Abstract
Pneumonia is the second leading reason for hospitalization of medicare beneficiaries. The mortality rate is high, especially in the elderly. In this study, we aimed to determine the risk factors associated with severe pneumonia in the elderly. Retrospective study was conducted and data of old patients with severe pneumonia were collected. They were divided into two groups: the experiment group (death group) and the control (living group). The general situation, underlying diseases, laboratory tests, types of etiology, imaging analysis and treatment situation of patients were analyzed and compared. Univariate analysis and logistic multivariate regression analysis were used to screen the related and independent risk factors for the diagnosis of severe pneumonia in the elderly. In univariate analysis, there were many factors had statistical significance including chronic kidney disease, electrolyte disturbance, low phosphorus and so on. Result of logistic multivariate regression analysis showed pro-BNP level and serum prealbumin were independent risk factors. In sputum culture, the relevance ratio of acinetobacter baumannii was the highest in gram negative bacteria followed by klebsiella pneumoniae. In gram positive bacteria, the relevance ratio of staphylococcus aureus was the highest. In conclusion, the analysis on risk factors for severe pneumonia has great clinical significance on improving the prognosis.
Keywords: Severe pneumonia, risk factors, single factor analysis, logistic multivariate regression analysis, prognosis
Introduction
Pneumonia is the second leading reason for hospitalization of medicare beneficiaries and accounts for more than 600,000 medicare hospitalizations yearly [1,2]. It is common and potentially serious infected. The elderly are more susceptible to pneumonia and more likely to die from this infection than younger populations. Older patients with pneumonia requiring hospitalization are more likely to develop complications necessitating longer hospital stays [3]. Pneumonia in the elderly happens fast and the prognosis is poor, and elderly are susceptible to severe Pneumonia. The mortality rate for severe pneumonia is as high as 20% [4]. The principal cause of the death is respiratory insufficiency [5]. According to the study of Kitamoto et al, 83% of deaths from pneumonia were to organic dysfunctions in the respiratory tract and alveoli; 48% of deaths were from respiratory insufficiency due to progression of pneumonia, and 35% were attributable to protracted respiratory insufficiency despite suppressed development of pneumonic lesions [6]. Any intervention that would affect pneumonia mortality is of great public health importance [7]. Mycoplasma pneumoniae and Chlamydia pneumoniae are the two most common atypical pathogens [8]. The etiology of severe pneumonia requiring mechanical ventilation in the very elderly has been imprecise because of lack of comprehensive studies and low yield of diagnostic approach [9].
Severe CAP is a clinical entity with particular characteristic as concerns aetiology, diagnosis, treatment and prognosis. Prognosis factors of severe CAP have been identified in some CAP studies. The prognosis factors found in these studies were the following: radiographic spread of pneumonia, septic shock, simplified acute physiology score (SAPS) more than 13, streptococcus pneumoniae or enterobacteriaceae infections, bacteria, non-pneumonia-related complications, anticipated death within 5 years and ineffective initial antibiotic therapy [10-13]. Most of the recognized factors are merely a reflection of the baseline characteristics of patients or of the severity of pneumonia and its complications or outcome [14]. The severity of the underlying illness, high SAPS scores, age, bacteremia, septic shock, radiographic spread of pneumonia and the development of complications during ICU stay are examples of this [15].
The aim of the proposed study was to identify the risk factors that have major significance in predicting mortality in the elderly with severe pneumonia. Univariate analysis and logistic multivariate regression analysis were used.
Material and methods
Patients and definitions
Hospitalized patients (age ≥65) of 60 cases from March 1, 2011 to March 3, 2013 treated for severe pneumonia were assigned to this retrospective study. Of them, 30 cases were set as living group (control group) and the other 30 were death group (experiment group). CAP was defined as an acute illness associated with a new infiltrate on a chest radiogram in an out-patient department or within 48 h of hospitalization, accompanied by the presence of respiratory symptoms (cough, sputum production, wheezing, dyspnea, or chest pain) with or without fever. Diagnosis of community acquired pneumonia (CAP) refers to “the diagnosis and treatment guidelines for CAP” of Respiratory Society, Chinese Medical Association.
Diagnosis of hospital acquired pneumonia (HAP) obeys “the diagnosis and treatment guidelines for HAP” of Respiratory Society, Chinese Medical Association. Diagnosis of severe pneumonia complies with the diagnostic criteria revised by American IDSA/ATS.
Patients behave as the followings were excluded: (1) patients died after admission to hospital 24 h or left hospital without cure; (2) treatment was interrupted or given up by families during therapeutic process when patients were not cured; (3) patients with severe immunosuppression (such as hemeopathy, Acquired Immune Deficiency Syndrome and after bone marrow transplantation); (4) clinical data is not complete.
In experiment group, CAP was 24 cases and HAP 6 cases, 22 cases (73.3%) of male and 8 cases (26.7%) of female. Age of patients in this group ranged from 72 to 93 years and the average age ± SD was 79.7±7.6 years. In the control group, there were CAP of 16 cases and HAP of 14 cases, 20 cases (66.7%) of male and 10 cases (33.3%) of female. Age of patients in this group ranged from 72 to 93 years and the average age ± SD was 84.5±5.3 years. Within 24 h of admission, the following data were recorded: age, sex, underlying disease, cardio-pulmonary function, blood routine examination, blood biochemical indexes, bacteriological monitoring, imaging manifestations, blood gas analysis, using condition of antibacterial agents in the initial, mechanical ventilation and so on.
Clinical examination
All selected patients were conducted with treatment and monitor as follows. Monitoring programs included vital signs, hearts and lungs function indices (brain natriuretic peptide, pulmonary function test and so on), Blut-Routine-Untersuchung, C reactive protein, blood, liver and kidney function, blood glucose, blood electrolytes, coagulation indicator, thyroxine level, arterial blood gas analysis, chest X-ray or CT, electrocardiographic examination, etiological examination (blood culture, secrets of deep endotracheal obtained by fiber bronchoscope or sputum-sucking tube via the artificial airway, the first bite of sputum culture collected in morning cough after rinse your mouth vigorously with water).
We can comprehensively assess the severity scale for infection, general condition, the functional status of each visceras by monitoring results above. So a series of therapeutic plans can be set up, therapeutic effect and prognosis could be evaluated. Treatments included timely anti-infection, early mechanical ventilation, application of vasoactive drugs, acid-base equilibrium or electrolyte disturbance correction by adding liquid, treatment for basic diseases and so on.
