Abstract
Pulmonary fibrosis (PF) leads to chronic inflammation and accumulation of macrophages, neutrophils, and lymphocytes in the alveoli. The factors involved in the development of PF include reactive oxygen species and tissue remodelling regulators. The present study demonstrates the effect of andrographolide on bleomycin (BLM)-induced PF in Sprague-Dawley rats. We investigated the total bronchoalveolar lavage fluid protein (BALF) and hydroxyproline (HYP) content along with the level of oxidative stress markers like malondialdehyde (MDA) and GSH/GSSG ratio. In addition, the levels of MMP-1 and TIMP-1 were also analysed. The results revealed an increase in BALF protein, HYP, and MDA contents and decrease in GSH/GSSG ratio of the lungs in animals treated with BLM. However, andrographolide treatment caused a reversal of the BLM induced changes after 20 or 40 days. Treatment with andrographolide suppressed oxidative stress with the decrease of MDA and the increase of the GSH/GSSG ratio. Andrographolide also improved the BLM mediated changes in the MMP-1/TIMP-1 ratio. Therefore, andrographolide has a potential therapeutic effect in the prevention of PF.
Keywords: Hydroxyproline, bronchoalveolar, inflammation, macrophages, lymphocytes
Introduction
Pulmonary fibrosis, formation of excessive fibrous tissue leads to chronic inflammation and accumulation of macrophages, neutrophils, and lymphocytes in the lung alveoli [1]. Reactive oxygen species (ROS) like superoxide, peroxides, peroxynitrite, and hydroxyl radicals are believed to play a vital role in the lung inflammatory processes [2]. It is reported that the ROS generated by phagocytes on activation induce pulmonary fibrosis [1,3,4]. There is formation of lesions in alveolar septa, proliferation of fibroblast and myofibroblast in lung parenchyma, and deposition of excessive extracellular matrix macromolecule in PF [2,5,6]. Nuclear accidents, total body irradiation for hematopoietic stem cell transplantation, thoracic radiotherapy for lung, breast, thymoma, and lymphoma carcinoma is accompanied by pulmonary fibrosis [7,8]. It is reported that radiation-induced pulmonary fibrosis is accompanied by chronic oxidative stress [9]. The currently available drugs only decrease acute pneumonitis for 2-3 months after irradiation but fail to suppress fibrosis [10]. Therefore, discovery of a novel molecule for the prevention and treatment of PF is highly desired.
Bleomycin (BLM) used for the treatment of different carcinomas through oxidative DNA damage leads to the development of PF due to cysteine hydrolase, BLM in-activator deficiency in lungs [11]. BLM-induced pulmonary fibrosis strategies, therefore, involve use of antioxidants like N-acetylcysteine (NAC) and bilirubin [12,13]. Matrix metalloproteinases (MMPs) and their specific inhibitors, TIMPs have a vital role in inflammatory conditions [14,15]. MMP-1 has been reported to be present in lung samples from IPF (idiopathic pulmonary fibrosis) patients and are believed to be responsible for the degradation of type III collagen [16]. However, TIMP-1 induced by different agents like BLM in lung tissues decreases the ratio of MMP/TIMP and induce fibrogenicity [5,17-19].
Andrographolide (Figure 1) a member of labdane diterpene lactone family was isolated from the plant Andrographis paniculata [20]. Traditionally the plant extract was used for the treatment of upper respiratory tract infection [21,22]. Andrographolide has shown promising antiviral [23], anti-cancer [24], hepatoprotective [25] and anti-inflammatory [26,27] activities. In addition, andrographolide is also believed to promote Nrf2 nuclear translocation human endothelial cell line EA.hy926 [28]. Thus, the present study was devised to investigate the effect of andrographolide on pulmonary fibrosis in a rat model of lung injury induced by BLM treatment.
Figure 1.
