Abstract
The present study has investigated the effects of mobile phone electromagnetic radiation (EMR) on fertility in rats. The purpose of this study was to explore the capability of polyphenolic-rich Moringa oleifera leaf extract in protecting rat testis against EMR-induced impairments based on evaluation of sperm count, viability, motility, sperm cell morphology, anti-oxidants (SOD & CAT), oxidative stress marker, testis tissue histopathology and PCNA immunohistochemistry. The sample consisted of sixty male Wistar rats which were divided into four equal groups. The first group (the control) received only standard diet while the second group was supplemented daily and for eight weeks with 200 mg/kg aqueous extract of Moringa leaves. The third group was exposed to 900 MHz fields for one hour a day and for (7) days a week. As for the fourth group, it was exposed to mobile phone radiation and received the Moringa extract. The results showed that the EMR treated group exhibited a significantly decrease sperm parameters. Furthermore, concurrent exposure to EMR and treated with MOE significantly enhanced the sperm parameters. However, histological results in EMR group showed irregular seminiferous tubules, few spermatogonia, giant multinucleated cells, degenerated spermatozoa and the number of Leydig cells was significantly reduced. PCNA labeling indices were significant in EMR group versus the control group. Also, EMR affects spermatogenesis and causes to apoptosis due to the heat and other stress-related EMR in testis tissue. This study concludes that chronic exposure to EMR marked testicular injury which can be prevented by Moringa oleifera leaf extract.
Keywords: Infertility, electromagnetic radiation (EMR), Moringa oleifera, PCNA, sperm parameters.
Introduction
Despite the diversity of habitat and cultures around the world, an augment in male infertility has been observed in recent decades and apparently it constitutes an international phenomenon [1]. The true incidence of male infertility is unknown due to the enormous variability in infertility extensiveness. However, in 30-50% of sub-fertile couples the male partner has sub-optimal semen liquid quality, either because of poorly motile sperm, low sperm count or sperm with abnormal size and shape (morphology). In more than 50% of infertility cases in male, the etiology remains unknown and the infertility is classified as idiopathic. Evaluation of male infertility must go far beyond a simple analysis of semen which has to be complemented with a comprehensive history and physical examination as well as relevant genetic, endocrine and other investigations. The testis comprises two distinct compartments, the seminiferous tubules (the spermatogenesis site) and the Leydig cells (the testosterone source). The process of sperms production is regulated by genes located on the Y chromosome and takes approximately (70) days to complete starting from the spermatocyte stage. Furthermore, 12-21 days are required for the transport of sperm from the testis through the epididymis to the ejaculatory duct. During passage through the epididymis, sperms mature further to develop the capacity for sustained motility. The long time required for sperm development and transit implies that the results of a semen analysis reflect conditions existing many weeks earlier. Semen includes secretions contributed by the prostate, the seminal vesicles and the distal vas deferens [2].
It has long been suggested that the major cause of male infertility may be attributed to oxidative stress and the factors that are capable of compromising it by inducing the same in the testicular tissue are ionizing radiation, occupational toxins, altered atmospheric circumstances as well as many infectious conditions, oxygen metabolism, inflammation and life style stress [3].
With the increase in the use of electromagnetic wave techniques, the biological effects of electromagnetic pulses (EMPs) have come into focus. Environmental, occupational and accidental exposure to electromagnetic radiation (EMR) affect human health; in particular, its effect on the genital system and the mechanisms underlying this have been the topic of research [4]. The incidence of infertility among men exposed to electromagnetic waves has shown a gradual increase over recent years. Also, the reproductive value of male subjects working in high voltage electric stations, mobile communication base stations and other electromagnetic environments has significantly decreased [5]. The rapid growth of mobile communication has been accompanied by an increase in radiofrequency (RF) electromagnetic radiation (EMR) [6]. Consequently, public-concerns have been elevated by the possible hazardous health effect of exposure to RF-EMR emitted from mobile telephones. Many studies have suggested that mobile phone use is a risk factor of brain tumors [7]. Although evidence from human studies and animal studies is limited, the International Agency for Research on Cancer (IARC) classifies RF-EMR as “possibly carcinogenic to humans being” [8].
