Abstract
Lung cancer is the most leading cause of cancer-related death worldwide, with non-small-cell lung cancer (NSCLC) accounting for over 80% of all lung cancer cases. Patients with NSCLC are mostly treated with platinum-based chemotherapy. Chemoresistance is a leading cause of chemo-therapy failure in NSCLC treatment. Recent studies have shown that dysregulation of microRNAs might modulate the resistance of cancer cells to anti-cancer drugs, yet the modulation mechanism is not fully understood. In this paper, we try to test whether miR-192 regulates chemo-resistance in human carcinoma A549 mice model by targeting Bcl-2. Mice model of human lung adenocarcinoma was built up, and was used for gemcitabine and cisplatin combined chemotherapy. MTT assay, real-time RT-PCR, western blotting assay were used to investigate miR-192 expression levels, cell viability ratio and Bcl-2 protein expression levels. MiR-192 expression level in A549 cells is significantly higher than in normal human bronchial epithelial cells. MiR-192 inhibitor treated tumor exhibits sensitivity to cisplatin and gemcitabine therapy. Bcl-2 mRNA and protein expression levels up-regulated in miR-192 inhibitor treated tumor. Bcl-2 is a key regulator for miR-192 related chemotherapy resistance. In this study, we demonstrate that miR-192 regulates chemoresistance for gemcitabine and cisplatin combined chemotherapy in human adenocarcinoma lung cancer A549 cells, and Bcl-2 is the target of miR-192.
Keywords: MicroRNA, chemoresistance, Bcl-2, pathways
Introduction
Lung cancer is the most leading cause of cancer-related death worldwide. Non-small-cell lung cancer (NSCLC) accounts for over 80% of all lung cancer cases [1]. The prognosis for NSCLC remains poor despite advances in the methodologies of diagnosis and chemotherapy. The 5-year survival rate is only 11% [2]. Although Surgery excision is the most effective method for NSCLC treatment, only a few portion of patients can get benefits from it due to the difficulties to diagnose early stage NSCLC. NSCLC patients are often treated with platinum-based chemotherapy. However, patients often develop chemoresistance which leads to chemo-therapy failure [3]. Methods to alleviate chemoresistance are of great interest in NSCLC treatment [4,5].
MicroRNAs (miRNAs) are short non-coding RNAs that negatively regulate target gene expressions by binding to 3’-untranslated regions of its messenger RNAs leading to degradation or translational suppression [6-8]. Recent studies have shown that miRNAs might modulate chemoresistance of cancer cells although the modulation mechanisms are not clear [9-11]. Several miRNAs were found to promote the NSCLC oncogenesis such as miR-10b, miR-150 and miR-205 [12-14]. On the contrary, miRNA-16, miRNA-140 and miRNA-223 could suppress tumorigenesis [15-17]. MiRNA-192 was associated with oncogenesis of lung cancer, gastric cancer, and colorectum [18,19]. However, whether miR-192 has any effects on chemoresistance in NSCLC is not known.
The Bcl-2 family proteins play an important role in apoptosis through the balance of anti-apoptotic proteins (e.g., Bcl-2, Bcl-XL, Mcl-1) and pro-apoptotic proteins (e.g., Bak, Bax, Bad, Bid) [20]. Bcl-2 proteins are over-expressed in a variety of tumors, which can allow cancer cells escape from apoptosis [21,22]. Bcl-2 was considered as prognostic impact factor and aggression factor in NSCLC [23,24]. Whether Bcl-2 is related to chemotherapy drug resistance is unknown. In this paper, we try to test whether miR-192 regulates chemo-resistance in human carcinoma A549 mouse model by targeting Bcl-2.
Materials and methods
Xenograph tumor model in mice
All animal work was approved by the Animal Care Committee of Soochow University in accordance with institutional and Chinese government guidelines for animal experiments. All injections on animals were performed under anesthesia with inhaled isoflurane. Mice were housed in a temperature controlled environment with lights from 06:00-20:00 hour cycle and with water and food freely available. All efforts were made to minimize animal suffering and to reduce experimental animal numbers.
