Abstract
Purpose: There is no consensus regarding the clinical course and prognosis for oral squamous cell carcinoma (SCC) in young patients. In this study, we report our experience with the management of oral squamous cell carcinoma, with a special focus on patients younger than 40 years old. Methods: From 2005 to 2012, all patients were treated for oral (gingiva, the anterior 2/3 of tongue, floor of the mouth and buccal) SCC in our medical center were enrolled. Results: We enrolled 430 patients, of whom 31 (7.2%) patients (19 male and 12 female) were younger than 40 years. Among the patients under 40, tumors were most commonly found on the tongue (15; 48.4%); there was recurrence in 10 (52.6%) male patients, and six (31.6%) male patients died of disease; and there was recurrence in seven (58.3%) female patients, while three (25.0%) female patients died of disease. In young patients with tongue carcinoma, five (33.3%) died of the disease and four (25%) young patients with carcinoma at other sites died of the disease. The older (>40 years old) patient group had similar rates of recurrence-free survival and disease-specific survival to the young (<40 years old) patient group. Conclusion: In the young group, patients with tongue carcinoma tended to have a poorer prognosis than patients with carcinoma at other sites. The clinical course and prognosis were similar between the two groups, but younger patients were more likely to have a recurrence.
Keywords: Squamous cell carcinoma, young patients, oral cavity, prognosis
Introduction
Oral squamous cell carcinoma (SCC) mostly occurs after the forth decade of life. Previous studies have reported that the incidence of oral SCC in young patients ranged from 0.4% to 3.9% [1,2], but we noted a higher incidence in this study. There is a considerable controversy about the clinical course and prognosis of young patients with oral SCC: some reports [3,4] state that young patients have better clinical results than older patients and other reports [5] noted a significant decrease in survival rate in the younger group, but more recent studies [6-9] show a similar prognosis between the younger and older groups. To date, no study has focused on the prognostic differences between tongue carcinoma and carcinoma at other sites in young patients.
In this study, we assess the data obtained from 430 of our patients with oral SCC. We focus on patients younger than 40 years old and compare this group to patients older than 40 years.
Materials and methods
The China Medical University institutional research committee approved our study and all participants signed an informed consent agreement.
From 2005 to 2012, 486 patients were treated for oral (gingiva, the anterior 2/3 of tongue, floor of the mouth and buccal) SCC at Department of Plastic Surgery, China Medical University, and Department of Head Neck, Henan Tumor Hospital. Due to lack of follow-up, 56 patients were excluded from the study. The medical records for the remaining 430 patients were reviewed and information on demographic data, tobacco and alcohol consumption, tumor stage, node stage, differentiation, treatment, recurrence and survival was collected. These patients were split into two groups: one group of patients who were younger than 40 years and another group who were older than 40 years.
The chi-squared test was used to evaluate the significance of the variables, the Kaplan-Meier method was used to analyze the recurrence-free survival (RFS) rate and the disease-specific survival (DSS) rate. Statistical analysis was conducted using SPSS 13.0. A P<0.05 was considered significant.
Results
The study consisted of 296 males and 134 females and the mean age was 58.7 (range: 22-94) years. In the younger group, there were 31 patients (19 male and 12 female): 14 (45.2%) patients had a history of tobacco use and eight (25.8%) patients had a history of alcohol consumption. Six (19.4%) tumors were staged as T1, 16 (51.6%) tumors were staged as T2 and 9 (29.0%) tumors were staged as T4. Most (45.2%) of the tumors were well differentiated. The most common site for tumors was the tongue, followed by the gingiva and the floor of the mouth (Table 1). The distribution of tumor stage, location, differentiation and node stage in male patients were comparable to those in female patients (all P>0.05; Table 2).
Table 1.