Observation index
In this study, the patients were investigated by retrospective case-control method. All information about patients was recorded respectively including age, sex, underlying disease (chronic obstructive pulmonary disease, cardiovascular and cerebrovascular diseases, chronic liver disease, chronic renal insufficiency, diabetes, malignancy, Benign Prostatic Hyperplasia), number of red and white blood cell, thrombocyte, hemoglobin, neutrophil count, lymphocyte numbers, various indicators for blood biochemistry, blood gas analysis, D-Dimer, thyroid function index, C reactive protein, brain natriuretic peptide. The worst value of acute physiology parameters after admission in hospital 24 h were taken as the indexes above. At the same time, respiratory rate, state of consciousness, mechanical ventilation, chest radiogram, pathogenic species, therapeutic schedule by initial antibiotic, application or not of hormone, ACEI and thymosin, whether invasive procedure performed (cutting stomach tube, catheter, deep vein catheterization) and whether long-term bed or aspiration factors exist.
Statistical analysis
SPSS13.0 software was used for total analysis. Univariate analysis included all tests, comparing single parameters between two patient groups (living and death). Continuous variables were expressed by means ± SD and categorical variables by frequencies and percentages. The χ2 test was used for categorical variables. Continuous parameters with normal (Gaussian) division were compared by Student’s t-test.
The factors considered as significant mortality predictors in univariate analysis were tested by multivariate analysis, applying the method of logistic regression. In the final model, each input parameter had to have significant influence on mortality. The OR was set, together with a 95% CI for it.
All statistical tests were carried out at a significance level of α=0.05. By specifying this level, we had a 5% chance of erroneously rejecting our null hypothesis when, in fact, the null hypothesis was true. The SPSS statistical program for Windows, version 8, was used.
Results
Underlying disease situations
In this study, most of the elderly patients merging cardiovascular and cerebrovascular diseases, diabetes, chronic obstructive pulmonary disease, tumour, chronic liver and kidney diseases, great surgery and so on. Of those, hypertension, cardiopathy and cerebrovascular disease were more important and the result was shown in Figure 1.
Figure 1.
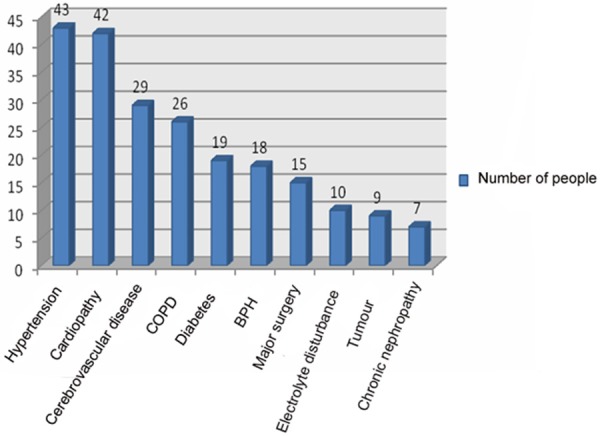
Common underlying diseases of severe pneumonia in elderly.
Comparison of pathogen detection situation
Sputum of 54 patients was collected successfully and sputum bacteria culture of 46 patients (76.7%) presented positive. Of the 46 patients, twenty-seven cases contained two and above mixed bacteria infection (58.7%) and 20 cases merged fungal infection (43.5%). Gram-negative and positive bacteria were isolated from the medium of sputum, respectively. In gram-negative bacteria, relevance ratio of acinetobacter baumannii (AB) was the highest (18.4%) and followed by klebsiella pneumoniae (11.4%). In gram positive bacteria, staphylococcus aureus (SA) had the largest detection rate (9.8%). There were 25 cases of blood culture inspected successfully and 7 presented positive (28%). Among them, klebsiella pneumoniae had the highest detection rate and it was 38.5%. In this study, 316 strains of bacteria etiology and 47 strains of fungus were separated. Of all the inspected samples, there were 111 strains of drug-resistance bacteria and it occupied 35.1 percents of bacteria etiology. Of all the drug-resistance bacteria, multiresistant acinetobacter baumannii (MDRAb) had the highest percentage (40.5%) and the next was Methicillin-resistant staphylococcus aureus (MRSA) (24.3%). The results of total bacteria situation including bacteria detected out in sputum and blood medium were shown in Figure 2. Bacteria situation detected in sputum and blood medium was shown in Figures 3 and 4, respectively.
Figure 2.
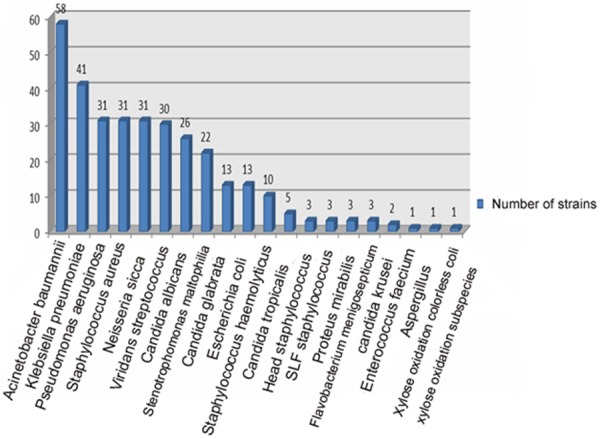
The check out situation of total bacteria etiology in elderly severe pneumonia.
Figure 3.
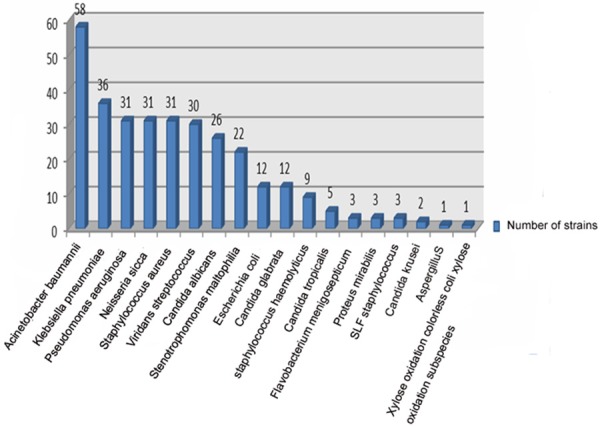
The check out situation of etiology from sputum culture in elderly severe pneumonia.
Figure 4.