Herbal drugs offer an alternative to the synthetic compounds and have been considered either nontoxic or toxic. This has given driving force to screen for their radio-protective ability. Herbal plants and products represent important source of natural anti-oxidants and they have been used, in several traditional systems of medicine, for thousands of years for treating various ailments (including infertility) all over the world [9]. Figure 1 represents the mobile phone exposure effects on human male fertility pattern by affecting spermatogenesis and hormonal level [10].
Figure 1.
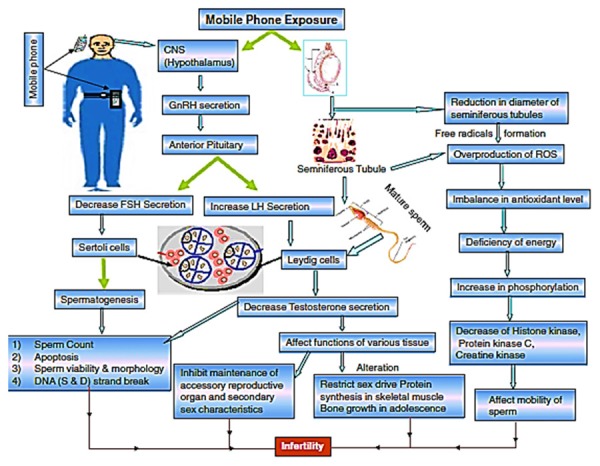
Possible pathways between central nervous system (CNS) and testis for the mechanism of damage caused to spermatozoa by electromagnetic waves (EMW) emitted from cell phones [10].
Moringa oleifera (MO) Lam (Family: Moringaceae) is a highly valued plant in tropical and subtropical countries where it is mostly cultivated. The leaves are highly nutritious, being a good source of β-carotene, riboflavin, nicotinic acid, folic acid, pyridoxine, protein, vitamins A, B, C and E, amino acids, various phenolic compounds and minerals [11]. MO leaves are highly nutritious, being a significant source of sterols, alkaloids, glycosides, phenolic and flavonoids [12]. Almost all the parts of these plants have been used for various ailments in the indigenous medicine of South Asia, including the treatment of inflammation and infectious diseases along with gastrointestinal cardiovascular, hematological and hepatorenal disorders [13]. Moringa Oleifera has anti-inflammatory, anti-tumor, cholesterol lowering, anti-hypertensive, anti-diabetic, anti-oxidant and hepatoprotective effects [14]. Leaves of Moringa Oleifera have been reported to regulate thyroid status and possess radio-protective effect. The plant has also been reported to be hepatoprotective against anti-tubercular drug and acetaminophen [15,16]. Therefore, the objective of this study is to evaluate the possible protective effect of aqueous extract of Moringa Oleifera against testis damage induced by mobile phone electromagnetic radiation in rats.
Materials and methods
The subjects of this research were sixty, two-month-old male Sprague-Dawley rats, weighing 150-180 g. These rats were kept in an air-conditioned room (20-25°C) and subjected to a 12/12 h day light/darkness cycle with free access to food and water. All the ethical protocols and guidelines for animal handling and treatment were followed and supervised by the animal facilities, Faculty of Science, Princess Nourah bint Abdulrahman University in compliance with the national standards published in the Guide for the Care and Use of Laboratory Animals.
The rats were divided into four equal groups; 15 rats each group. The 1st group (the control group) received only standard diet. The 2nd group was supplemented daily and for eight weeks with 200 mg/kg aqueous extract of MO leaves [17]. The 3rd group was exposed to 900 MHz continuous RF/MW fields for one hour every day and for 7 days a week. The 4th group was exposed to mobile phone electromagnetic radiation and received the Moringa Oleifera extract.