Female Balb/c nude mice (3 months old, 180-220 grams) were purchased from Shanghai SLAC Laboratory Animal Co., LTD (Shanghai, China). Mice were housed singly in polyethylene cages with hardwood-chip bedding and given free access to food and water. Five million A549 cells were subcutaneously injected in the right flank of 25 mice with inhaled isoflurane.
Mice body weights and tumor growth rates were monitored twice a week for 3 weeks. The tumor volume was calculated with the formula (long dimension) × (short dimension)2/2. Two mice died within one week after the study started. Only 18 mice carrying tumors reached approximately 200 mm3, and body weights ranged from 160-240 grams were included into this study. Five mice were sacrificed with inhaling carbon monoxide in their home cage due to either too low body weights or tumor sizes are too big. These 18 mice were randomly divided into two groups (n = 9 mice/each group), and treated with either vehicle or gemcitabine plus cisplatin by tail vein injection. The dose and timetable of injection was shown in Figure 4A. The body weight and survival of the nude mice were monitored twice per week throughout the experiments. The mice were sacrificed by decapitation to collect tumor samples for further analysis.
Figure 4.
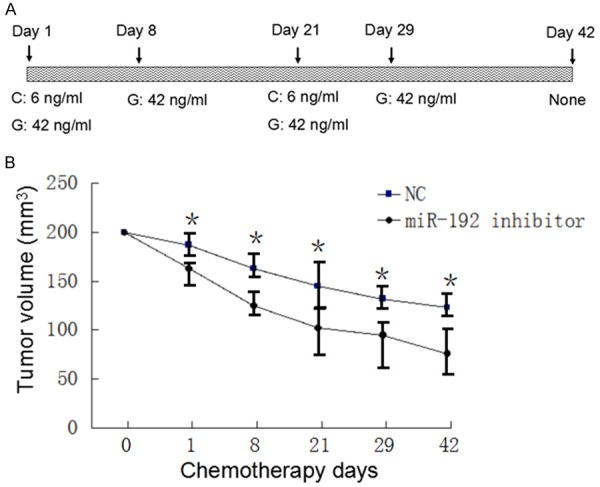
MiR-192 inhibitor treated tumor exhibits sensitivity to ciaplatin and gemcitabine therapy. A. Demonstrates timetable and drug doses of Gemcitabine and cisplatin combined chemotherapy. B. Shows tumor sizes shrank during chemotherapy. MiR-192 treated human lung cancer tumor decreased significantly comparing with negative control group (P < 0.05).
Cell culture
The human lung adenocarcinoma cell line A549 was purchased from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). Normal human bronchial epithelial cells (NHBE) (CloneticsTM) were maintained in a culture medium according to the protocol provided by CloneticsTM. A549 cell line was cultured in DMEM containing 10% fetal bovine serum (Gibco, Invitrogen, Carlsbad, CA, USA), 100 units/ml penicillin, and 100 mg/ml streptomycin at 37°C in a 5% CO2 humidified incubator.
Drugs and reagents
Cisplatin was purchased from QiLu Pharmaceutical (Jinan, China). Gemcitabine was purchased from HaoSeng Pharmaceutical (Jiangsu, China). MiR-192 inhibitor, Bcl-2 siRNA, and their negative control oligonucleotides were obtained from Invitrogen (Carlsbad, CA, USA). These were used to transfect A549 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the instructions provided by the manufacture.
Western blot
The proteins were loaded onto a 4% SDS denaturing polyacrylamide stacking gel, and separated on a 10% SDS denaturing polyacrylamide running gel, then transferred onto a nitrocellulose membrane. Antibody to Bcl-2 and GAPDH was incubated overnight at 4°C (Abcam, Cambridge, MA, USA). The membrane was washed and incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (Abcam, Cambridge, MA, USA). Protein expression was assessed by enhanced chemiluminescence and exposure to chemiluminescent film.