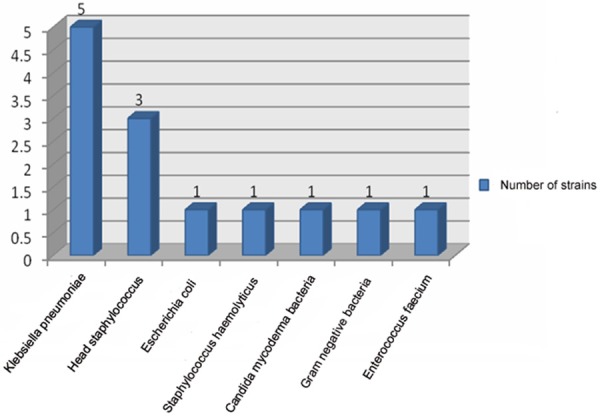
The check out situation of etiology from blood culture in elderly severe pneumonia.
To identify the differences between the death and living patients, comparison of positive rate of blood culture, infection rate of phlegm fungi and multiple resistant bacteria (MDRO) between the experiment and control group were compared. The results were shown in Table 1 and Figure 5.
Table 1.
Comparison of the check out situation of pathogen in patients with elderly severe pneumonia between experiment group and the control
| Group | Test statistics quantity | P | |||
|---|---|---|---|---|---|
|
| |||||
| Control n (%) | Experiment n (%) | ||||
| Blood bacteria culture | No bacteria | 28 (93.3) | 19 (63.3) | χ2=7.954 | 0.005 |
| Bacterial positive | 2 (6.7) | 11 (36.7) | |||
| Sputum fungus culture | No fungus | 22 (73.3) | 8 (26.7) | χ2=13.067 | 0.000 |
| Fungus positive | 8 (26.7) | 22 (73.3) | |||
| Multiple drug-resistant bacteria in sputum culture (MDRO) | None | 23 (76.7) | 7 (23.3) | χ2=17.067 | 0.000 |
| Have | 7 (23.3) | 23 (76.7) | |||
Figure 5.
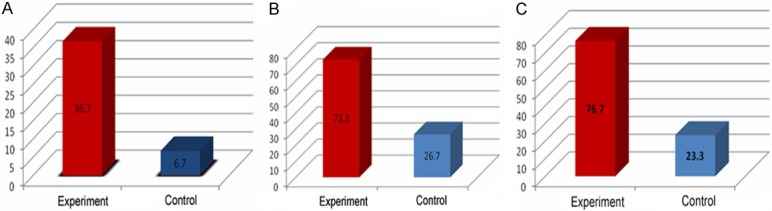
Comparison of positive rate in patients with elderly severe pneumonia between experiment group and the control. A: Comparison of positive rate in blood culture; B: Comparison of positive rate in sputum culture; C: Comparison of positive rate of multiple resistant bacteria isolated from sputum culture.
Risk factors analysis
Patients of 60 cases in experiment and control group were analyzed to screen risk factors. First, univariate analysis was performed and factors such as general situation of patients, underlying disease, main medical history, auxiliary examination, situation of medication and index of laboratory examination were taken into consideration. Factor with P<0.05 has statistical significance. Analysis on general situation of patients and merged underlying disease was shown in Table 2. Analysis on main medical history, auxiliary examination and situation of medication was shown in Table 3. Analysis on index of laboratory examination was shown in Table 4.
Table 2.
Single factor analysis of risk factors in elderly severepneumonia (general condition and merged underlying disease)
| General condition | Living group (%) (n=30) | Death group (%) (n=30) | Test statistics quantity | P | |
|---|---|---|---|---|---|
| Sex | Male | 20 (66.7) | 22 (73.3) | χ2=0.317 | 0.573 |
| Female | 10 (33.3) | 8 (26.7) | |||
| Onset age (x̅±s) | 84.2±5.3 | 82.6±5.3 | t=1.146 | 0.256 | |
| Complicated hypertension (x̅±s) | 23 (76.7) | 20 (66.7) | χ2=0.739 | 0.390 | |
| Complicated COPD (x̅±s) | 13 (43.3) | 13 (43.3) | χ2=0 | 1.000 | |
| Complicated cerebrovascular disease (x̅±s) | 17 (56.7) | 12 (40.0) | χ2=1.379 | 0.240 | |
| Complicated cardiovascular disease (x̅±s) | 18 (60.0) | 24 (80.0) | χ2=2.857 | 0.091 | |
| Complicated diabetes (x̅±s) | 9 (30.0) | 10 (33.3) | χ2=0.077 | 0.781 | |
| Complicated chronic nephrosis (x̅±s) | 1 (3.3) | 6 (20.0) | χ2=4.043 | 0.044 | |
| Complicated major surgery (x̅±s) | 9 (30.0) | 6 (20.0) | χ2=0.800 | 0.371 | |
| Complicated tumor (x̅±s) | 5 (16.7) | 4 (13.3) | χ2=0.131 | 0.718 | |
| Complicated electrolyte disturbance (x̅±s) | 1 (3.3) | 9 (30.0) | χ2=7.680 | 0.006 | |
| Low phosphorous | 13 (43.3) | 24 (85.7) | χ2=11.262 | 0.001 | |
| Normal phosphorous | 17 (56.7) | 4 (14.3) | |||
| Non-simultaneous low magnesium and phosphorous | 23 (76.7) | 14 (50.0) | χ2=4.459 | 0.035 | |
| Meanwhile low magnesium and phosphorous | 7 (23.3) | 14 (50.0) | |||
| Low magnesium | 8 (27) | 24 (80) | χ2=17.14 | 0.000 | |
| Normal magnesium | 22 (73) | 6 (20) | |||
| Complicated BPH (±s) | 10 (33.3) | 8 (26.7) | χ2=0.317 | 0.573 | |
Table 3.