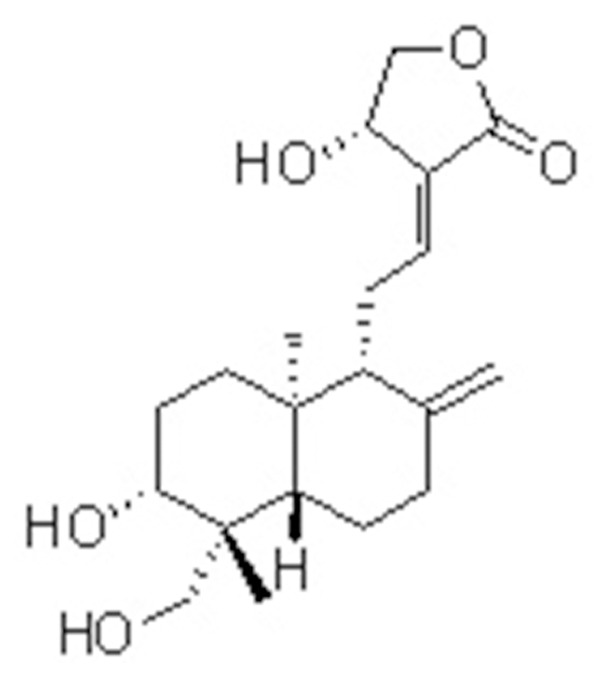
Structure of andrographolide.
Materials and methods
Materials
Andrographolide and bleomycin A5 hydrochloride (BLM) were purchased from Sigma (St. Louis, MO, USA). Glutathione (GSH), 5’, 5’-dithiobis-2-nitrobenzoic acid (DTNB) and other common chemicals were purchased from Merck (New Jersey, USA).
Animals
Ten week old male, adult SpragueDawley rats (Chengdu Dashuo Biological Technology Co., Ltd., Chengdu, China) were maintained according to the guidelines of the National Institutes of Health and Academy of Military Medical Sciences for the Care and Use of Laboratory Animals. Rats were provided with pathogen-free water and food with a 12 h light/dark cycle. The study was approved by the Committee on the Ethics of Animal Experiments of the Affiliated Hospital of Academy of Military Medical Sciences.
Pulmonary fibrosis induction and treatment
Among 3 groups of rats with 20 each, 2 groups were administered bleomycin hydrochloride (5 mg/kg body weight) and the control group received saline. The treatment group of rats were intraperitoneally injected 10 mg/kg andrographolide once daily and the control group received same volume of saline. The animals were sacrificed at 10, 20 and 40 days after BLM treatment. The parameters used to analyse pulmonary fibrosis (PF) were lung histology, hydroxyproline (HYP) content, oxidative stress marker levels and total protein in bronchoalveolar lavage fluid (BALF).
Histological assessment
For examination of histopathologic changes left lungs from all the three groups were collected 10, 20, and 40 days after BLM treatment. The sections were fixed in paraformaldehyde and embedded in paraffin. The extent of fibrosis was analysed using haematoxylin and eosin and Masson’s trichrome stain. Semiquantitative grading system was used for the extent and severity of interstitial fibrosis. Ten sites were examined for each lung section to assign grades to the PF. Normal lung was assigned, grade 0; minimal fibrous thickening, grade 1; moderate thickening, grade 2-3; increased fibrosis with definite damage to lung architecture, grade 4-5; severe distortion of structure, grade 6-7 and total fibrous obliteration, grade 8. The mean score of all fields was taken as the fibrosis score of that lung section.
Protein content in BALF and hydroxyproline measurement
Rats were anaesthetized, sacrificed and the right lung was washed with saline to collect BALF. Lowry’s method was used to determine total protein concentration in BALF supernatants (g/L). For HYP content measurement homogenized lung tissue was hydrolyzed in 6 N HCl for 18 h at 110°C and neutralized with 2.5 M NaOH. After mixing 2 ml aliquots with citrate buffer, 1 ml chloramine T (0.05 M), and 1 ml perchloric acid (3.15 M) were added to quench the reaction. The samples were then incubated with 1 ml dimethylaminobenzaldehyde at 80°C and absorbance was measured at 560 nm.