Quantitative RT-PCR
RNAs were collected from 1 mm3 cancer tissue. Total RNAs were extracted using RNeasy columns (Qiagen) according to the manufacture instructions. Total RNA (1 g) was reverse transcribed into cDNA (Clontech). SYBR green real-time PCR kit (Applied Biosystems) was used to quantify gene expression levels. Data were analyzed with SDS Relative Quantification Software version 2.2.2 (Applied Biosystems). Ct values were exported into Excel software for data analysis. miR192 forward primer is: GTGGACCTGACCTGCCGTCT; miR192 reverse primer is: GGAGGAGTGGGTGTCGCTGT. GAPDH forward primer is: AAGGGAAGGTTGCTGGATAGG; GAPDH reverse primer: CACATCCACCTCCTCCACATC.
MTT assay
Cells transfected with miR-192 inhibitor or siRNA-Bcl2 were seeded into 96-well plates at 6000 cells per well. After growing overnight, cells were treated with different concentrations of cisplatin and gemcitabine. After 24 hours of treatment, 20 ml of 5 mg/ml MTT reagent (Sigma-Aldrich, St. Louis, MO, USA) was added and incubated in the dark for 4 hours. The absorbance of the plate was measured in a microplate reader at a wavelength of a 570-nm reference. Each treatment was carried out in triplicate.
Statistical analysis
The data from the experiments at different time points for the different treatment groups were analysed for statistical significance with the SPSS 13.0 statistical software, analysis among different groups used One-way ANOVA, T test was used to compare two different groups. P < 0.05 was considered as significance.
Results
MiR-192 expression level in A549 cells is significantly higher than in NHBE cells
MiR-192 expression levels in A549 and normal human bronchial epithelial cells (NHBE) were compared by qRT-PCR. The expression levels of miR-192 in A549 cells was significantly higher than in NHBE cells (P < 0.01, Figure 1). This result suggests that miR-192 may have a role in human lung cancer tumorigenesis.
Figure 1.
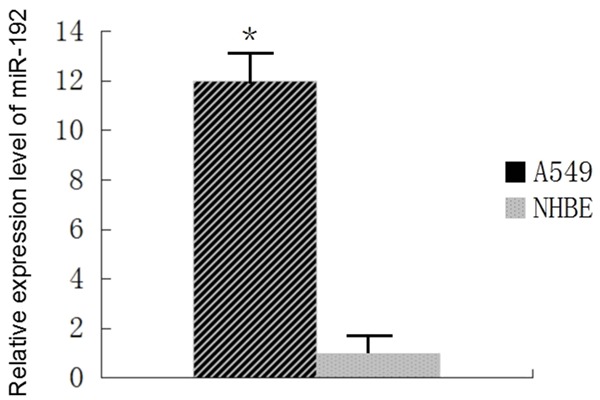
MiR-192 is up-regulated in A549 cells comparing with NHBE. MiR-192 expression levels in A549 and normal human bronchial epithelial cells (NHBE) were compared by Qrt-PCR. The expression levels of miR-192 in A549 cells was significantly higher than in NHBE cells (P < 0.01).
MiR-192 inhibitor suppress miR-192 expression levels in A549 cells
We transfected miR-192 inhibitor and its negative control oligonucleotides into A549 cells. Transfection with miR192 inhibitor suppressed miR-192 expression level compared with the control group (Figure 2). This result suggests that miR-192 inhibitor works properly to suppress miR-192 expression in A549 cells.
Figure 2.
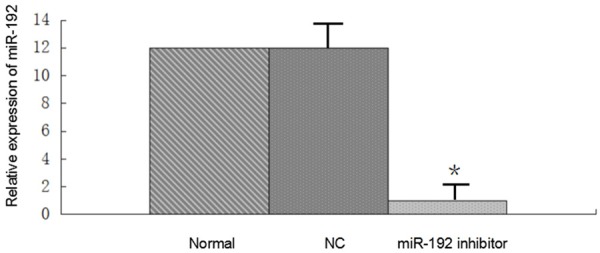
MiR-192 inhibitor suppress miR-192 expression levels in A549 cells. MiR-192 inhibitor and its negative control oligonucleotides (NC) into A549 cells. Transfection with miR192 inhibitor suppressed miR-192 expression level compared with the negative control group (P < 0.01). This result suggests that miR-192 inhibitor works properly to suppress miR-192 expression in A549 cells.