Single factor analysis of risk factors in elderly severe pneumonia (main medical history, auxiliary examination and situation of medication)
| Group | Test statistics quantity | P | |||
|---|---|---|---|---|---|
|
| |||||
| Living group n (%) | Death group n (%) | ||||
| Thyroid function normal or not | Normal | 15 (50) | 2 (7) | χ2=13.87 | 0.000 |
| Abnormal | 15 (50) | 28 (93) | |||
| Number of affected lung lobe in chest | <3 | 15 (50) | 12 (40) | χ2=0.60 | 0.436 |
| >3 | 15 (50) | 18 (60) | |||
| Hydrothorax degree | No | 22 (73) | 13 (43) | χ2=5.78 | 0.055 |
| A little | 6 (20) | 11 (37) | |||
| Hydrops | 2 (7) | 6 (20) | |||
| Unilateral and bilateral hydrothorax | No | 22 (73) | 13 (43) | χ2=5.57 | 0.062 |
| Unilatera | 4 (13) | 8 (27) | |||
| Bilateral | 4 (13) | 9 (30) | |||
| Liver function | Normal | 25 (83) | 14 (47) | χ2=8.86 | 0.003 |
| Abnormal | 5 (17) | 16 (53) | |||
| Alimentary tract hemorrhage | No | 28 (93) | 22 (73) | χ2=4.32 | 0.038 |
| Yes | 2 (7) | 8 (27) | |||
| State of consciousness | Unconsciousness | 9(30) | 29 (97) | χ2=28.70 | 0.000 |
| Clear consciousness | 21 (70) | 1 (3) | |||
| Suction right or wrong | No | 15 (50) | 2 (7) | χ2=13.87 | 0.000 |
| Yes | 15 (50) | 28 (93) | |||
| Nasal feeding diet | No | 30 (100) | 13 (43) | χ2=23.72 | 0.000 |
| Yes | 0 (0) | 17 (57) | |||
| Bucking | No | 26 (87) | 21 (70) | χ2=2.45 | 0.117 |
| Yes | 4 (13) | 9 (30) | |||
| Dysphagia | No | 30 (00) | 23 (77) | χ2=7.92 | 0.005 |
| Yes | 0 (0) | 7 (23) | |||
| Gastrointestinal decompression | No | 30 (100) | 29 (97) | χ2=1.16 | 0.313 |
| Yes | 0 (0) | 1 (3) | |||
| Fasting | No | 30 (100) | 29 (97) | χ2=1.16 | 0.313 |
| Yes | 0 (0) | 1 (3) | |||
| After fracture operation | No | 30 (100) | 29 (97) | χ2=1.16 | 0.313 |
| Yes | 0 (0) | 1 (3) | |||
| Long-term bed | No | 12 (40) | 1 (3) | χ2=11.88 | 0.001 |
| Yes | 18 (60) | 29 (97) | |||
| Application of antimicrobial agents for the first time after admission | Inappropriate | 3 (10) | 15 (50) | χ2=11.42 | 0.001 |
| Appropriate | 27 (90) | 15 (50) | |||
| Invasive ventilation | No | 2 (22) | 5 (21) | χ2=0.75 | 0.931 |
| Yes | 7 (78) | 19 (79) | |||
| Mechanical ventilation | No | 21 (70) | 4 (13) | χ2=19.81 | 0.000 |
| Yes | 9 (30) | 26 (87) | |||
| Application of hormone | No | 17 (57) | 10 (33) | χ2=3.29 | 0.069 |
| Yes | 13 (43) | 20 (67) | |||
| Invasive procedure | No | 15 (50) | 3 (10) | χ2=11.42 | 0.001 |
| Yes | 15 (50) | 27 (90) | |||
| Trachea cannula | No | 24 (80) | 13 (43) | χ2=8.53 | 0.003 |
| Yes | 6 (20) | 17 (57) | |||
| Stomach tube | No | 15 (50) | 4 (13) | χ2=9.31 | 0.002 |
| Yes | 15 (50) | 26 (87) | |||
| Catheter | No | 22 (73) | 15 (50) | χ2=3.45 | 0.063 |
| Yes | 8 (27) | 15 (50) | |||
| Deep vein catheterization | No | 24 (80) | 13 (43) | χ2=8.53 | 0.003 |
| Yes | 6 (20) | 17 (57) | |||
| ACEI | Nonuse | 17 (57) | 26 (87) | χ2=6.64 | 0.010 |
| Use | 13 (43) | 4 (13) | |||
| Thymosin | Nonuse | 9 (30) | 20 (67) | χ2=8.75 | 0.004 |
| use | 21 (70) | 10 (33) | |||
| Thymosin | <4 weeks | 11 (52) | 1 (10) | χ2=5.12 | 0.024 |
| >4 weeks | 10 (48) | 9 (90) | |||
Table 4.
Single factor analysis of risk factors in elderly severe pneumonia (index of laboratory examination)
| General condition | Living group | Death group | Test statistics quantity | P |
|---|---|---|---|---|
| Hb (g/L) | n=30 | n=28 | t=2.86 | 0.006 |
| 113.36±21.94 | 95.85±24.63 | |||
| PLT (*109/L) | n=30 | n=27 | t=1.73 | 0.088 |
| 188.13±64.23 | 150.25±98.35 | |||
| CRP (mg/L) | n=30 | n=30 | t=-3.86 | 0.000 |
| 53.9±51.37 | 113.01±66.24 | |||
| BNP (pg/ml) | n=14 | n=9 | t=-2.34 | 0.029 |
| 110.36±115.54 | 285.16±240.98 | |||
| proBNP (pg/ml) | n=29 | n=29 | t=-4.04 | 0.000 |
| 1308.67±1576.45 | 8530.71±9487.93 | |||
| WBC (×109/L) | n=30 | n=30 | t=-2.90 | 0.005 |
| 9.05±3.32 | 14.71±10.14 | |||
| N% | n=30 | n=30 | t=0.46 | 0.641 |
| 75.21±11.66 | 72.16±33.65 | |||
| L% | n=30 | n=29 | t=3.96 | 0.000 |
| 15.33±9.03 | 6.86±7.27 | |||
| Albumin (g/l) | n=29 | n=30 | t=6.52 | 0.000 |
| 35.83±4.86 | 27.43±5.01 | |||
| Prealbumin (mg/L) | n=30 | n=30 | t=4.63 | 0.000 |
| 172.27±74.48 | 96.27±50.23 | |||
| Serum uric acid (umol/L) | n=30 | n=30 | t=-1.55 | 0.126 |
| 266.64±118.52 | 322.07±155.52 | |||
| Homocysteine (umol/L) | n=14 | n=11 | t=0.35 | 0.725 |
| 16.36±6.3 | 15.47±5.99 | |||
| Blood glucose (mmol/L) | n=30 | n=30 | t=-2.06 | 0.044 |
| 6.27±2.36 | 8.22±4.63 | |||
| Renal function (Ccr ml/min) | n=30 | n=30 | t=2.21 | 0.031 |
| 61.24±24.39 | 45.22±31.32 | |||
| D-D dimer (mg/L) | n=30 | n=27 | t=-2.22 | 0.030 |
| 1.73±1.56 | 3.54±4.65 |
Based on univariate analysis, there were factors had statistical significance and they were: chronic kidney disease, electrolyte disturbance, low phosphorus, low magnesium and phosphorus, low or normal magnesium, thyroid function, liver function, gastrointestinal bleeding, conscious state, have a correct or wrong suction, nasal feeding, difficulty in swallowing, long-term bed, application of antimicrobial agents for the first time after admission, with and without mechanical ventilation, invasive procedure, trachea cannula, stomach tube, deep vein catheterization, ACEI, hymopeptides, hymopeptides greater than 4 weeks, Hb, CRP, proBNP, proBNP, WBC, L%, albumin, prealbumin, blood glucose, renal function, DD dimmer.