Lung tissue oxidant and antioxidant analysis
We used thiobarbituric acid to estimate the lung MDA (malondialdehyde) levels and CBB (Coomassie brilliant blue G250) staining for total protein concentration. For reduced glutathione (GSH), 100 μl BALF sample was mixed with 100 μl 10% TCA and then centrifuged for 15 min. The supernatant (50 μl) was mixed with 50 μl formaldehyde, 1.8 ml of 0.1 M phosphate buffer, 100 μl 0.1% o-phthalaldehyde and incubated for 45 min. Fluorospectrophotometer was then used to measure the GSH level. The level of GSSG was determined by using the above method with a minor modification of 0.1 M NaOH instead of 0.1 M phosphate buffer.
RNA preparation and RT-PCR of MMP-1 and TIMP-1
The total RNA from deep frozen lung homogenates of rats was isolated using TRIzol reagent. The Super Script II reverse transcriptase was used to reverse transcribe first strand cDNA from the total RNA. The primers for the genes of interest were subjected to PCR amplification. Using total RNA (2 μg), RT-PCR for MMP-1, TIMP-1 and β-actin control was performed using Gene Amp PCR System 9700 (Perkin Elmer Applied Biosystems, Foster City, CA). The amplified PCR products (5 μl) were separated by electrophoresis on a 1.2% agarose gel containing ethidium bromide, and were photographed under UV light (Bio-Rad Gel system). The intensity of each cDNA band was quantitated and normalized to the corresponding β-actin cDNA band intensity (internal control) using a Bio-Rad Gel Doc 2000 System.
Immunohistochemical detection of MMP-1 and TIMP-1
Deparaffinised lung sections (2 µ) were treated with hydrogen peroxide for 45 min and then washed thrice with PBS. The sections were treated with 1.5% normal goat serum for 45 min, then washed in PBS and incubated with primary antibodies like rabbit anti-MMP-1, -TIMP-1, and a negative control antibody (Boster, P. R. China) overnight at 4°C. The sections were then incubated with a biotinylated anti-rabbit IgG (secondary antibody; Boster) for 30 after PBS washing. Diaminobenzidine-hydrogen peroxide solution was used to develop the reaction, the sections were counterstained with haematoxylin and eosin and then mounted.
Statistical analysis
All the results are expressed as mean of ± S. D. Two-way ANOVA and Student’s t-tests were used for data analysis. The SPSS version 11.0 statistical software (SPSS, Inc., Chicago, IL) was used for data analyses. The differences were considered statistically significant at P < 0.05.
Results
Effect of andrographolide on pulmonary fibrosis histopathology
Light microscopic examination of the lung sections revealed absence of bleeding sites and signs of congested edema in andrographolide-treated rats after 40 days of BLM-treatment compared with BLM-treated and control group (Figure 2A). The lung sections showed the presence of excess of inflammatory cells in alveoli and interstitium, large fibrous areas and traction bronchiectasis after 40 days in BLM-group compared to control group (Figure 2B). However, treatment with andrographolide caused a significant decrease in the extent of fibrosis compared to BLM group (Figure 2B). The estimation of overall grades of lung fibrosis by numerical scoring after 40 days showed a significant decrease in scores of fibrosis in the lung sections of the andrographolide-treated group compared to BLM group (Table 1).
Figure 2.
