CD4+ T cells (× 109/L) | 0.49 ± 0.35 |
| The proportion of CD4+ T cells | 47.32 ± 12.57 |
| The ratio of CD4+/CD8+ T cells | 1.13 ± 0.57 |
| The number of neutrophil granulocytes (× 109/L) | 3.19 ± 3.78 |
| The proportion of neutrophil granulocytes | 53.59 ± 23.01 |
| the proportion of total lymphocytes | 37.21 ± 20.93 |
As that could be observed in Figure 6, the results showed that the proportion of CD14+CD16+ monocytes was inversely correlated with absolute number of CD4+ T cells (r = -0.419, P = 0.002) and the proportion of CD4+ T cells (r = -0.370, P = 0.008). It was also inversely correlated with ratio of CD4+/CD8+ T cells (r = -0.310, P = 0.028) and the proportion of peripheral blood total lymphocytes (r = -0.322, P = 0.023). While it was positively correlated with number of neutrophil granulocytes (r = 0.348, P = 0.013) and the proportion of neutrophil (r = 0.385, P = 0.006).
Figure 6.
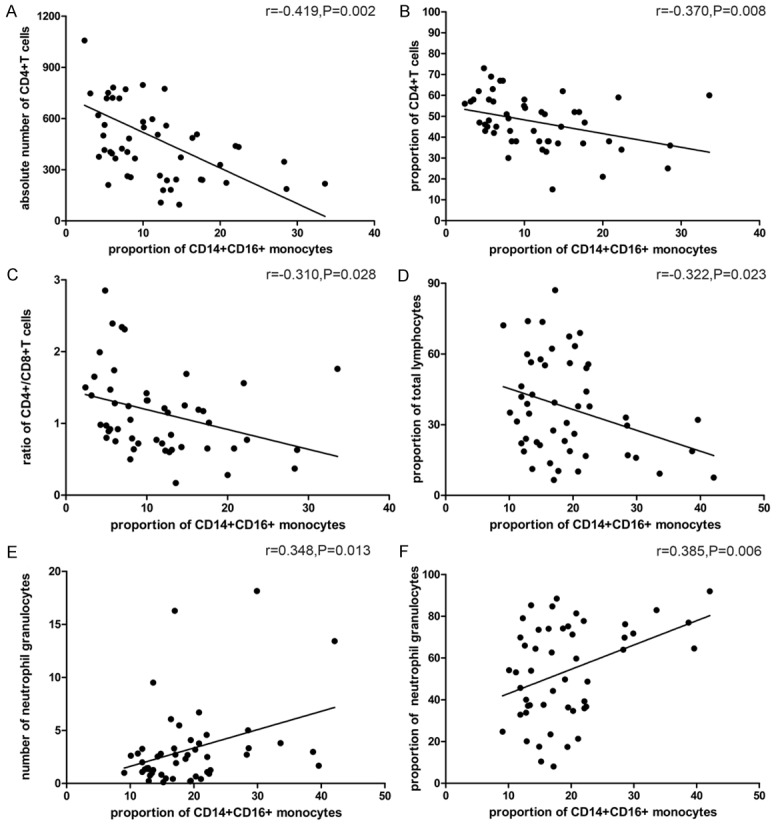
Correlation between the proportion of CD14+CD16+ monocytes and absolute number of CD4+ T cells (A), the proportion of CD4+ T cells (B), the ratio of CD4+/CD8+ T cells (C), the proportion of total lymphocytes (D), number of neutrophil granulocytes (E) and the proportion of neutrophil granulocytes (F).
Discussion
Monocytes are basis of the innate immune system and adaptive immunity. Based on the expression of lipopolysaccharide (LPS) receptor CD14 and the FcγIII receptor CD16, human peripheral blood monocytes have been divided to two subsets as CD14+CD16+ and CD14+CD16+ monocytes [3]. However, heterogeneity has also been found existing in CD14+CD16+ monocytes, which were further segregated into two subpopulations: the intermediate subset expresses relatively higher levels of CD14 coupled with lower CD16 expression, while the non-classical subset expresses lower CD14 but higher CD16 (CD14+CD16++) [7]. Later the International Consensus Statement on Monocyte Nomenclature acknowledged the major three subsets of monocytes: classical (CD14++CD16-), intermediate (CD14++CD16+) and non-classical (CD14+CD16++) monocytes [11].