Multivariate analysis was then conducted using factors which had statistical significance in univariate analysis. The result was shown in Table 5. As shown, pro-BNP level and low magnesium were independent risk factors for elderly severe pneumonia.
Table 5.
Logistic regression analysis for elderly severepneumonia
| Risk factors | Estimate of parameter | Standard error | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| proBNP (pg/ml) | 0.001 | 0.000 | 5.059 | 0.024 | 1.001 | 1.000-1.001 |
| Prealbumin (mg/L) | 0.032 | 0.020 | 2.580 | 0.108 | 0.968 | 0.931-1.007 |
| Multiple resistant bacteria in sputum culture (MDRO) | -2.616 | 1.761 | 2.206 | 0.138 | 0.073 | 0.002-2.308 |
| Low magnesium | 4.870 | 2.450 | 3.951 | 0.047 | 130.304 | 1.071-15858.749 |
| Conscious state | 3.629 | 2.594 | 1.957 | 0.162 | 37.681 | 0.233-6088.79 |
| Whether application of antimicrobial agents for the first time after admission appropriate | 4.293 | 2.311 | 3.450 | 0.063 | 73.186 | 0.789-6789.461 |
| Constant | -4.519 | 3.871 | 1.363 | 0.243 | 0.011 | - |
Discussion
Severe pneumonia in elder is difficult to treat commonly in pneumology department and often involves in multiple organs [16]. It is serious, has a rapid progression and the morality is high [4,17]. Due to immune system and Respiratory defense function slow down and multiple underlying diseases accompanied, the occurrence rate in pneumonia especially severe pneumonia is high [9,18]. Moreover, the morbidity increases with the aging trend of population, which is the main cause of death in the elderly [19]. To improve the prognosis and reduce mortality, 60 cases of patient with severe pneumonia were collected for retrospective study and we screened independent risk factors by univariate and multivariate analysis. Results showed there were multiple risk factors influence the prognosis of severe pneumonia in elderly such as underlying diseases, the distribution characteristics of pathogen, mechanical ventilation, cardiac insufficiency, the application of antimicrobial agents for the first time after admission, application of ACEI, thymosin α1 and some serum test parameters and so on. Among them, pro-BNP level and low magnesium were independent risk factors for severe pneumonia in elder.
Chronic underlying disease is one of the most important risk factors in elder pneumonia [20]. 60 to 90 percent of elder pneumonia patients in hospital have one or more underlying disease and many chronic diseases occur with age increase [21]. Moreover, the immune function of patients also descends which lead to the infection rate of lung and mortality increase. In this study, the number of patients with chronic underlying disease in experiment group was significantly higher than that in the control (P<0.05), which was similar to the results of overseas studies [22]. So for elderly patients, we should adopt comprehensive prevention and treatment measures for various reasons, treat underlying disease actively to cut off the vicious cycle chain.
In the 60 patients, sputum culture was conducted in 54 patients and 316 strains of pathogenic bacteria were separated. Pathogenic bacteria were not checked out only in 8 cases and positive rate in experiment group and the control was 89.3% and 80.8%, respectively, which were much higher than that in related reports [23]. Those may be due to most patients came from Intensive Care Unit (ICU) and emergency observation ward, and sputum was absorbed from trachea of patients by aseptic inhale phlegm pipe and send to bioclean room in bacterium-free pipe, which reduce the interference factors and improve the positive detection rate. Gram-negative bacteria play a major role in the 2 groups. In experiment group, acinetobacter baumannii was the most and in the control the main pathogenic bacteria were neisseria gonorrhoeae, pseudomonas aeruginosa, acinetobacter baumannii followed by staphylococcus aureus, escherichia coli and klebsiella pneumoniae. In drug-resistance bacteria, the percent of multiresistant Acinetobacter baumannii was the highest (40.5%) followed by methicillin resistant staphylococcus aureus (MRSA) (24.3%) and Extended Spectrum Beta-Lactamases (ESBLs) (8.1%). Acinetobacter baumannii is a species of non-fermentative gram-negative bacteria commonly found in water and soil [24]. It is an important and common pathogen causing nosocomial infections, especially in immunocompromised patients [25]. It has emerged as a highly troublesome pathogen for many institutions globally and a significant opportunistic pathogen that is associated with a serious underlying disease [26,27]. Acinetobacter baumannii often causes a wide range of infections and is a common cause of nosocomial pneumonia [28,29]. MRSA is a major cause of hospital-acquired infections that are becoming increasingly difficult to combat because of emerging resistance to all current antibiotic classes [30]. It is increasingly a cause of nosocomial and community-onset infection with unknown national scope and magnitude [31]. It is reported associated with severe pneumonia in elder [31]. Therefore, based on the result of etiology, we should select antibiotics reasonably, timely and correctly. Studies showed rational antibiotic treatment can rectify risk factors which improve the diagnosis of severe pneumonia in elder [32].