Characteristics of patients
| Young group (n=31) | Old group (n=399)a | P-valueb | |
|---|---|---|---|
| Sex | |||
| Male | 19 (61.3) | 277 (69.4) | 0.346 |
| Female | 12 (38.7) | 122 (30.6) | |
| Habits | |||
| Tobacco | 14 (45.2) | 252 (63.2) | 0.047 |
| Alcohol | 8 (25.8) | 168 (42.1) | 0.075 |
| Tumor stage | |||
| T1 | 6 (19.4) | 52 (13.0) | 0.097 |
| T2 | 16 (51.6) | 182 (45.6) | |
| T3 | 0 | 52 (13.0) | |
| T4 | 9 (29.0) | 113 (28.3) | |
| Node stage | |||
| N0 | 28 (90.3) | 295(73.9) | 0.043 |
| N1 | 1 (3.2) | 77 (19.3) | |
| N2 | 2 (6.5) | 26 (6.5) | |
| Tumor differentiation | |||
| Well | 14 (45.2) | 204 (51.1) | 0.711 |
| Moderately | 8 (25.8) | 104 (26.1) | |
| Poorly | 9 (29.0) | 91 (22.8) | |
| Tumor location | |||
| Gingiva | 8 (25.8) | 83 (20.8) | 0.206 |
| Tongue | 15(48.4) | 161 (40.4) | |
| Floor of the mouth | 7 (22.6) | 89 (22.3) | |
| Buccal | 1 (3.2) | 66 (16.5) | |
| Treatment | |||
| Surgery | 25 | 305 | 0.505 |
| Surgery+RA | 6 | 70 | |
| Surgery+RA+CH | 0 | 24 |
node stage of 1 patient was unknown;
Chi-square test;
Data are number (%) of patients; RA: radiation therapy; CH: chemotherapy.
Table 2.
Information of young patients based on sex
| Male (n=19) | Female (n=12) | P-valuea | |
|---|---|---|---|
| Tumor stage | |||
| T1 | 5 (26.3) | 1 (8.3) | 0.617 |
| T2 | 9 (47.4) | 7 (58.3) | |
| T3 | 0 | 0 | |
| T4 | 5 (26.3) | 4 (33.3) | |
| Tumor location | |||
| Gingiva | 6 (31.6) | 2 (16.7) | 0.111 |
| Tongue | 6 (31.6) | 9 (75.0) | |
| Floor of the mouth | 6 (31.6) | 1 (8.3) | |
| Buccal | 1 (5.3) | 0 | |
| Tumor differentiation | |||
| Well | 8 (42.1) | 6 (50.0) | 1.000 |
| Moderately | 5 (26.3) | 3 (25.0) | |
| Poorly | 6 (31.6) | 3 (25.0) | |
| Node stage | |||
| N0 | 18 (94.7) | 10 (83.3) | 0.480 |
| N1 | 0 | 1 (8.3) | |
| N2 | 1 (5.3) | 1 (8.3) |
Chi-square test;
Data are number (%) of patients.
In the older group, there were 277 males and 122 females: 252 (63.2%) patients had a history of tobacco use and 168 (42.1%) patients had a history of alcohol consumption. A total of 52 (13.0%) tumors were staged as T1, 182 (45.6%) tumors were staged as T2, 52 (13.0%) tumors were staged as T3 and 113 (28.3) tumors were staged as T4. Most (51.1%) tumors were well differentiated. The most common site for tumors was the tongue, followed by the floor of the mouth and the gingiva (see Table 1).
Figure 1 shows the RFS rates for both the young and old groups; there was no significant difference between the groups (P=0.296). In the younger group, 17 patients had locoregional recurrences and no distant metastasis occurred; in the older group, 164 patients had locoregional recurrences and 22 patients had distant metastasis. Figure 2 shows the DSS rates for the two groups and there was no significant difference (p=0.708). In the younger group, nine patients died of disease; in the older group, 114 patients died of disease.
Figure 1.
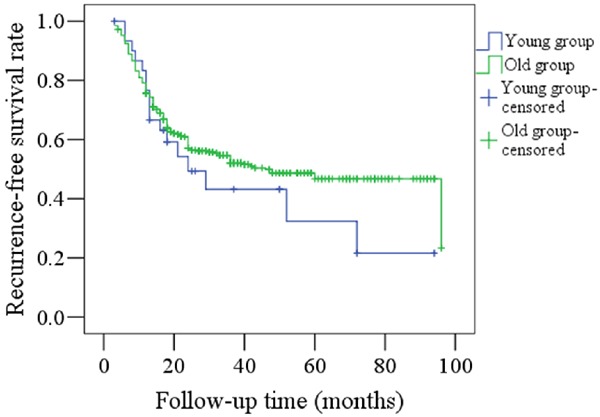
Comparison of RFS rates between young and old patients (P=0.296).
Figure 2.
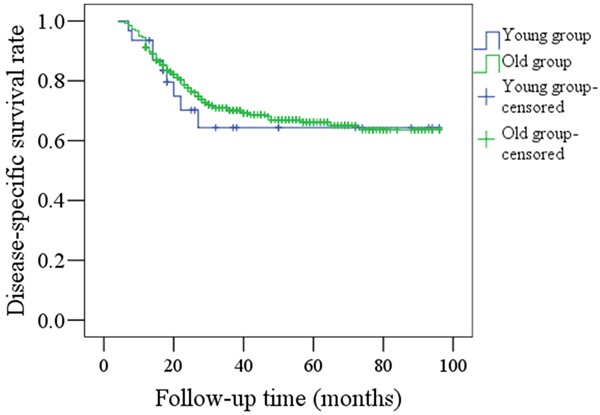
Comparison of DSS rates between young and old patients (P=0.708).