MiR-192 is not involved in human lung adenocarcinoma tumorigenesis
A549 cells without any treatment, A549 cells with miR-192 inhibitor treatment, and with miR-192 inhibitor negative control treatment cells were subcutaneously injected in the right flank of 5 weeks old Balb/c female mice. Tumor growth was monitored by measuring the tumor size twice a week for 3 weeks after treatment. The tumor volume was calculated with the formula (long dimension) × (short dimension)2/2. Figure 3A and 3B show the tumor size around 200 mm3. Sizes and volumes of tumors did not show significant difference with or without miR-192 inhibitor treatment (P > 0.05, Figure 3C). This result suggests that miR-192 is not involved in human lung adenocarcinoma tumorigenesis.
Figure 3.
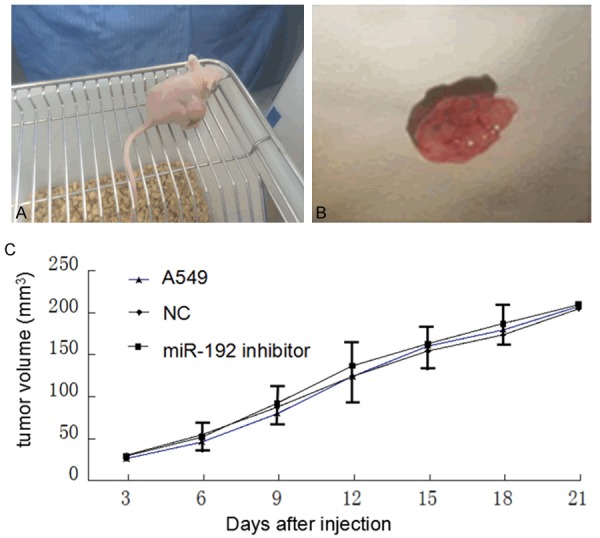
MiR-192 is not involved in human lung adenocarcinoma tumorigenesis. A. Shows that Balb/c mice carried human lung tumor; B. Shows the tumor tissue after dissected from its host; C. Shows that tumor growth rates did not show significant difference between miR-192 inhibitor treated and not treated groups (P > 0.05). NC stands for negative control of miR-192 inhibitor.
MiR-192 inhibitor treated tumor exhibits sensitivity to cisplatin and gemcitabine therapy
The mice carried miR-192 inhibitor treated tumor or miR-192 inhibitor negative control nucleotide treated tumor were treated with gemcitabine and cisplatin combined chemotherapy. Cisplatin concentration range was 6 ng/ml. Gemcitabine concentration was 42 ng/ml. Detailed chemotherapy timetable was shown in Figure 4A. Tumor sizes were measured every week. The results showed that miR-192 inhibitor treated tumor was more sensitive to chemotherapy comparing with negative control group (P < 0.05, Figure 4B).
Bcl-2 mRNA and protein expression levels up-regulated in miR-192 inhibitor treated tumor
Total RNAs were extracted from miR-192 inhibitor treated tumor and miR-192 inhibitor negative control treated tumor tissues. Bcl-2 mRNA level was over-expressed in miR-192-suppressed tumor compared with controls (Figure 5A). During chemotherapy, Bcl-2 mRNA levels were over-expressed compared with controls (data not shown). Bcl-2 protein expression level was up-regulated compared with controls (Figure 5B). These suggest that Bcl-2 is a target of miR-192.
Figure 5.