Monocytes can give rise to macrophages and dendritic cells, and the pathway of the latter has drawn increasing attention [1]. Previously the whole genome expression analysis found several biologic and functional differences between the two monocyte subsets, the results suggested CD14+CD16+ monocytes may be linked to myeloid and granulocyte lineage, and showed high antimicrobial potential [5,12]. On the other hand, CD14+CD16+ monocytes seemed more advanced in differentiation, and had more dendritic cell and macrophage characters [5]. Randolph et al. [13] also demonstrated the CD14+CD16+ monocytes could preferentially migrate and develop into dendritic cells with superior allo-stimulatory capacity. Later this subset was found to develop preferentially into CD1b+ dendritic cells with high antigen-presenting capacity [14]. These data support the conclusion that CD14+CD16+ monocytes and their dendritic cells progeny are superior antigen presenting cells.
Furthermore, CD14+CD16+ monocytes showed higher surface expression of fractalkine receptor CX3CR1 and macrophage inflammatory protein 1α (MIP-1α)/RANTES receptor CCR5, but lacked monocyte chemotactic protein-1 (MCP-1) receptor CCR2 [7,15,16]. Meanwhile, function studies revealed that this subset expressed higher levels of pro-inflammatory TNF-α, IL-1β, IL-6 and IL-12 but lower level of anti-inflammatory IL-10 compared with CD14+CD16- cells [10,17-20]. In summary, CD14+CD16+ monocytes are recognized as pro-inflammatory monocytes based on the high expression of pro-inflammatory cytokines and high potency in antigen presentation.
Among the three monocyte subsets, the intermediates had been demonstrated the highest capacity to present antigen to T cells [21]. Zawada et al. [22] confirmed they were superior with respect to antigen-specific induction of IL-12 and IFN-γ as well as with respect to induction of alloantigen-induced T cell proliferation. Furthermore, the intermediate monocytes showed angiogenic properties as they express the angiopoetin-2 receptor Tie-2, suggesting a crucial role of these cells in the process of angiogenesis. The non-classical monocytes had been reported the highest expression of CX3CR1 [23]. As CX3CL1 could mediate arrest and migration of CD14+CD16+ cells, the non-classical monocytes may contribute to triggering integrin-mediated migration of leukocytes into surrounding tissues under inflammatory conditions [7,24]. Classical monocytes were found to express lots of genes linked to the phagocytosis process, according to the highest phagocytic capacity they showed [18,22]. Moreover, they also expressed highest antimicrobial proteins, which indicated the classical monocytes to be first line of innate immune defense against microbial pathogens [12].
As CD14+CD16+ monocyte subset has been labeled proinflammatory, the proportion of these monocytes has been found pronounced expansion in various kinds of disease, especially in infectious and inflammatory diseases, just like bacterial infections (e.g. sepsis, tuberculosis), viral infections (e.g. HBV, HCV, HIV, Dengue fever), autoimmune diseases (e.g. Crohn’s disease, rheumatoid arthritis), atherosclerosis, Kawasaki disease, diabetes and asthma [9,10]. Of note, in almost all circumstances of these diseases described above, expansion of both intermediates and non-classical monocytes were observed. But when come to the question that which subpopulation expanded more, we got different results from different studies. The difference may be mainly due to the different ways of gating strategy to segregate the subpopulations. In order to corroborate the crucial role played by non-classical and intermediate monocytes, explicit gating strategy must be declared by the nomenclature committee and more studies with larger patient cohorts under more disease circumstances are still in request.
In the study, we found out the CD14+CD16+ monocyte subset, including both intermediate and non-classical monocytes, in patients with AL expanded significantly in numbers, and the proportion of this subset changed as the disease progressed. Our results coincided with the characteristic of CD14+CD16+ monocytes in other disease process and suggested their clinical significance in assessing AL process. What’s more, as the intermediate subpopulation showed its significant difference even between the groups of patients in remission and normal controls, we speculate this subpopulation is more sensitive in indicating the severity of AL.