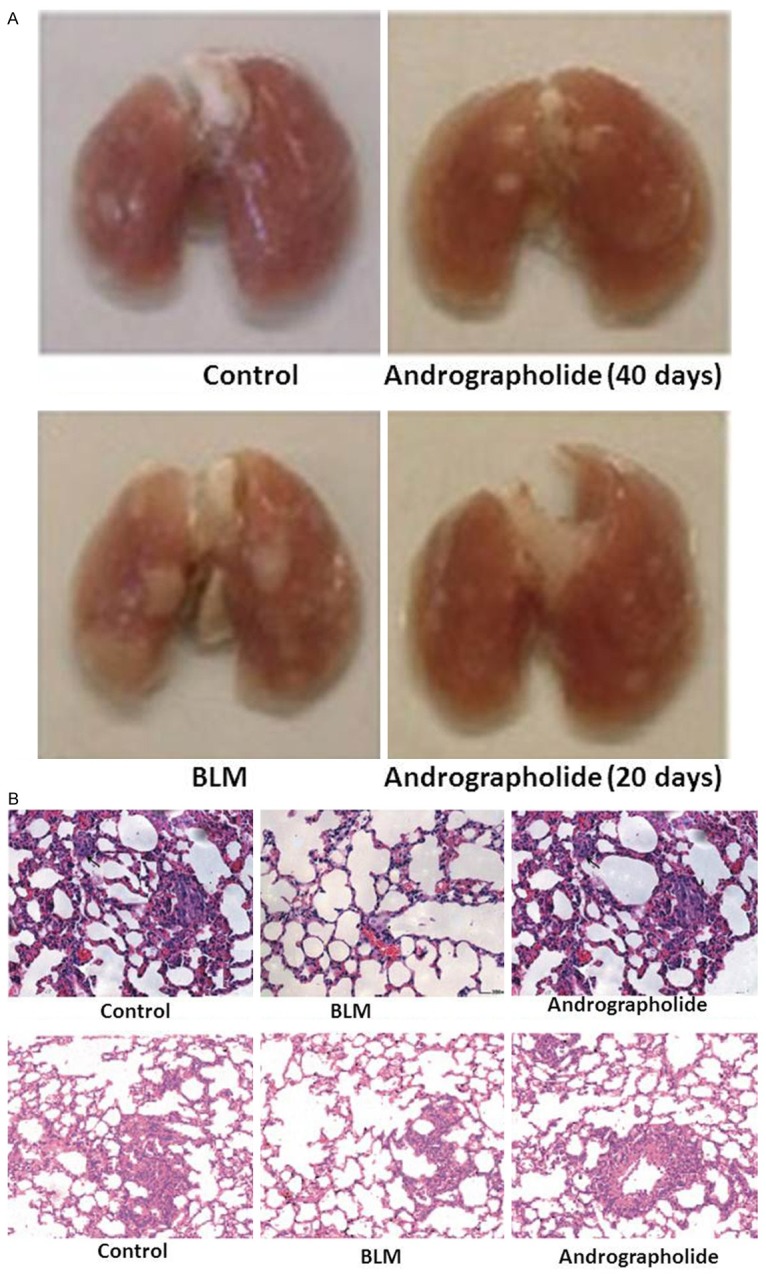
A. Effect of andrographolide on the lung appearance after 80 days of BLM. Andrographolide-treated animals showed no lung collapse and gray fibrous nodules. B. Representative photomicrographs of lung histology in the control, BLM and andrographolide groups. Lung tissues were obtained after 40 days treatment, and stained with haematoxylin and eosin and Masson’s trichrome to evaluate histological changes of the lungs.
Table 1.
Pulmonary fibrosis grades after 40 days of treatment
| Groups | Grade of fibrosis |
|---|---|
| Control | 0.91 ± 0.05 |
| BLM | 5.45 ± 0.26 |
| Andrographolide | 1.83 ± 0.53 |
Effect of andrographolide on BALF Protein and lung hydroxyproline content
Treatment of rats with BLM leads to a significant increase in BALF protein expression (Figure 3A). Andrographolide-treatment caused a decrease in BALF proteins; however the decrease was significant after 20 days of the treatment. Since increased BALF protein concentration is an important marker of alveolar edema, therefore, andrographolide has a great potential for ameliorating lung edema. Andrographolide treatment also leads to a significant decrease in the BLM-induced HYP content after 20 days in lung homogenates. Since lung HYP concentration reflects collagen deposition in lungs, thus andrographolide has a protecting effect against pulmonary fibrosis induced by BLM (Figure 3B).
Figure 3.