All animals in control and experimental groups were housed collectively in polycarbonate cages 30 × 40 × 40 cm (W × L × H). The experimental group was continually exposed to EMR from mobile phone. The radiation of microwave was manufactured by a mobile test phone (model NOKIA 3110; Nokia Mobile Phones Ltd.). A 900 MHz EMR near-field signal for GSM (Mobile communication at 900 MHz for Global System, continuous wave, analog phone) system was used. The mobile phone was located in the cage center, while the distance between mobile phone from the bottom of cage was 3 cm and maximal distance from the cage corners was 28.2 cm [18].
Semen parameters
The semen characteristics such as sperm morphology and sperm count were analyzed using the following procedures: The animals were sacrificed by using CO2 inhalation then the cauda epididymides (both left and right) were taken out. The cauda epididymides were nicked in several places using a scalpel blade in such a way that it extends into but not through the lumen of the duct and blood vessels are avoided. The tubule segment was immersed in 10 mL of PBS buffer facilitating dispersion into the buffer which was maintained at 37ºC. The segment was allowed to disperse for 40 min. After 40 min, 10 µL of each sample was loaded into a clean glass slide and the smear was carried out. Then, it was dipped in methanol and allowed to dry and it proceeded with H&E staining for alive or dead sperms. Sperm morphology was analyzed under light microscopy for abnormalities. As for sperm count, 2 mL of PBS was added to 0.5 mL of sample prepared as above, mixed gently and maintained for 10 minutes at 37°C. 10 µL of prepared sample was taken and loaded in the hemocytometer and the spermatozoa were counted under a light microscope. The final sperm count calculation was done using the following formula:
Total count = (Mean count × dilution factor)/(Voiume of one primary square)
The total count number of sperms/g of epididymis was calculated [19]. In order to determine the mean, all these procedures for sperm count, morphology and motility were repeated for both the control and experimental groups.
Anti-oxidants and oxidative stress
CAT enzyme activity in the blood was measured using the speed reduction of hydrogen peroxidase [20]. Total SOD activity in the plasma samples was determined according to Sun’s method [21]. Blood malondialdehyde (MDA) was determined by the double heating method of Draper and Hadley [22].
Histopathological studies
Preparation of paraffin sections
For histological preparations, animals were sacrificed and left testis was dissected out. It was cut and instantly fixed in 10% neutral buffer formalin for 24 h. After fixation, testes tissues were dehydrated in ascending series of ethyl alcohol 70%, 80%, 90% and 96% for half hour each, then in two changes of absolute ethyl alcohol for half hour each. Tissues were cleared by immersion in xylol for twenty minutes (two changes) then impregnated in paraplast paraffin (three changes) at 60°C for three hours and embedded in paraplast wax. Histological sections 4 µm thick were prepared using the microtome and stained with hematoxylin and eosin [23].
Immunohistochemistry
The selected paraffin blocks for immunohistochemical examination were stained with monoclonal antibody PCNA (LabVision, USA, avidin-biotin peroxidase method). Sections were deparaffinized in xylol and rehydrated with graded alcohols. PCNA antigen retrieval was enhanced using a microwave technique. Sections were covered with in 0.01 M Citrate buffer (pH 6) and microwaved (Gibson’s oven, USA) with full power to boiling, then coMOEd for at least 20 min at room temperature. Endogenous peroxidase effect was suppressed with 10 min. incubation in 3% hydrogen peroxide in phosphate buffer saline (PBS, 10 mM Na2PO4, 140 mM NaCL, pH 7.2). The sections were washed in three changes (2 min each) and started the immunostaining protocol. Immunohistochemical staining was performed using streptavidin-biotin method by Histostainplus kit (Zymed, USA) which contains 10% nonimmune serum, secondary antibody (biotinylated) and streptavidin-peroxidase. Sections were washed with PBS several times and incubated with blocking serum to block the unspecific binding which resulted from electrostatic or hydrophobic interaction between the antibody and tissue contains for 10 min. The removed of excess of blocking serum was done, then the histological sections covered with primary antibody PCNA, in the proper dilution (1:50) for one hour at room temperature. The secondary antibody was used as a second layer for 10 min, after the sections were washed with PBS and streptavidin-peroxidase complex was applied for 10 min. Sections were washed with PBS and the signal of peroxidase was developed in 0.05% diaminobenzidine (DAB) and 0.01% hydrogen peroxide in Phosphate Buffer Saline (PBS). The sections were lightly counter stained in hematoxylin before dehydration and mounting with per-mount. Negative control slides were performed where substitution of the primary antibody by PBS was done with parallel sections. Also slides with normal positive reactivity tissues of the antibody (taken from the pamphlet) were used in the same run. Nuclear stain was seen in brown positive cases of PCNA expression.