Whether applying antimicrobial agents for the first time after admission appropriately is a key factor for the diagnosis of severe pneumonia. If antibiotic used inappropriately, the drug-fast of antibiotic would increase which prolong hospital stays and the mortality. Reports at home and abroad showed the morality of patients treated by combing antibacterial agents is lower than that by single agent [33,34]. Studies showed the treatment to infection resulted by multiresistant Acinetobacter baumannii by a large dose of sulbactam or sulbactam with another antibiotic is effective [35,36]. So, for elderly patients, especially with basic liver and kidney diseases, we should adjust the dosage accordingly. In experiment group, more patients had respiratory failure and were treated with mechanical ventilation than the control. The morality of patients with respiratory failure was also higher. This may be because the organizational structure of respiratory tract changed, the immune function decreased and many chronic underlying diseases combined, which led to the easy transformation of pneumonia to severe pneumonia [37]. Study showed the pH of arterial blood below 7.35 and PaO2 below 60mmHg could increase morality, which indicated the lack level of oxygen could influence the prognosis of severe pneumonia in elder [38]. As result showed, pro-BNP was independent risk factor for the diagnosis of severe pneumonia in elder and morality in patients with cardiac insufficiency is markedly higher. Many studies have showed cardiac insufficiency is important risk factor for the diagnosis of severe pneumonia in elder [39]. But we should also consider the percent of patients with basic heart disease. In most studies, the ratio ranged from 11% to 23% [9,40]. In our study, the value was 66.7% which may be resulted from cardiac insufficiency. Hypomagnesemia was risk factor for the diagnosis of severe pneumonia in elder obtained in our study. Magnesium is an important constituent of the intracellular space that affects a number of intracellular and whole body functions [41]. Homeostasis of magnesium levels is tightly regulated and depends on the balance between intestinal absorption and renal excretion [42]. Magnesium deficiency is important feature of severe infection and septic shock [43]. In patients with severe pneumonia, hypomagnesemia were found in many cases [44]. Phosphorus concentration of patients in experiment group was much lower than that in the control and indicated a risk factor for the diagnosis of severe pneumonia in elder. Patients with pneumonia demonstrated lower levels (P<0.01) of calcium, phosphorus [44]. phosphorus levels were useful in identifying mimics of swine influenza pneumonia [45]. When phosphorus concentration in blood was low, the phagocytosis and chemotactic function of macrophages and granulocyte reduced which progressed further and resulted in the poor prognosis [46,47]. So we should pay much attention on the diagnosis of magnesium and phosphorus concentration.
CRP, ALB, sPA and D-Dimer were found the risk factors for the diagnosis of severe pneumonia in elder in univariate analysis. C-reactive protein (CRP) is an ancient highly conserved molecule and a member of the pentraxin family of proteins [48]. Elevated serum levels of CRP have been widely considered to be nonspecific but sensitive markers of the acute inflammatory response [49]. Its level can be elevated in the acute phase of bacterial infection and kept almost the same when infected by virus [50]. Therefore, we concluded the increase of CRP level is associated with bacterial infection. Serum prealbumin (sPA), a negative acute-phase reactant, has a role as a clinical index of patient status after inflammation, trauma, or surgery that is gaining recognition [51]. In our study, the level of sPA in experiment group was significantly lower than the control which indicated there was a negative linear correlation between sPA level and infection severity. D-Dimer is a specific derivative of cross-linked fibrin [52]. D-Dimer levels are directly related to the intra-and extra-vascular coagulation that occurs in acute and chronic lung damage in patients with community-acquired pneumonia (CAP) [53]. Raised D-Dimer concentrations can often be observed [54,55].
In conclusion, pro-BNP and hypomagnesemia were independent risk factors for the diagnosis of severe pneumonia in elder. MDRO infection, fungal infection and bacteremia were microbiological risk factors. The application of thymosin α1 can improve the prognosis. The determination of CRP, Hb, WBC, lymphocytes percentage, BNP, pro-BNP, albumin and serum prealbumin has important clinical significance for the prognosis. Other risk factors also need many researches to verify the importance and independent risk factors can be screened.
Acknowledgements
This work was supported by Shanghai Municipal Bureau of Health Scientific Research Fund Project (No. 2010082), Shanghai Cadres Steer Clear Scientific Research Subject (No. 2011GB24) and Research Grant from Shanghai Hospital Development Center (No. SHDC12013901).
Disclosure of conflict of interest
None.
References
- 1.Bauer TT, Ferrer R, Angrill J, Schultze-Werninghaus G, Torres A. Ventilator-associated pneumonia: incidence, risk factors, and microbiology. Semin Respir Infect. 2000;15:272–9. doi: 10.1053/srin.2000.20938. [DOI] [PubMed] [Google Scholar]
- 2.Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med. 2005;33:2184–2193. doi: 10.1097/01.ccm.0000181731.53912.d9. [DOI] [PubMed] [Google Scholar]
- 3.Fein A, Niederman M. Severe pneumonia in the elderly. Clin Geriatr Med. 1994;10:121–143. [PubMed] [Google Scholar]
- 4.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr, Musher DM, Niederman MS, Torres A, Whitney CG Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawai S, Ochi M, Nakagawa T, Goto H. Antimicrobial therapy in community-acquired pneumonia among emergency patients in a university hospital in Japan. J Infect Chemother. 2004;10:352–358. doi: 10.1007/s10156-004-0350-2. [DOI] [PubMed] [Google Scholar]
- 6.Kiyokawa K, Kawai S. Efficacy of steroid therapy on adult patients with severe pneumonia. J Infect Chemother. 2010;16:266–271. doi: 10.1007/s10156-010-0049-5. [DOI] [PubMed] [Google Scholar]