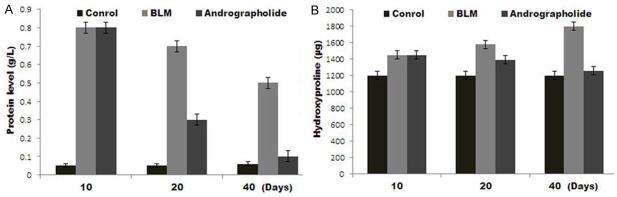
Andrographolide treatment suppressed BLM-induced total proteins and hydroxyproline. A. Total proteins in BALF were measured after 10, 20 and 40 days in rats treated with BLM with or without andrographolide treatment. B. Lung HYP content was measured spectrophotometrically after 10, 20 and 40 days in the BLM and andrographolide treatment groups. Data are the mean ± SEM of three independent observations.
Effect of andrographolide on oxidative stress markers, MDA content and GSH/GSSG ratio
In the rats treated with BLM, MDA level was increased after 20 days compared to that of control group (Figure 4A). Andrographolide-treatment caused a significant decrease in the BLM-induced lung tissue MDA content, suggesting a vital role of andrographolide in reducing BLM-mediated oxidative stress. Treatment of rats with BLM resulted in a decrease in the GSH/GSSG ratio (a decrease in GSH accompanied by an increase in GSSG) after 10, 20 and 40 days of the exposure. Andrographolide treated rats showed a significant increase in BLM-induced GSH/GSSG ratio reduction after 10, 20 and 40 days of the treatment (Figure 4B). Therefore, treatment with andrographolide resulted in a significant enhancement in the GSH/GSSG ratio in the lung compared with that in the BLM group.
Figure 4.
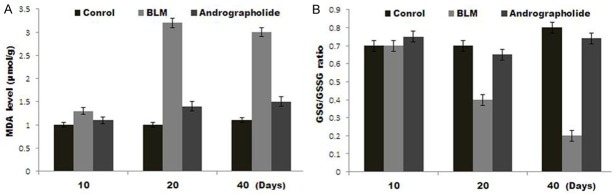
Effect of andrographolide on BLM-modulated malondialdehyde (MDA) and the GSH/GSSG ratio 10, 20 and 40 days after BLM administration. A. Reduction of MDA levels in lung tissue after 40 days of BLM administration in rats treated with intraperitoneal andrographolide. B. Enhancement of BLM-induced decreases in the GSH/GSSG ratio at day 20 and 40 by andrographolide. Data are presented as the mean ± SEM.
Effect of andrographolide on MMP-1 and TIMP-1 expression
The results from Western blot analysis revealed an increase of both MMP-1 and TIMP-1 mRNA levels after day 10 which persisted for 40 days following BLM exposure (Figure 5A-C). However, andrographolide treatment caused a significant decrease in the levels of both MMP-1 and TIMP-1 mRNA (Figure 5A-C). These results indicate that bleomycin injury led to a significant increase in TIMP-1, while andrographolide could lower its expression after 10 days.
Figure 5.
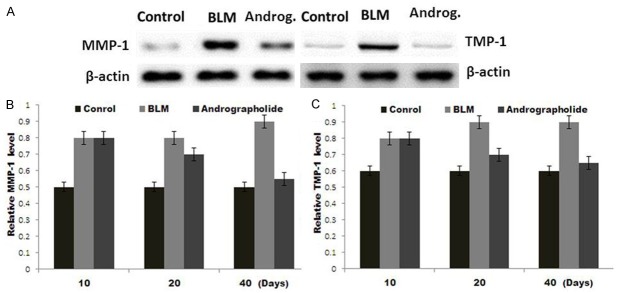
Western blot analysis of mRNA expression levels of MMP-1 and TIMP-1. A. Total RNA was isolated from the lung tissue of the control, BLM and andrographolide groups for Western blot analysis at day 10, 20 and 40. B. The relative MMP-1 and C. TIMP-1 mRNA levels after 10, 20 and 40 days in each group were estimated using densitometry and were normalized to control β-actin.