Scoring for PCNA expressions: Each section was counted manually at high power (× 400) after identifying at low power (× 100) the representative areas with the highest concentration of stained cells according to the recommendation of Cohen et al. [24]. About 1000 cells/slide were counted in each of five microscopic fields from well-labeled areas to determine the average of PCNA labeling index (PCNA LI). PCNA LI was expressed as the number of labeled cells (positive staining) as a percentage of the total number of cells counted in each specimen. The results are expressed as mean plus or minus standard deviation (PCNA LI = Mean + SD).
Statistical analysis
Data were analyzed using a commercially available statistics software package (SPSS® for Windows, v. 9.0, Chicago, USA). Statistical significance was determined at the level of P < 0.05 using the Student’s t test.
Results
This study investigated the effects of EMR on testes of rats. At the end of the study it was observed that the testicular effect was predominant. The decrease of epididymal sperm counts in the 3rd group and was found to be statistically significant in comparison with the control group (P < 0.001). The results of epididymal sperm counts in all groups are shown in Figure 2. The sperm count was 220.1 + 24.9, 223.5 + 26.1, 80.3 + 18. 3 and 182.7 + 28.3 in control, MOE, EMR and MOE + EMR groups respectively.
Figure 2.
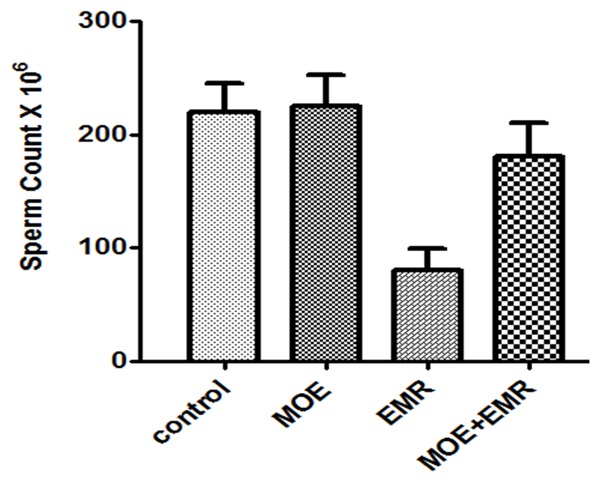
The Spermatocytes counts × 106 in the control and experimental groups.
EMR exposures led to significant increase in the types of abnormal sperm cells observed which were pyriform head, detached head, coiled tail, and multiple abnormalities, where the highest numbers were 3.43 ± 0.89, 4.6 ± 0.59, 14.8 ± 1.99 and 2.99 ± 3.01 respectively in the group exposed to EMR as shown in Table 1. The results of live to death ratio in the smear test showed that the sperm with highest ratio obtained in the control group while the lowest ratio was obtained in the group exposed to EMR. The sperm motility was significantly decreased, whereas the frequency percentage of dead spermatozoa was significantly (P < 0.001) increased in the EMR exposed animals and amelioration was in EMR + MOE group, when compared with the control animals. The results presented in Figure 3 show the comparison between the measured values for gross motility and life to death ratio respectively.
Table 1.