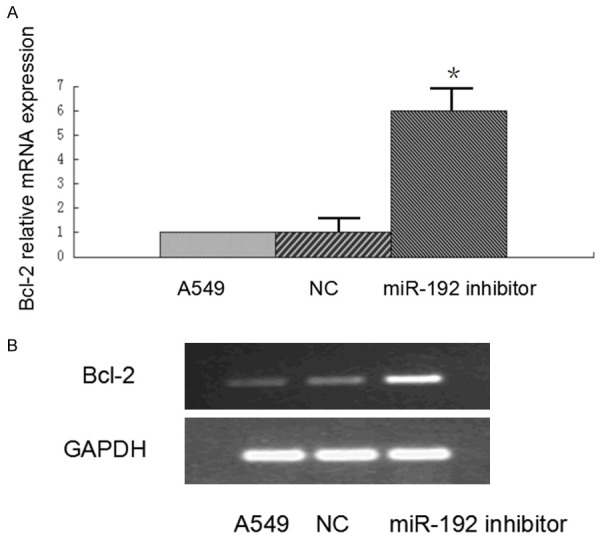
Bcl-2 mRNA and protein expression levels up-regulated in miR-192 inhibitor treated tumor. Total RNAs were extracted from miR-192 inhibitor treated tumor and miR-192 inhibitor negative control treated tumor tissues. Bcl-2 mRNA level was over-expressed in miR-192-suppressed tumor compared with controls (P < 0.01, A). Bcl-2 protein expression level was up-regulated compared with controls (B).
Bcl-2 is a key regulator for miR-192 related chemotherapy resistance
MTT assay was used to test whether Bcl-2 is responsible for miR-192 related chemotherapy resistance. A549 cells were transfected with Bcl-2 siRNA, miR-192 inhibitor or both. MTT assay was performed, cells were treated with gemcitabine combined with cisplatin overnight. The lowest cell viability was from miR-192 inhibitor transfected cells, the highest cell viability was from Bcl-2 siRNA transfected cells. Co-transfected cells viability was slightly lower than Bcl-2 siRNA transfected cells (Figure 6). These results suggest that Bcl-2 is a key regulator for miR-192 related resistance for gemcitabine and cisplatin combined chemotherapy.
Figure 6.
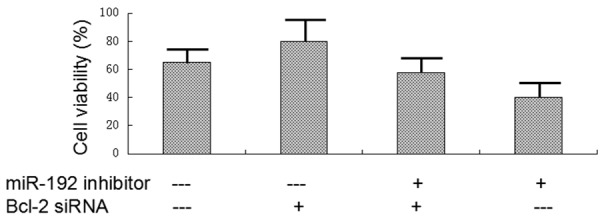
Bcl-2 is a key regulator for miR-192 related chemotherapy resistance. A549 cells were transfected with Bcl-2 siRNA, miR-192 inhibitor or both. MTT assay was performed, cells were treated with gemcitabine combined with cisplatin overnight. The lowest cell viability was from miR-192 inhibitor transfected cells, the highest cell viability was from Bcl-2 siRNA transfected cells. Co-transfected cells viability was slightly lower than Bcl-2 siRNA transfected cells. These result suggest that Bcl-2 is a key regulator for miR-192 related resistance for gemcitabine and cisplatin combined chemotherapy.
Discussion
The efficacy of chemotherapeutic agents is often limited by chemoresistance in the treatment of NSCLC. Gemcitabine is a nucleoside analog in which the hydrogen atoms on the 2’ carbon of deoxycytidine are replaced by fluorine atoms [25]. The triphosphate analogue of gemcitabine replaces one of the building blocks of cytidine during DNA replication. The process arrests tumor growth, resulting in apoptosis. Cisplatin is a platinum-based compound that forms intra- and inter-strand addicts with DNA [26,27]. Gemcitabine combined with cisplatin is widely used in the treatment of NSCLC despite of development of drug resistance. The molecular mechanisms leading to chemoresistance for gemcitabine plus cisplatin therapy are widely unknown.