Effect of andrographolide on BLM-induced MMP-1 and TIMP-1 protein expression
The examination of lung sections showed higher level of immunostaining for MMP-1 at 40 days after bleomycin exposure in BLM group compared to control group of animals. However, treatment with andrographolide led to a slight increase in MMP-1 immunostaining (Figure 6). The level of immunostaining for MMP-1 inhibitor TIMP-1 was significantly increased in rats injected with bleomycin compared to the control rats. Treatment of the BLM-treated rats with andrographolide caused a significant decrease in the level of dark-brown TIMP-1 staining (Figure 6). Thus andrographolide improves the MMP-1/TIMP-1 ratio.
Figure 6.
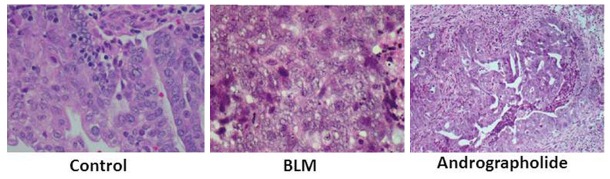
Immunohistochemical analysis of MMP-1 and TIMP-1 in lung sections obtained after 20 days from control and BLM-treated rats with or without andrographolide treatment.
Discussion
Cancer chemotherapy leads to the development of pulmonary fibrosis (PF) resulting in the deposition of excessive collagen. ROS-activated inflammatory cells induce expression of a variety of intracellular oxidative enzymes and synthesis of reactive nitrogen species (RNS) to remove necrotic tissue [18,19]. Change in oxidant/antioxidant balance in the lung increases tissue damage.
The currently available treatment strategies involve use of glucocorticoid and some cytotoxin-like medicine, but the need for a novel molecule to treat pulmonary fibrosis is unmet. The present study demonstrates the effect of andrographolide in an animal model of bleomycin (BLM)-induced PF. The results demonstrate that andrographolide effectively reduced lung injury induced by BLM-treatment in rats. The mechanism of andrographolide action for suppression of PF is not fully understood, but it appears to act through inhibition of radicles formation. Andrographolide leads to inhibition of collagen accumulation and improves MMP-1/TIMP-1 ratio. It is reported that BLM activates NF-κB resulting in the generation of ROS [13]. However, inhibition of NF-κB activation by antioxidants suppresses pulmonary fibrosis [27,28]. The results from the present study reveal inhibition of NF-κB activation by andrographolide. We observed an increase in the level of MDA, a key marker of lipid peroxidation in BLM-treated rats but the level was decreased in the andrographolide treated group. Glutathione (GSH), an antioxidant in lungs protects lung cells against oxidative damage [29], however BLM treatment caused a decrease in the level of GSH. The andrographolide treated rats showed a significant increase in the GSH/GSSG ratio.
The MMPs play a vital role in the regulation of tissue remodelling through ECM turnover in both the normal and pathological conditions. Suppression of PF by inhibition of MMPs using MMP-inhibitor, batimastat indicates a vital role of MMPs in BLM-induced PF [16]. In this study, both MMP-1 mRNA and protein were decreased after 20 or 40 days BLM treatment. On the other hand, BLM exposure led to a significant increase in TIMP-1, while andrographolide lowered its expression after 20 or 40 days.
In conclusion, andrographolide can be a potent agent in the prevention of BLM-induced oxidative lung damage and human IPF.
Disclosure of conflict of interest
None.