The sperm abnormalities in the control and experimental groups (mean ± SD)
| Groups | Sperm abnormalities% | |||
|---|---|---|---|---|
|
| ||||
| Pyriform head | Detached head | Coiled tail | Multiple | |
| Control | 0.9 ± 0.03 | 0.9 ± 0.35 | 4.50 ± 0.45 | 0.63 ± 0.15 |
| MOE | 0.8 ± 0.02 | 0.7 ± 0.22 | 4.10 ± 0.30 | 0.50 ± 0.09 |
| EMR | 3.43 ± 0.89* | 4.6 ± 0.59* | 14.8 ± 1.99* | 2.99 ± 3.01* |
| EMR + MOE | 1.1 ± 0.32 | 1.3 ± 0.98 | 6.99 ± 1.27* | 1.3 ± 0.88 |
P ≤ 0.05 is considered significant, P > 0.05 is considered non-significant.
Significant.
Figure 3.
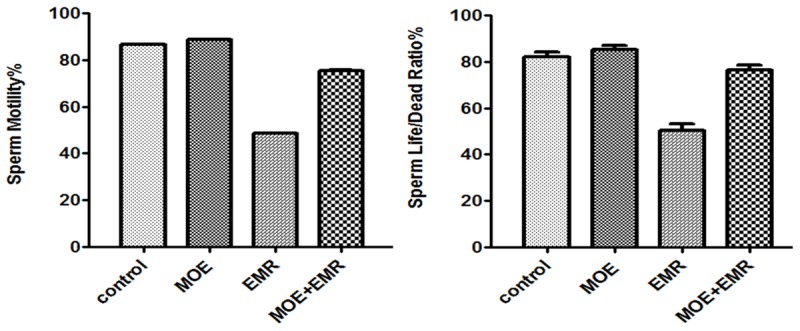
The motility of spermatocytes percentage (Above) and the sperm life/death ratio percentage (Bottom) in the control and experimental groups.
Table 2 shows the effects of EMR exposure for 8 weeks on plasma lipid peroxidation (Malondialdehyde). The results showed a significant increase in lipid peroxidation (MDA, P < 0.003) compared with the control group, where in case of animals which were exposed to EMR and treatment with MOE the results showed decrease in lipid peroxidation (MDA) similar to the control group. The catalase (CAT) and superoxide dismutase (SOD) capacity decreased significantly (P < 0.031 and P < 0.001, respectively) in animals that were exposed to EMR for 8 weeks when compared with control group. Also, there was an improvement of MDA, CAT and SOD in animals treated with MOE when compared with animals in the control group.
Table 2.
The Malondialdehyde, Catalase and Superoxide dismutase concentrations in the control and experimental groups (mean ± SD)
| Animal groups | Oxidative stress | Anti-oxidants | |
|---|---|---|---|
|
| |||
| MDA (nmole/ml) | CAT (IU) | SOD (IU) | |
| Control | 10.5 ± 0.17 | 17.02 ± 0.35 | 11.66 ± 0.17 |
| MOE | 9.1 ± 0.19 | 18.01 ± 0.4 | 12.05 ± 0.14 |
| EMR | 24.9 ± 3.5* | 8.09 ± 0.5* | 8.08 ± 0.16* |
| EMR + MOE | 12.9 ± 1.08 | 13.09 ± 0.3 | 10.34 ± 0.19 |
P ≤ 0.05 is considered significant, P > 0.05 is considered non-significant.
Significant.
Light microscopic results of testis
Examination of hematoxylin & eosin stained sections of control testes (Group I) showed many seminiferous tubules and interstitial tissue in-between containing clusters of Leydig cells with their vesicular nuclei and vacuolated acidophilic cytoplasm. Tubules were surrounded by well-defined basement membrane which was lined by a stratified epithelium consisted of Sertoli cells and spermatogenic cells. Sertoli cells were known from their large ovoid and vesicular nuclei distributed at intervals between spermatogenic cells. Spermatogenic cells included spermatogonia, primary spermatocytes, followed by early spermatids, late spermatids and finally spermatozoa which were seen in the lumen of the tubules (Figure 4A). Also the histopathological of the 2nd group, which received MOE, showed seminiferous tubules lined by spermatogonia resting on basement membrane, followed by early spermatids and finally late spermatids. The lumen of tubules contained spermatozoa. Leydig cells appeared in interstitial tissue near blood vessels in-between tubules. They appeared almost nearly similar to those of the 1st group (Figure 4B).