Figure 3 compares RFS rates between the tumor sites (the tongue vs other sites) in the younger group and again, the difference was not significant (P=0.667). Ten patients who had experienced tongue SCC had recurrences, whereas there were only seven recurrences in those patients who had experienced SCC at other sites.
Figure 3.
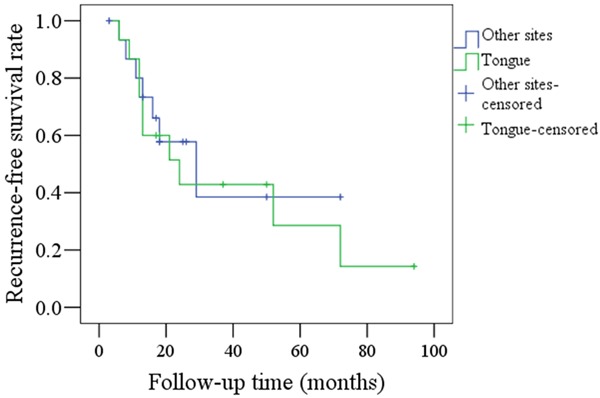
Comparison of RFS rates between tongue carcinoma and SCC at other sites in young patients (P=0.667).
DSS rates for tongue tumors and other sites in the younger group are shown in Figure 4. There was no significant difference between the sites (P=0.875). Five patients with tongue SCC died of disease and four patients with SCC at other sites died of disease.
Figure 4.
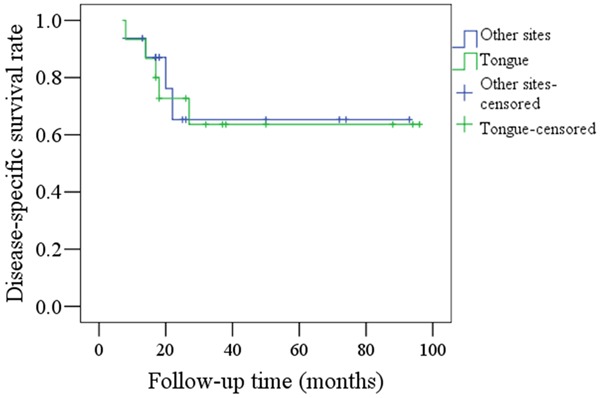
Comparison of DSS rates between tongue carcinoma and SCC at other sites in young patients (P=0.875).
There was no significant difference (P=0.149) in RFS rates between male and female patients in the younger group, as shown in Figure 5. A total of 10 male patients and seven female patients had recurrences.
Figure 5.
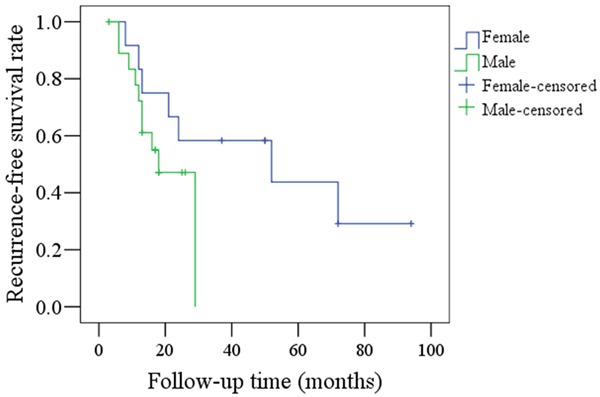
Comparison of RFS rates between female and male patients in the young group (P=0.149).
There was no significant difference (P=0.254) in DSS rates between male and female patients in the younger group, as shown in Figure 6. Six male patients and three female patients died of disease.
Figure 6.
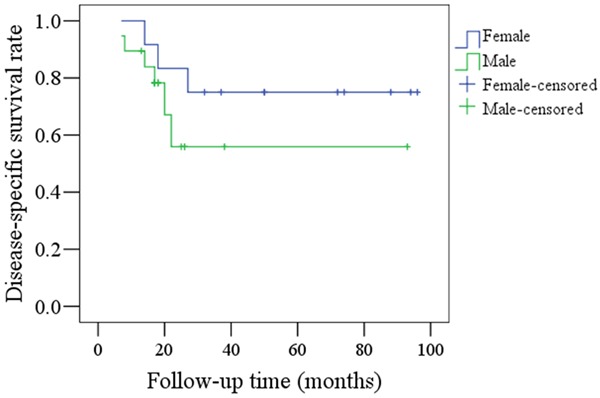
Comparison of DSS rates between female and male patients in the young group (P=0.254).