References
- 1.Ward PA, Hunninghake GW. Lung inflammation and fibrosis. Am J Respir Crit Care Med. 1998;157:S123–S129. doi: 10.1164/ajrccm.157.4.nhlbi-10. [DOI] [PubMed] [Google Scholar]
- 2.Crouch E. Pathobiology of pulmonary fibrosis. Am J Physiol. 1990;259:L159–L184. doi: 10.1152/ajplung.1990.259.4.L159. [DOI] [PubMed] [Google Scholar]
- 3.Strausz J, Muller-Quernheim J, Steppling H, Nagel M, Ferlinz R. Oxygen radical production by alveolar macrophages in sarcoidosis in relation to activity status of bronchoalveolar lavage lymphocytes. Pneumologie. 1990;44:222–223. [PubMed] [Google Scholar]
- 4.Rahman I, Skwarska E, Henry M, Davis M, O’Connor CM, FitzGerald MX, Greening A, MacNee W. Systemic and pulmonary oxidative stress in idiopathic pulmonary fibrosis. Free Radic Biol Med. 1999;27:60–68. doi: 10.1016/s0891-5849(99)00035-0. [DOI] [PubMed] [Google Scholar]
- 5.Braselmann S, McCormick F. Bcr and Raf form a complex in vivo via 14-3-3 proteins. EMBO J. 1995;14:4839–4848. doi: 10.1002/j.1460-2075.1995.tb00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katzenstein AL, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med. 1998;157:1301–1315. doi: 10.1164/ajrccm.157.4.9707039. [DOI] [PubMed] [Google Scholar]
- 7.Almeida C, Nagarajan D, Tian J, Leal SW, Wheeler K, Munley M, Blackstock W, Zhao W. The role of alveolar epithelium in radiation-induced lung injury. PLoS One. 2013;8:e53628. doi: 10.1371/journal.pone.0053628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epperly MW, Guo H, Gretton JE, Greenberger JS. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29:213–224. doi: 10.1165/rcmb.2002-0069OC. [DOI] [PubMed] [Google Scholar]
- 9.Robbins ME, Zhao W. Chronic oxidative stress and radiation-induced late normal tissue injury: a review. Int J Radiat Biol. 2004;80:251–259. doi: 10.1080/09553000410001692726. [DOI] [PubMed] [Google Scholar]
- 10.Mak JC. Potential role of green tea catechins in various disease therapies: progress and promise. Clin Exp Pharmacol Physiol. 2012;39:265–273. doi: 10.1111/j.1440-1681.2012.05673.x. [DOI] [PubMed] [Google Scholar]
- 11.Hayman MJ, Enrietto PJ. Cell transformation by the epidermal growth factor receptor and v-erbB. Cancer Cells. 1991;3:302–307. [PubMed] [Google Scholar]
- 12.Serrano-Mollar A, Closa D, Prats N, Blesa S, Martinez-Losa M, Cortijo J, Estrela JM, Morcillo EJ, Bulbena O. In vivo antioxidant treatment protects against bleomycin-induced lung damage in rats. Br J Pharmacol. 2003;138:1037–1048. doi: 10.1038/sj.bjp.0705138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang HD, Yamaya M, Okinaga S, Jia YX, Kamanaka M, Takahashi H, Guo LY, Ohrui T, Sasaki H. Bilirubin ameliorates bleomycin-induced pulmonary fibrosis in rats. Am J Respir Crit Care Med. 2002;165:406–411. doi: 10.1164/ajrccm.165.3.2003149. [DOI] [PubMed] [Google Scholar]
- 14.Corbel M, Belleguic C, Boichot E, Lagente V. Involvement of gelatinases (MMP-2 and MMP-9) in the development of airway inflammation and pulmonary fibrosis. Cell Biol Toxicol. 2002;18:51–61. doi: 10.1023/a:1014471213371. [DOI] [PubMed] [Google Scholar]
- 15.Corbel M, Caulet-Maugendre S, Germain N, Molet S, Lagente V, Boichot E. Inhibition of bleomycin-induced pulmonary fibrosis in mice by the matrix metalloproteinase inhibitor batimastat. J Pathol. 2001;193:538–545. doi: 10.1002/path.826. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda Y, Ishizaki M, Kudoh S, Kitaichi M, Yamanaka N. Localization of matrix metalloproteinases-1, -2, and -9 and tissue inhibitor of metalloproteinase-2 in interstitial lung diseases. Lab Invest. 1998;78:687–698. [PubMed] [Google Scholar]