Figure 4.
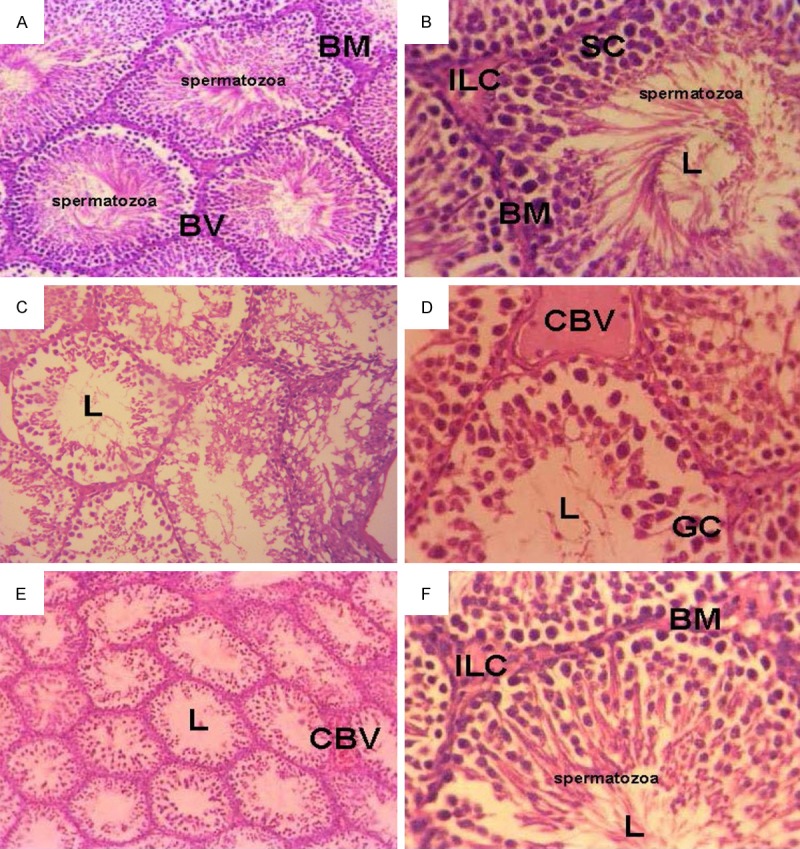
Micrograph of transverse section of testis of (A) control rats showed normal seminiferous tubule. The seminiferous tubule has well-developed basement membrane (BM), blood vessel (BV), numerous sertoli cells, spermatogonium, and spermatocytes in the lumen (L) of the tubule. (H&E × 200). (B) Rats injected with MOE, showed normal arrangement of seminiferous tubules which have numerous sertoli cells (SC), interstial Leydig cells (ILC) and different stages of the spermatocytes (H&E × 400). (C-E) Testes of rats treated with EMR, showed destructed seminiferous tubules associated with degenerated spermatogonic cells, giant cells (GC), congested blood vessel (CBV), absent interstitial leyding cells and free tubule lumen (L) spermatocytes (H&E × 400). (F) Testis of rats treated with EMR and MOE for 8 weeks showed the normal arrangement of seminiferous tubule cells which have numerous cells sertoli spermatogonium, spermatocytes (H&E × 400).