- 7.Sazawal S, Black RE Pneumonia Case Management Trials Group. Effect of pneumonia case management on mortality in neonates, infants, and preschool children: a meta-analysis of community-based trials. Lancet Infect Dis. 2003;3:547–556. doi: 10.1016/s1473-3099(03)00737-0. [DOI] [PubMed] [Google Scholar]
- 8.Samransamruajkit R, Jitchaiwat S, Wachirapaes W, Deerojanawong J, Sritippayawan S, Prapphal N. Prevalence of Mycoplasma and Chlamydia pneumonia in severe community-acquired pneumonia among hospitalized children in Thailand. Jpn J Infect Dis. 2008;61:36–9. [PubMed] [Google Scholar]
- 9.El-Solh AA, Sikka P, Ramadan F, Davies J. Etiology of severe pneumonia in the very elderly. Am J Respir Crit Care Med. 2001;163:645–651. doi: 10.1164/ajrccm.163.3.2005075. [DOI] [PubMed] [Google Scholar]
- 10.Moine P, Sikka P, Ramadan F, Davies J. Severe community-acquired pneumonia. Etiology, epidemiology, and prognosis factors. French Study Group for Community-Acquired Pneumonia in the Intensive Care Unit. CHEST Journal. 1994;105:1487–1495. doi: 10.1378/chest.105.5.1487. [DOI] [PubMed] [Google Scholar]
- 11.Ortqvist A, Sterner G, Nilsson JA. Severe community-acquired pneumonia: factors influencing need of intensive care treatment and prognosis. Scand J Infect Dis. 1984;17:377–386. doi: 10.3109/13813458509058778. [DOI] [PubMed] [Google Scholar]
- 12.Fang GD, Fine M, Orloff J, Arisumi D, Yu VL, Kapoor W, Grayston JT, Wang SP, Kohler R, Muder RR, et al. New and emerging etiologies for community-acquired pneumonia with implications for therapy: a prospective multicenter study of 359 cases. Medicine (Baltimore) 1990;69:307–316. doi: 10.1097/00005792-199009000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Leroy O, Santré C, Beuscart C, Georges H, Guery B, Jacquier JM, Beaucaire G. A five-year study of severe community-acquired pneumonia with emphasis on prognosis in patients admitted to an intensive care unit. Intensive Care Med. 1995;21:24–31. doi: 10.1007/BF02425150. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz M, Ewig S, Torres A, Arancibia F, Marco F, Mensa J, Sanchez M, Martinez JA. Severe community-acquired pneumonia: risk factors and follow-up epidemiology. Am J Respir Crit Care Med. 1999;160:923–929. doi: 10.1164/ajrccm.160.3.9901107. [DOI] [PubMed] [Google Scholar]
- 15.Torres A, El-Ebiary M. Prognosis factors in severe community-acquired pneumonia: a step forward. Intensive Care Med. 1996;22:1288–1290. doi: 10.1007/BF01709539. [DOI] [PubMed] [Google Scholar]
- 16.Society AT. Guidelines for the management of adults with community-acquired pneumonia, Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163:1730–1754. doi: 10.1164/ajrccm.163.7.at1010. [DOI] [PubMed] [Google Scholar]
- 17.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan V, Angus DC, Griffin MF, Clermont G, Scott Watson R, Linde-Zwirble WT. Hospitalized community-acquired pneumonia in the elderly: age-and sex-related patterns of care and outcome in the United States. Am J Respir Crit Care Med. 2002;165:766–772. doi: 10.1164/ajrccm.165.6.2103038. [DOI] [PubMed] [Google Scholar]
- 19.El-Solh AA, Pietrantoni C, Bhat A, Aquilina AT, Okada M, Grover V, Gifford N. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167:1650–1654. doi: 10.1164/rccm.200212-1543OC. [DOI] [PubMed] [Google Scholar]
- 20.Kida K, Mizuuchi T, Takeyama K, Hiratsuka T, Jinno S, Hosoda K, Imaizumi A, Suzuki Y. Serum secretory leukoprotease inhibitor levels to diagnose pneumonia in the elderly. Am Rev Respir Dis. 1992;146:1426–1429. doi: 10.1164/ajrccm/146.6.1426. [DOI] [PubMed] [Google Scholar]
- 21.Roblot F, Godet C, Le Moal G, Garo B, Faouzi Souala M, Dary M, De Gentile L, Gandji JA, Guimard Y, Lacroix C, Roblot P, Becq-Giraudon B. Analysis of underlying diseases and prognosis factors associated with Pneumocystis carinii pneumonia in immunocompromised HIV-negative patients. Eur J Clin Microbiol Infect Dis. 2002;21:523–531. doi: 10.1007/s10096-002-0758-5. [DOI] [PubMed] [Google Scholar]
- 22.Marrie TJ. Community-acquired pneumonia in the elderly. Clin Infect Dis. 2000;31:1066–1078. doi: 10.1086/318124. [DOI] [PubMed] [Google Scholar]
- 23.Murdoch DR, Laing RT, Mills GD, Karalus NC, Town GI, Mirrett S, Reller LB. Evaluation of a rapid immunochromatographic test for detection of Streptococcus pneumoniae antigen in urine samples from adults with community-acquired pneumonia. J Clin Microbiol. 2001;39:3495–3498. doi: 10.1128/JCM.39.10.3495-3498.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fournier PE, Vallenet D, Barbe V, Audic S, Ogata H, Poirel L, Richet H, Robert C, Mangenot S, Abergel C, Nordmann P, Weissenbach J, Raoult D, Claverie JM. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet. 2006;2:e7. doi: 10.1371/journal.pgen.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai HT, Wang JT, Chen CJ, Chang SC. Association between antibiotic usage and subsequent colonization or infection of extensive drug-resistant Acinetobacter baumannii: a matched case-control study in intensive care units. Diagn Microbiol Infect Dis. 2008;62:298–305. doi: 10.1016/j.diagmicrobio.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 26.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins PG, Wisplinghoff H, Stefanik D, Seifert H. In vitro activities of the β-lactamase inhibitors clavulanic acid, sulbactam, and tazobactam alone or in combination with β-lactams against epidemiologically characterized multidrug-resistant Acinetobacter baumannii strains. Antimicrob Agents Chemother. 2004;48:1586–1592. doi: 10.1128/AAC.48.5.1586-1592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez-Hernández MJ, Pachón J, Pichardo C, Cuberos L, Ibáñez-Martínez J, García-Curiel A, Caballero FJ, Moreno I, Jiménez-Mejías ME. Imipenem, doxycycline and amikacin in monotherapy and in combination in Acinetobacter baumannii experimental pneumonia. J Antimicrob Chemother. 2000;45:493–501. doi: 10.1093/jac/45.4.493. [DOI] [PubMed] [Google Scholar]