- 17.Kolb M, Bonniaud P, Galt T, Sime PJ, Kelly MM, Margetts PJ, Gauldie J. Differences in the fibrogenic response after transfer of active transforming growth factor-beta1 gene to lungs of ‘fibrosis-prone’ and ‘fibrosis-resistant’ mouse strains. Am J Respir Cell Mol Biol. 2002;27:141–150. doi: 10.1165/ajrcmb.27.2.4674. [DOI] [PubMed] [Google Scholar]
- 18.Swiderski RE, Dencoff JE, Floerchinger CS, Shapiro SD, Hunninghake GW. Differential expression of extracellular matrix remodeling genes in a murine model of bleomycin induced pulmonary fibrosis. Am J Pathol. 1998;152:821–828. [PMC free article] [PubMed] [Google Scholar]
- 19.Madtes DK, Elston AL, Kaback LA, Clark JG. Selective induction of tissue inhibitor of metalloproteinase-1 in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2001;24:599–607. doi: 10.1165/ajrcmb.24.5.4192. [DOI] [PubMed] [Google Scholar]
- 20.Lim JC, Chan TK, Ng DS, Sagineedu SR, Stanslas J, Wong WS. Andrographolide and its analogues: verstaile bioactive molecules for combating inflammation and cancer. Clin Exp Pharmacol Physiol. 2012;39:300–310. doi: 10.1111/j.1440-1681.2011.05633.x. [DOI] [PubMed] [Google Scholar]
- 21.Coon JT, Ernst E. Androgaphis paniculata in the treatment of upper respiratory tract infection: a systematic review of safety and efficacy. Planta Med. 2004;70:93–298. doi: 10.1055/s-2004-818938. [DOI] [PubMed] [Google Scholar]
- 22.Chang J, Zhang RM, Zhang Y, Chen ZB, Zhang ZM, Xu Q, Yang YP, Long YY, Liu LL, Cai HY, Gao J, Lu N, Mao B, Wang L, Li TQ. Andrographolide drop-pill in the treatment of acute upper respiratory tract infection with external wind-heat syndrome: a multicenter and randomized controlled trial. J Chin Integr Med. 2008;6:1238–1245. doi: 10.3736/jcim20081206. [DOI] [PubMed] [Google Scholar]
- 23.Lin TP, Chen SY, Duh PD, Chang LK, Liu YN. Inhibition of the Epstein-Barr virus lytic cycle by andrographolide. Biol Pharm Bull. 2008;31:2018–2023. doi: 10.1248/bpb.31.2018. [DOI] [PubMed] [Google Scholar]
- 24.Lim JC, Chan TK, Ng DS, Sagineedu SR, Stanslas J, Wong WS. Andrographolide and its analogues: verstaile bioactive molecules for combating inflammation and cancer. Clin Exp Pharmacol Physiol. 2012;39:300–310. doi: 10.1111/j.1440-1681.2011.05633.x. [DOI] [PubMed] [Google Scholar]
- 25.Negi AS, Kumar JK, Luqman S, Shanker K, Gupta MM, Khanuja SP. Recent advances in plant hepatoprotectives: a chemical and biological profile of some important leads. Med Res Rev. 2008;28:746–772. doi: 10.1002/med.20115. [DOI] [PubMed] [Google Scholar]
- 26.Abu-Ghefreh AA, Canatan H, Ezeamuzie CI. In vitro and in vivo anti-inflammatory effects of andrographolide. Int Immunopharmacol. 2009;9:313–318. doi: 10.1016/j.intimp.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Bao Z, Guan SP, Cheng C, Wu S, Wong SH, Kemeny DM, Leung BP, Wong WS. A novel antiinflammatory role for andrographolide in asthma via inhibition of the nuclear factor-kB pathway. Am J Respir Crit Care Med. 2009;179:657–665. doi: 10.1164/rccm.200809-1516OC. [DOI] [PubMed] [Google Scholar]
- 28.Yu AL, Lu CY, Wang TS, Tsai CW, Liu KL, Cheng YP, Chang HC, Lii CK, Chen HW. Induction of heme oxygenase 1 and inhibition of tumor necrosis factor alpha-induced intercellular adhesion molecule expression by andrographolide in EA. hy926 cells. J Agric Food Chem. 2010;58:7641–7648. doi: 10.1021/jf101353c. [DOI] [PubMed] [Google Scholar]
- 29.Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J. 2000;166:534–554. doi: 10.1034/j.1399-3003.2000.016003534.x. [DOI] [PubMed] [Google Scholar]