But the histopathological stained sections of the 3rd group (EMR) showed irregular seminiferous tubules. Most of these tubules were depleted from their spermatogenic cells. They were lined by Sertoli cells, few spermatogonia and few primary spermatocytes with dark condensed nuclei. Giant multinucleated cells were seen inside the seminiferous tubules. Also, some degenerated spermatocytes could be detected in the lumen of the seminiferous tubules. The extended interstitial tissue seemed vacuolated with exudation and congested blood vessels (Figure 4C-E). Furthermore, the examination of stained sections of the 4th group (MOE + EMR) showed that the seminiferous tubules were lined by several layers of spermatogenic cells. Sertoli cells and Spermatogonia were seen resting on the basement membrane followed by primary spermatocytes, early and late spermatids nearly similar to the control group (Figure 4F).
PCNA immunohistochemical results
Mitotic activity of germ cells was evaluated by determining proliferating cell nuclear antigen (PCNA) and its labeling index (LI). The positive productions were brown granules located at the nuclear of spermatogonia and part of spermatocytes, where PCNA-positive cells were strongly detected in the spermatogonia and early-stage spermatocytes of the control and MOE treated rats (Figure 5A, 5B respectively). The positive expression of PCNA in seminiferous epithelium in experimental rats significantly decreased after EMR exposure (Figure 5C), the positive cells in every seminiferous in which only spermatogenous cells were stained were 58.9 ± 4.5, it was significant compared with that of the control animals’ group (82.1 ± 5.6) (P < 0.05). The percentage of PCNA-positive cells in regenerated tubules was markedly increased in case of EMR exposure animals treated with MOE group (Figure 5D). The PCNA labeling indices were shown in Table 3.
Figure 5.
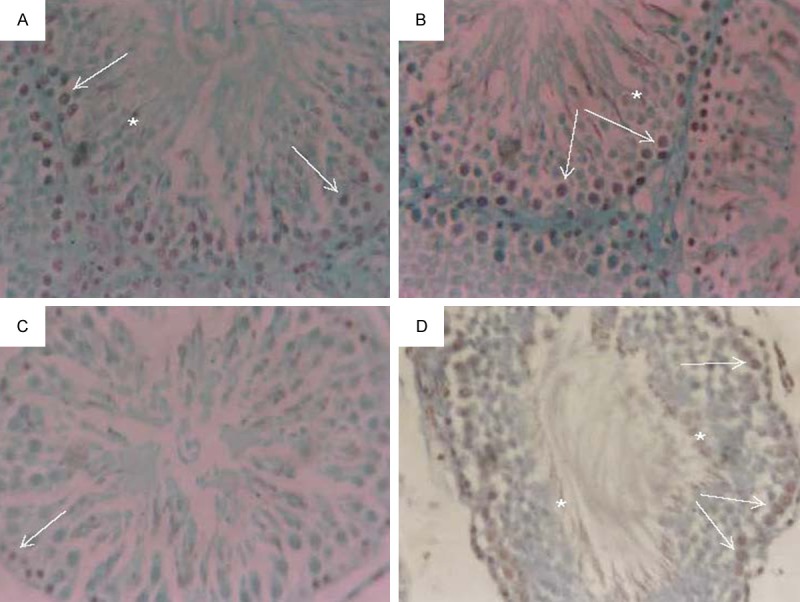
Micrograph of immunohistochemical analysis of paraffin-embedded testis using PCNA showing brown nuclear staining (A) control, (B) MOE injected animal group, (C) EMR exposure animal group and (D) EMR exposure animal treated with MOE group. Positive staining in spermatogonia (white arrows) and early-stage spermatocytes (*). (immunoperoxidase × 250).
Table 3.
The PCNA labeling index (LI) in the control and experimental groups (mean ± SD)
| Groups | PCNA LI (%) | P value |
|---|---|---|
| Control | 82.1 ± 5.6 | -- |
| MOE | 83.5 ± 6.1 | -- |
| EMR | 58.9 ± 4.5 | 0.011 |
| EMR + MOE | 68.9 ± 4.7 | 0.043 |
P ≤ 0.05 is considered significant, P > 0.05 is considered non-significant.