Discussion
Traditionally, oral SCC develops in older patients after decades of alcohol and tobacco abuse, but recent studies have shown an increased incidence, ranging from 1.3% to 18.2% [3-5,7,9,10]. In this study, we found that patients younger than 40 years old accounted for 7.2% of the total sample.
Previous articles have reported a predominance of oral SCC in young male patients [4,7-9,10-12]; similarly, we found a higher incidence of oral SCC in males from the younger group, with a ratio of 1.5 (males):1 (females). In contrast, Kuriakose et al. [5] reported a slight predominance of female oral SCC patients in the younger age group. These variations may be due to different geographic locations and social lifestyles between populations. Furthermore, no significant differences in RFS and DSS rates were observed between male and female patients in the younger group, though we did note poorer prognoses for young male patients (Figures 5, 6). Martin-Granizo et al. [9] reported that young male patients had a similar prognosis to female patients, but Vargas et al. [13] found that young women with tongue SCC had significantly higher rates of recurrent disease.
As reported by previous studies [8,10], we found that the tongue was the most common location for oral SCC in young adults, followed by the gingiva and the floor of the mouth. Only one patient had buccal mucosa carcinoma, despite a previous report [14] stating that this as the most common location in young patients; the habit of betel quid chewing, which is associated with buccal SCC, may account for this finding. In addition, although the difference was not significant, the tongue accounted for 75.0% of the tumor locations in young female patients compared to 31.6% in young male patients, suggesting that tongue SCC may be more common in young women. Moreover, in the young group, 10 (66.7%) of the 15 patients with tongue SCC developed locoregional recurrences compared to 43.8% of patients with tumors at other sites. Although this finding was not significant, it suggests that tongue SCC patients tend to have a poorer prognosis; the 5-year DSS rate in patients with tongue SCC was 63%, compared to 70% for patients with tumors at other sites.
Oral SCC is strongly linked to a history of tobacco and alcohol use. However, we found that the younger group had a higher percentage of nonsmokers than the older group, suggesting that the etiology and pathogenesis of oral SCC may be different in young patients. Previous studies have shown that oral SCC in the younger group may be associated with the expression of epidermal growth factor receptor, exposure to human papilloma virus and some other viral infections [4,15,16].
We found that T1 and T2 tumors were the most common stages for both groups which is consistent with other studies [4,8]. The majority of tumors were well or moderately differentiated and the differences between the groups were not significant. However, we found that the incidence of node metastasis was relatively low in both groups and it was even less frequent in young patients. This is in contrast to that reported by Kuriakose et al. [5], who reported that the incidence of nodal metastasis in T3 tumors was significantly higher in the younger group than in the older group.
At present, there is no consensus regarding the clinical course and prognosis for oral squamous cell carcinoma in young patients. Udeabor et al. [4] and Ho et al. [3] concluded that young adults may have a better prognosis, but Kuriakose et al. [5] believed that oral SCC in young patients behaved more aggressively and the authors suggested more radical treatments were necessary to control the disease. However, recent studies have shown that there is no significant difference in DSS in young patients with oral SCC compared to older patients [6-9]. In this study, we report that the prognosis of young patients strongly resembles that of older patients. The 5-year RFS rates in the young and old groups were 34% and 45%, respectively, but younger patients were more likely to suffer a recurrence; the 5-year DSS rates in the young and old groups were 66% and 64%, respectively.
In conclusion, the incidence of oral SCC in young patients was low and traditional risk factors such as tobacco were less common in the younger group. The incidence of node metastasis was relatively low in both groups and it occurred less frequently in young patients. In the younger group, patients with tongue SCC seemed to have a poorer prognosis than patients with SCC at other sites. The clinical course and prognosis were similar between the two groups, but younger patients were more likely to suffer from a recurrence.
Disclosure of conflict of interest
None.