MiRNAs are considered as oncogene or tumor suppressors [28-30]. Different miRNA has variant effects during cancer development. MiR-192 was considered as tumor suppressor in human chondrosarcoma [31], and involved in tumor blood vessel development, therefore it worked as oncogene [32]. The Bcl-2 family proteins play an important role in apoptosis. Our study demonstrated that miR-192 regulates chemoresistance to gemcitabine and cisplatin in A549 cells, and Bcl-2 is the target of miR-192. However, miR-192 is not involved in NSCLC tumorogeneis in this mouse human disease model. Whether miR-192 could be considered as chemosensitivity biomarker needs further investigations.
Previous studies demonstrated that deregulation of miRNAs such as miR-21, miR-503, miR-181a and miR-620 is related to drug resistance [33-35]. Many miRNAs and oncogene target pathways, such as miR7/Bcl2, miR-99b/FGF3, and miR196/HOXA5 have been demonstrated to participate in the tumorigenesis of lung cancer [36-38]. Whether miR-192 has function through other pathways other than Bcl-2 pathways need to be investigated. It is reasonable expectation that chemoresistance is related with apoptosis, which had never been reported before. We need to study chemoresistance of other lung cancer types besides NSCLC to further study this possible linkage.
Acknowledgements
This study was supported by the Shanghai Natural Science Foundation (No. 12ZR1428900) and Shanghai Health and Family Planning Commission Research fund (No. 201540158).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Verdecchia A, Francisci S, Brenner H, Gatta G, Micheli A, Mangone L, Kunkler I EUROCARE-4 Working Group. Recent cancer survival in Europe: a 2000-02 period analysis of EURO-CARE-4 data. Lancet Oncol. 2007;8:784–796. doi: 10.1016/S1470-2045(07)70246-2. [DOI] [PubMed] [Google Scholar]
- 3.Szakacs G, Materson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 4.Zheng S, Du Y, Chu H, Chen X, Li P, Wang Y, Ma Y, Wang H, Zang W, Zhang G, Zhao G. Analysis of MAT3 gene expression in NSCLC. Diagn Pathol. 2013;8:166. doi: 10.1186/1746-1596-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu Q, Lu S, Huang L, Wang T, Wan Y, Zhou CX, Zhang C, Zhang Z, Li X. The expression of V-ATPase is associated with drug resistance and pathology of non-small-cell lung cancer. Diagn Pathol. 2013;8:145. doi: 10.1186/1746-1596-8-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shenouda SK, Alahari SK. MicroRNA function in cancer: Oncogene or a tumor suppressor? Cancer Metastasis Rev. 2009;28:369–378. doi: 10.1007/s10555-009-9188-5. [DOI] [PubMed] [Google Scholar]
- 7.Wang Q, Wang S, Wang H, Li P, Ma Z. MicroRNAs: Novel biomarkers for lung cancer diagnosis, prediction and treatment. Exp Biol Med (Maywood) 2012;237:227–235. doi: 10.1258/ebm.2011.011192. [DOI] [PubMed] [Google Scholar]
- 8.Mallick R, Patnaik Sk, Yendamuri S. MicroRNAs and lung cancer: biology and applications in diagnosis and prognosis. J Carcinog. 2010;9:8. doi: 10.4103/1477-3163.67074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu W, Xu H, Zhu D, Zhi H, Wang T, Wang J, Jiang B, Shu Y, Liu P. miR-200bc/429 cluster modulates multidrug resistance of human cancer cell lines by targeting BCL2 and XIAP. Cancer Chemother Pharmacol. 2012;69:723–731. doi: 10.1007/s00280-011-1752-3. [DOI] [PubMed] [Google Scholar]