Immunoregulation also had been taken through different lymphocyte subsets [25]. Human peripheral mature lymphocytes are all of CD3+ phenotype, which include CD4+ helper T cells and CD8+ cytotoxic T cells. Detecting and monitoring the proportion of lymphocytes and the ratio of CD4+/CD8+ T cells may be beneficial not only to understand immune function, but also to assist in the clinical diagnosis, to provide evidences for the pathogenesis of the diseases as well as to determine clinical treatment forms [26-28]. For instance, it indicates the immune function would be in low level when the number of CD4+ T cells decreases or the number of CD8+ T cells increases [29].
In consideration of this, we detected the numbers and proportions of peripheral blood lymphocytes and their subsets as well as neutrophil granulocytes, and investigated the relevance between the proportion of CD14+CD16+ monocytes and results of these cells. We found proportion of this monocyte subset was inversely related to the proportion of CD4+ T cells and the ratio of CD4+/CD8+ T cells but positively related to the proportion of neutrophil granulocytes. The results suggested these indexes were similar indicators with respect to immune function assessment.
To conclude, our results indicate a crucial role of CD14+CD16+ monocyte in AL progression and provide further insights into their function in regulating immune responses. Monitoring the proportion of CD14+CD16+ monocytes seems valuable in assessing the severity of AL disease so as to provide advice or guidance for clinical treatment.
Acknowledgements
The work was supported by a grant from the Natural Science Foundation of Shandong Province, China (2012zrb14170).
Disclosure of conflict of interest
None.
References
- 1.Volk HD, Reinke P, Docke WD. Clinical aspects: from systemic inflammation to ‘immunoparalysis’. Chem Immunol. 2000;74:162–177. doi: 10.1159/000058753. [DOI] [PubMed] [Google Scholar]
- 2.Dayyani F, Belge KU, Frankenberger M, Mack M, Berki T, Ziegler-Heitbrock L. Mechanism of glucocorticoid-induced depletion of human CD14+CD16+ monocytes. J Leukoc Biol. 2003;74:33–39. doi: 10.1189/jlb.1202612. [DOI] [PubMed] [Google Scholar]
- 3.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–2534. [PubMed] [Google Scholar]
- 4.Mobley JL, Leininger M, Madore S, Baginski TJ, Renkiewicz R. Genetic evidence of a functional monocyte dichotomy. Inflammation. 2007;30:189–197. doi: 10.1007/s10753-007-9036-0. [DOI] [PubMed] [Google Scholar]
- 5.Ancuta P, Liu KY, Misra V, Wacleche VS, Gosselin A, Zhou X, Gabuzda D. Transcriptional profiling reveals developmental relationship and distinct biological functions of CD16+ and CD16- monocyte subsets. BMC Genomics. 2009;10:1471–2164. doi: 10.1186/1471-2164-10-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, Kourilsky P, Wong SC. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118:e16–31. doi: 10.1182/blood-2010-12-326355. [DOI] [PubMed] [Google Scholar]
- 7.Ancuta P, Rao R, Moses A, Mehle A, Shaw SK, Luscinskas FW, Gabuzda D. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J Exp Med. 2003;197:1701–1707. doi: 10.1084/jem.20022156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 9.Wong KL, Yeap WH, Tai JJ, Ong SM, Dang TM, Wong SC. The three human monocyte subsets: implications for health and disease. Immunol Res. 2012;53:41–57. doi: 10.1007/s12026-012-8297-3. [DOI] [PubMed] [Google Scholar]
- 10.Ziegler-Heitbrock L. The CD14+CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 11.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 12.Zhao C, Zhang H, Wong WC, Sem X, Han H, Ong SM, Tan YC, Yeap WH, Gan CS, Ng KQ, Koh MB, Kourilsky P, Sze SK, Wong SC. Identification of novel functional differences in monocyte subsets using proteomic and transcriptomic methods. J Proteome Res. 2009;8:4028–4038. doi: 10.1021/pr900364p. [DOI] [PubMed] [Google Scholar]