- 30.Enright MC, Robinson DA, Randle G, Feil EJ, Grundmann H, Spratt BG. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA) Proc Natl Acad Sci U S A. 2002;99:7687–7692. doi: 10.1073/pnas.122108599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Francis JS, Doherty MC, Lopatin U, Johnston CP, Sinha G, Ross T, Cai M, Hansel NN, Perl T, Ticehurst JR, Carroll K, Thomas DL, Nuermberger E, Bartlett JG. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis. 2005;40:100–107. doi: 10.1086/427148. [DOI] [PubMed] [Google Scholar]
- 32.Gutiérrez F, Masiá M. Improving outcomes of elderly patients with community-acquired pneumonia. Drugs Aging. 2008;25:585–610. doi: 10.2165/00002512-200825070-00005. [DOI] [PubMed] [Google Scholar]
- 33.Empey PE, Jennings HR, Thornton AC, Rapp RP, Evans ME. Levofloxacin failure in a patient with pneumococcal pneumonia. Ann Pharmacother. 2001;35:687–690. doi: 10.1345/aph.10229. [DOI] [PubMed] [Google Scholar]
- 34.Waterer GW, Somes GW, Wunderink RG. Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia. Arch Intern Med. 2001;161:1837–1842. doi: 10.1001/archinte.161.15.1837. [DOI] [PubMed] [Google Scholar]
- 35.Hsueh PR, Teng LJ, Chen CY, Chen WH, Yu CJ, Ho SW, Luh KT. Pandrug-resistant Acinetobacter baumannii causing nosocomial infections in a university hospital, Taiwan. Emerg Infect Dis. 2002;8:827–32. doi: 10.3201/eid0808.020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins C, Murtough SM, Williamson E, Hiom SJ, Payne DJ, Russell AD, Walsh TR. Resistance to antibiotics and biocides among non-fermenting Gram-negative bacteria. Clin Microbiol Infect. 2001;7:308–315. doi: 10.1046/j.1198-743x.2001.00253.x. [DOI] [PubMed] [Google Scholar]
- 37.Williams JV, Harris PA, Tollefson SJ, Halburnt-Rush LL, Pingsterhaus JM, Edwards KM, Wright PF, Crowe JE Jr. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoogewerf M, Oosterheert JJ, Hak E, Hoepelman IM, Bonten MJ. Prognostic factors for early clinical failure in patients with severe community-acquired pneumonia. Clin Microbiol Infect. 2006;12:1097–1104. doi: 10.1111/j.1469-0691.2006.01535.x. [DOI] [PubMed] [Google Scholar]
- 39.Shann F, MacGregor D, Richens J, Coakley J. Cardiac failure in children with pneumonia in Papua New Guinea. Pediatr Infect Dis J. 1998;17:1141–1143. doi: 10.1097/00006454-199812000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Yoshimoto A, Nakamura H, Fujimura M, Nakao S. Severe community-acquired pneumonia in an intensive care unit: risk factors for mortality. Intern Med. 2005;44:710–716. doi: 10.2169/internalmedicine.44.710. [DOI] [PubMed] [Google Scholar]
- 41.Kelepouris E, Agus Z. Hypomagnesemia: renal magnesium handling. Semin Nephrol. 1998;18:58–73. [PubMed] [Google Scholar]
- 42.Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth HW, Konrad M. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002;31:166–170. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- 43.Sunenshine RH, Tan ET, Terashita DM, Jensen BJ, Kacica MA, Sickbert-Bennett EE, Noble-Wang JA, Palmieri MJ, Bopp DJ, Jernigan DB, Kazakova S, Bresnitz EA, Tan CG, McDonald LC. A multistate outbreak of Serratia marcescens bloodstream infection associated with contaminated intravenous magnesium sulfate from a compounding pharmacy. Clin Infect Dis. 2007;45:527–533. doi: 10.1086/520664. [DOI] [PubMed] [Google Scholar]
- 44.Sankaran RT, Mattana J, Pollack S, Bhat P, Ahuja T, Patel A, Singhal PC. Laboratory abnormalities in patients with bacterial pneumonia. Chest. 1997;111:595–600. doi: 10.1378/chest.111.3.595. [DOI] [PubMed] [Google Scholar]
- 45.Cunha BA, Syed U, Mickail N, Strollo S. Rapid clinical diagnosis in fatal swine influenza (H1N1) pneumonia in an adult with negative rapid influenza diagnostic tests (RIDTs): diagnostic swine influenza triad. Heart Lung. 2010;39:78–86. doi: 10.1016/j.hrtlng.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eisenberg S, Ravesloot L, Koets AP, Grünberg W. Influence of feeding a low-phosphorus diet on leucocyte function in dairy cows. J Dairy Sci. 2014;97:5176–5184. doi: 10.3168/jds.2014-8180. [DOI] [PubMed] [Google Scholar]
- 47.Calder PC, Bond JA, Harvey DJ, Gordon S, Newsholme EA. Uptake and incorporation of saturated and unsaturated fatty acids into macrophage lipids and their effect upon macrophage adhesion and phagocytosis. Biochem J. 1990;269:807–814. doi: 10.1042/bj2690807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Du Clos TW. Function of C-reactive protein. Ann Med. 2000;32:274–278. doi: 10.3109/07853890009011772. [DOI] [PubMed] [Google Scholar]
- 49.Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–2168. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- 50.Sasaki K, Fujita I, Hamasaki Y, Miyazaki S. Differentiating between bacterial and viral infection by measuring both C-reactive protein and 2’-5’-oligoadenylate synthetase as inflammatory markers. J Infect Chemother. 2002;8:76–80. doi: 10.1007/s101560200010. [DOI] [PubMed] [Google Scholar]
- 51.Connors LH, Gertz MA, Skinner M, Cohen AS. Nephelometric measurement of human serum prealbumin and correlation with acute-phase proteins CRP and SAA: results in familial amyloid polyneuropathy. J Lab Clin Med. 1984;104:538–545. [PubMed] [Google Scholar]
- 52.Bounameaux H, Cirafici P, de Moerloose P, Schneider PA, Slosman D, Reber G, Unger PF. Measurement of D-dimer in plasma as diagnostic aid in suspected pulmonary embolism. Lancet. 1991;337:196–200. doi: 10.1016/0140-6736(91)92158-x. [DOI] [PubMed] [Google Scholar]
- 53.Arslan S, Ugurlu S, Bulut G, Akkurt I. The association between plasma D-dimer levels and community-acquired pneumonia. Clinics (Sao Paulo) 2010;65:593–597. doi: 10.1590/S1807-59322010000600006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di Nisio M, Squizzato A, Rutjes AW, Büller HR, Zwinderman AH, Bossuyt PM. Diagnostic accuracy of D-dimer test for exclusion of venous thromboembolism: a systematic review. J Thromb Haemost. 2007;5:296–304. doi: 10.1111/j.1538-7836.2007.02328.x. [DOI] [PubMed] [Google Scholar]
- 55.Snijders D, Schoorl M, Schoorl M, Bartels PC, van der Werf TS, Boersma WG. D-dimer levels in assessing severity and clinical outcome in patients with community-acquired pneumonia. A secondary analysis of a randomised clinical trial. Eur J Intern Med. 2012;23:436–441. doi: 10.1016/j.ejim.2011.10.019. [DOI] [PubMed] [Google Scholar]