- 10.Hummel R, Hussey DJ, Haier J. MicroRNAs: predictors and modifiers of chemo- and radiotherapy in different tumour types. Eur J Cancer. 2010;46:298–311. doi: 10.1016/j.ejca.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 11.Shenouda SK, Alahari SK. MicroRNA function in cancer: oncogene or a tumor suppressor? Cancer Metastasis Rev. 2009;28:369–378. doi: 10.1007/s10555-009-9188-5. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Li M, Zhang G, Pang Z. MicroRNA-10b overexpression promotes non-small-cell lung cancer cell proliferation and invasion. Eur J Med Res. 2013;18:41. doi: 10.1186/2047-783X-18-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang N, Wei X, Xu L. miR-150 promotes the proliferation of lung cancer cells by targeting P53. FEBS Lett. 2013;587:2346–2351. doi: 10.1016/j.febslet.2013.05.059. [DOI] [PubMed] [Google Scholar]
- 14.Lei L, Huang Y, Gong W. miR-205 promotes the growth, metastasis and chemoresistance of NSCLC cells by targeting PTEN. Oncol Rep. 2013;30:2897–2902. doi: 10.3892/or.2013.2755. [DOI] [PubMed] [Google Scholar]
- 15.Ke Y, Zhao W, Xiong J, Cao R. Downregulation of miR-16 promotes growth and motility by targeting HDGF in non-small-cell lung cancer cells. FEBS Lett. 2013;587:3153–3157. doi: 10.1016/j.febslet.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Nian W, Ao X, Wu Y, Huang Y, Shao J, Wang Y, Chen Z, Chen F, Wang D. miR-223 functions as a potent tumor suppressor of the LEwis lung carcinoma cell line by targeting insulin-like growth factor-1 receptor and cyclin-dependent kianse 2. Oncol Lett. 2013;6:359–366. doi: 10.3892/ol.2013.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan Y, Shen Y, Xue L, Fan H. miR-140 suppresses tumor growth and metastasis of non-small-cell lung cancer by targeting insulin-like growth factor 1 receptor. PLoS One. 2013;8:e73604. doi: 10.1371/journal.pone.0073604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Y, Fan Y. MiR-215/192 participates in gastric cancer progression. Clin Transl Oncol. 2015;17:34–40. doi: 10.1007/s12094-014-1194-6. [DOI] [PubMed] [Google Scholar]
- 19.Chen Q, Ge X, Zhang Y, Xia H, Yuan D, Tang Q, Chen L, Pang X, Leng W, Bi F. Plasma miR-122 and miR-192 as potential novel biomarkers for the early detection of distant metastasis of gastric cancer. Oncol Rep. 2014;31:1863–1870. doi: 10.3892/or.2014.3004. [DOI] [PubMed] [Google Scholar]
- 20.Gross A, McDonnell JM, Korsmeyer SJ. Bcl-2 family members and the mitochondrial in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 21.Adams JM, Cory S. The Bcl-2 switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amundson SA, Myers TG, Scudiero D, Kitada S, Reed JC, Fornace AJ Jr. An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 2000;60:6101–6110. [PubMed] [Google Scholar]
- 23.Schmidt LH, Gorlich D, Spieker T, Rohde C, Schuler M, Mohr M, Humberg J, Sauer T, Thoenissen NH, Huge A, Voss R, Marra A, Faldum A, Müller-Tidow C, Berdel WE, Wiewrodt R. Prognostic impact of Bcl-2 depends on tumor histology and expression of MALAT-1 IncRNA in Non-Small-Cell Lung Cancer. J Thorac Oncol. 2014;9:1294–1304. doi: 10.1097/JTO.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 24.Xu LF, Wu ZP, Chen Y, Zhu QS, Hamidi S, Navab R. MicroRNA-21 (miR-21) regulates cellular proliferation, invasion, migration, and apoptosis by targeting PTEN, RECK and Bcl-2 in lung squamous carcinoma, Gejiu City, China. PLoS One. 2014;9:e103698. doi: 10.1371/journal.pone.0103698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cerqueira NM, Fernandes PA, Ramos MJ. Understanding ribonucleotie reductase inactivation by gemcitabine. Chemistry. 2007;13:8507–8515. doi: 10.1002/chem.200700260. [DOI] [PubMed] [Google Scholar]