- 13.Randolph GJ, Sanchez-Schmitz G, Liebman RM, Schakel K. The CD16(+) (Fcgamma-RIII(+)) subset of human monocytes preferentially becomes migratory dendritic cells in a model tissue setting. J Exp Med. 2002;196:517–527. doi: 10.1084/jem.20011608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krutzik SR, Tan B, Li H, Ochoa MT, Liu PT, Sharfstein SE, Graeber TG, Sieling PA, Liu YJ, Rea TH, Bloom BR, Modlin RL. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med. 2005;11:653–660. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 16.Weber C, Belge KU, von Hundelshausen P, Draude G, Steppich B, Mack M, Frankenberger M, Weber KS, Ziegler-Heitbrock HW. Differential chemokine receptor expression and function in human monocyte subpopulations. J Leukoc Biol. 2000;67:699–704. doi: 10.1002/jlb.67.5.699. [DOI] [PubMed] [Google Scholar]
- 17.Robbins SH, Walzer T, Dembele D, Thibault C, Defays A, Bessou G, Xu H, Vivier E, Sellars M, Pierre P, Sharp FR, Chan S, Kastner P, Dalod M. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008;9:R17. doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais JP, D’Cruz D, Casanova JL, Trouillet C, Geissmann F. Human CD14 dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, Espevik T, Ziegler-Heitbrock L. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–3542. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 20.Szaflarska A, Baj-Krzyworzeka M, Siedlar M, Weglarczyk K, Ruggiero I, Hajto B, Zembala M. Antitumor response of CD14+/CD16+ monocyte subpopulation. Exp Hematol. 2004;32:748–755. doi: 10.1016/j.exphem.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 21.Grage-Griebenow E, Zawatzky R, Kahlert H, Brade L, Flad H, Ernst M. Identification of a novel dendritic cell-like subset of CD64(+)/CD16(+) blood monocytes. Eur J Immunol. 2001;31:48–56. doi: 10.1002/1521-4141(200101)31:1<48::aid-immu48>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Zawada AM, Rogacev KS, Rotter B, Winter P, Marell RR, Fliser D, Heine GH. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood. 2011;118:e50–61. doi: 10.1182/blood-2011-01-326827. [DOI] [PubMed] [Google Scholar]
- 23.Rogacev KS, Seiler S, Zawada AM, Reichart B, Herath E, Roth D, Ulrich C, Fliser D, Heine GH. CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur Heart J. 2011;32:84–92. doi: 10.1093/eurheartj/ehq371. [DOI] [PubMed] [Google Scholar]
- 24.Imhof BA, Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol. 2004;4:432–444. doi: 10.1038/nri1375. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimura K, Laird LS, Chia CY, Meckel KF, Slansky JE, Thompson JM, Jain A, Pardoll DM, Schulick RD. Live attenuated Listeria monocytogenes effectively treats hepatic colorectal cancer metastases and is strongly enhanced by depletion of regulatory T cells. Cancer Res. 2007;67:10058–10066. doi: 10.1158/0008-5472.CAN-07-0573. [DOI] [PubMed] [Google Scholar]
- 26.Krupnick AS, Kreisel D, Szeto WY, Popma SH, Amin KM, Moore JS, Rosengard BR. Multiparameter flow cytometric approach for simultaneous evaluation of T lymphocyte-endothelial cell interactions. Cytometry. 2001;46:271–280. doi: 10.1002/cyto.1168. [DOI] [PubMed] [Google Scholar]
- 27.Li CH, Kuo WH, Chang WC, Huang SC, Chang KJ, Sheu BC. Activation of regulatory T cells instigates functional down-regulation of cytotoxic T lymphocytes in human breast cancer. Immunol Res. 2011;51:71–79. doi: 10.1007/s12026-011-8242-x. [DOI] [PubMed] [Google Scholar]
- 28.Wang ZK, Yang B, Liu H, Hu Y, Yang JL, Wu LL, Zhou ZH, Jiao SC. Regulatory T cells increase in breast cancer and in stage IV breast cancer. Cancer Immunol Immunother. 2012;61:911–916. doi: 10.1007/s00262-011-1158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matkowski R, Gisterek I, Halon A, Lacko A, Szewczyk K, Staszek U, Pudelko M, Szynglarewicz B, Szelachowska J, Zolnierek A, Kornafel J. The prognostic role of tumor-infiltrating CD4 and CD8 T lymphocytes in breast cancer. Anticancer Res. 2009;29:2445–2451. [PubMed] [Google Scholar]