References
- 1.Cusumano RJ, Perskey MS. Squamous cell carcinoma of the oral cavity and oropharynx in young adults. Head Neck Surg. 1988;10:229–234. doi: 10.1002/j.1930-2398.1988.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 2.Lingen M, Sturgis EM, Kies MS. Squamous cell carcinoma of the head and neck in nonsmokers: clinical and biologic characteristics and implications for management. Curr Opin Oncol. 2001;13:176–182. doi: 10.1097/00001622-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Ho HC, Lee MS, Hsiao SH, Hwang JH, Hung SK, Chou P, Lee CC. Squamous cell carcinoma of the oral cavity in young patients: a matchedpair analysis. Eur Arch Otorhinolaryngol. 2008;265:S57–S61. doi: 10.1007/s00405-007-0496-5. [DOI] [PubMed] [Google Scholar]
- 4.Udeabor SE, Rana M, Wegener G, Gellrich NC, Eckardt AM. Squamous cell carcinoma of the oral cavity and the oropharynx in patients less than 40 years of age: a 20-year analysis. Head Neck Oncol. 2012;4:28. doi: 10.1186/1758-3284-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuriakose M, Sankaranarayanan M, Nair MK, Cherian T, Sugar AW, Scully C, Prime SS. Comparison of oral squamous cell carcinoma in younger and older patients in India. Eur J Cancer B Oral Oncol. 1992;28B:113–120. doi: 10.1016/0964-1955(92)90038-3. [DOI] [PubMed] [Google Scholar]
- 6.van Monsjou HS, Lopez-Yurda MI, Hauptmann M, van den Brekel MW, Balm AJ, Wreesmann VB. Oral and oropharyngeal squamous cell carcinoma in young patients: the Netherlands Cancer Institute experience. Head Neck. 2013;35:94–102. doi: 10.1002/hed.22935. [DOI] [PubMed] [Google Scholar]
- 7.Ribeiro AC, Silva AR, Simonato LE, Salzedas LM, Sundefeld ML, Soubhia AM. Clinical and histopathological analysis of oral squamous cell carcinoma in young people: a descriptive study in Brazilians. Br J Oral Maxillofac Surg. 2009;47:95–98. doi: 10.1016/j.bjoms.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Sasaki T, Moles DR, Imai Y, Speight PM. Clinical-pathological features of squamous cell carcinoma of the oral cavity in patients <40 years of age. J Oral Pathol Med. 2005;34:129–133. doi: 10.1111/j.1600-0714.2004.00291.x. [DOI] [PubMed] [Google Scholar]
- 9.Martin-Granizo R, Rodriguez-Campo F, Gonzalez FJ. Squamous cell carcinoma of the oral cavity in patients younger than 40 years. Otolaryngol Head Neck Surg. 2007;117:268–275. doi: 10.1016/s0194-5998(97)70185-2. [DOI] [PubMed] [Google Scholar]
- 10.Iamaroon A, Pattanaporn K, Pongsiriwet S, Wanachantararak S, Prapayasatok S, Jittidecharaks S, Chitapanarux I, Lorvidhaya V. Analysis of 587 cases of oral squamous cell carcinoma in northern Thailand with a focus on young people. Int J Oral Maxillofac Surg. 2004;33:84–88. doi: 10.1054/ijom.2003.0503. [DOI] [PubMed] [Google Scholar]
- 11.Llewellyn CD, Linklater K, Bell J, Johnson NW, Warnakulasuriya KA. Squamous cell carcinoma of the oral cavity in patients aged 45 years and under: a descriptive analysis of 116 cases diagnosed in the South East of England from 1990 to 1997. Oral Oncol. 2003;39:106–114. doi: 10.1016/s1368-8375(02)00026-x. [DOI] [PubMed] [Google Scholar]
- 12.Chitapanarux I, Lorvidhaya V, Sittitrai P, Pattarasakulchai T, Tharavichitkul E, Sriuthaisiriwong P, Kamnerdsupaphon P, Sukthomya V. Oral cavity cancers at a young age: analysis of patient, tumour and treatment characteristics in Chiang Mai University Hospital. Oral Oncol. 2006;42:83–88. doi: 10.1016/j.oraloncology.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Vargas H, Pitman KT, Johnson JT, Galati LT. More aggressive behavior of squamous cell carcinoma of the anterior tongue in young women. Laryngoscope. 2000;110:1623–1626. doi: 10.1097/00005537-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Chen YK, Huang HC, Lin LM, Lin CC. Primary oral squamous cell carcinoma: an analysis of 703 cases in southern Taiwan. Oral Oncol. 1999;35:173–179. doi: 10.1016/s1368-8375(98)00101-8. [DOI] [PubMed] [Google Scholar]
- 15.Thomas L, Moore EJ, McGree ME, Olsen KD, Kasperbauer JL, Erickson LA, Schembri-Wismayer DJ. Prognostic features, human papillomavirus status, and epidermal growth factor receptor expression in oral squamous cell carcinoma in young adults. Am J Otolaryngol. 2012;33:650–656. doi: 10.1016/j.amjoto.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Salem A. Dismissing links between HPV and aggressive tongue cancer in young patients. Ann Oncol. 2010;21:13–17. doi: 10.1093/annonc/mdp380. [DOI] [PubMed] [Google Scholar]