- 26.Judson I, Kelland LR. New developments and approaches in the platinum arena. Drugs. 2000;59(Suppl 4):29–36. doi: 10.2165/00003495-200059004-00004. [DOI] [PubMed] [Google Scholar]
- 27.Reed JC. Mechanisms of apoptosis avoidance in cancer. Curr Opin Oncol. 1999;11:68–75. doi: 10.1097/00001622-199901000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Meng F, Glaser SS, Francis H, DeMorrow S, Han Y, Passarini JD, Stokes A, Cleary JP, Liu X, Venter J, Kumar P, Priester S, Hubble L, Staloch D, Sharma J, Liu CG, Alpini G. Functional analysis of microRNAs in human hepatocellular cancer stem cells. J Cell Mol Med. 2012;16:160–173. doi: 10.1111/j.1582-4934.2011.01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farazi TA, Spitzer JI, Morozov P, Tuschi T. MiRNAs in human cancer. J Pathol. 2011;223:102–115. doi: 10.1002/path.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah AA, Leidinger P, Blin N, Meese E. miRNA: small molecules as potential novel biomarkeres in cancer. Curr Med Chem. 2010;17:4427–4432. doi: 10.2174/092986710794182980. [DOI] [PubMed] [Google Scholar]
- 31.Galoian KA, Guettouche T, Issac B, Qureshi A, Temple HT. Regulation of onco and tumor suppressor miRNAs by mTORC1 inhibitor PRP-1 in human chondrosarcoma. Tumour Biol. 2014;35:2335–2341. doi: 10.1007/s13277-013-1309-7. [DOI] [PubMed] [Google Scholar]
- 32.Hong JP, Li XM, Li MX, Zheng FL. VEGF suppresses epithelial-mesenchymal transition by inhibiting the expression of Smad3 and miR-192, a Smad3-dependent microRNA. Int J Mol Med. 2013;31:1436–1442. doi: 10.3892/ijmm.2013.1337. [DOI] [PubMed] [Google Scholar]
- 33.Qiu T, Zhou L, Wang T, Xu J, Wang J, Chen W, Zhou X, Huang Z, Zhu W, Shu Y, Liu P. miR-503 regulates the resistance of non-small cell lung cancer cells to cisplatin by targeting Bcl-2. Int J Mol Med. 2013;32:593–598. doi: 10.3892/ijmm.2013.1439. [DOI] [PubMed] [Google Scholar]
- 34.Gao W, Lu X, Liu L, Xu J, Feng D, Shu Y. MiRNA-21: a biomarker predictive for platinum-based adjuvant chemotherapy response in patients with non-small cell lung cancer. Cancer Biol Ther. 2012;13:330–340. doi: 10.4161/cbt.19073. [DOI] [PubMed] [Google Scholar]
- 35.Galluzzi L, Morselli E, Vitale I, Kepp O, Senovilla L, Criollo A, Servant N, Paccard C, Hupé P, Robert T, Ripoche H, Lazar V, Harel-Bellan A, Dessen P, Barillot E, Kroemer G. miR-181a and miR-630 regulate cisplatin-induced cancer cell death. Cancer Res. 2010;70:1793–1803. doi: 10.1158/0008-5472.CAN-09-3112. [DOI] [PubMed] [Google Scholar]
- 36.Kang J, Lee SY, Lee SY, Kim YJ, Park JY, Kwon SJ, Na MJ, Lee EJ, Jeon HS, Son JW. microRNA-99b acts as a tumor suppressor in non-small cell lung cancer by directly targeting fibroblast growth factor 3. Exp Ther Med. 2012;3:149–153. doi: 10.3892/etm.2011.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu XH, Lu KH, Wang KM, Sun M, Zhang EB, Yang JS, Yin DD, Liu ZL, Zhou J, Liu ZJ, De W, Wang ZX. MicroRNA-196a promotes non-small cell lung cancer cell proliferation and invasion through targeting HOXA5. BMC Cancer. 2012;12:348. doi: 10.1186/1471-2407-12-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiong S, Zheng Y, Jiang P, Liu R, Liu X, Chu Y. MicroRNA-7 inhibits the growth of human non-small cell lung cancer A549 cells through targeting BCL-2. Int J Biol Sci. 2011;7:805–814. doi: 10.7150/ijbs.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]