Discussion
Most men of reproductive age in high or middle-income countries own mobile phones. Along with this increase in ownership of mobile phone, there is egoism over the vital effects of mobile phone exposure on human health. Mobile telephones emit electromagnetic radiation (EMR), a low-level radiofrequency (RF), at a frequency of between 800 and 2200 MHz [25], which the human body can absorb. Mobile phones are legally limited to a specific absorption rate (SAR) of 2.0 W/kg [26], and currently, most have a SAR of ~ 1.4 W/kg [25]. At this low frequency of EMR is not likely to ionize atoms or molecules [27]. Mobile phones and other electromagnetic devices that emit RF-EMR radiation are detrimental to human fertility [28].
Moringa oleifera micronutrients contain anti-tumor, anti-epileptic, anti-diuretic, anti-inflammatory and venomous bite characters. Moringa Oleifera extract (MOE) contains specific plant pigments with demonstrated powerful anti-oxidative ability such as vitamins C, E, A, caffeoylquinic acids, carotenoids-lutein, alpha-carotene and beta carotene, kaempferol, quercetin, rutin [29,30]. In the present study, MOE was used as anti-electromagnetic radiation induced infertility in rats.
Results of the present study showed that long-term use of GSM mobile phone can cause hypospermatogenesis and maturation arrest in spermatozoa in the testes of rats compared to their matched control. At high intensities, RF radiation has heating properties leading to thermal effects, increase in tissue or body temperature on exposure to EMR can cause reversible disruption of spermatogenesis [31]. Wang et al. [32] suggested that Leydig cells are among the most susceptible cells to EMR and injury to these cells may affect the spermatogenesis and its parameters. As Leydig cells produce spermatozoa and if the Leydig cells are damaged, they ultimately affect the spermatogenesis. Ozguner et al. [33] demonstrated a decrease in seminiferous tubular diameter and epithelium thickness after applying a radio-frequency generator of 869-894 MHz. Also, Salama et al. [34] conducted a study on the effects of exposure to a mobile phone radiation on the testicular function and structure in adult rabbit. They reported a fall in the sperm concentration appeared in the phone group in the 6th week and it became statistically significant with increased duration of exposure from week 6 to week 8 compared with the control group. Moreover, there was a more significant decline in the motile, viability and increase in abnormalities sperm population which were in agreement with current study.
The results of the present study are in line with all these findings. Agarwal et al. [35] suggested that the use of cell phones is associated with a decrease in semen parameters. The decrease in the sperm viability, sperm count, motility and the morphology of sperms were related to the duration of exposure to cell phones. Similarly, in the present animal model study, hypospermatogenesis and maturation arrest in the spermatozoa were found in rats exposed to EMR.
The remarkable reduction in the activities of CAT and SOD concomitantly occurred in rats exposed to EMR (group 3) indicated that those animals suffered from oxidative stress. Ahmadpoor et al. [36] announce that oxidative stress occurred as a consequence of imbalance between body anti-oxidant capacity and reactive oxygen species (ROS). Thus, this Phenomenon could happen as a result of increased radical oxygen species (ROS) generation, impaired anti-oxidant defense system or a combination of both. SOD and CAT are enzymes naturally developed by the mammalian body as endogenous anti-oxidant system to deal with the intermediates of the reactive oxygen. SOD breakdowns the radicals of superoxide anion to H2O2 and H2O [37]. CAT catalyses the decomposition of H2O2 to H2O and oxygen [38]. The present study found obvious increase in the activities of SOD and CAT accompanied with a decrease in lipid peroxidation (MDA) as identified by an adduction of thiobarbituric acid reactive substances (TBARS) in the group 4. All these findings suggest that MOE is a potent anti-oxidant content.
Histological examination showed a decrease in the diameter of seminiferous tubules in the phone group compared to the control group. The findings of this study are compatible with Saunders and Kowalczuk’s [39,40] findings, which indicate that due to the primer effect of microwave, heat, damages the testicular tissue. Also, reduction in the amount of Leydig cells can clarify the damaging effect of the magnetic field in Leydig cells. The findings indicate that EMR caused a serious testicular degeneration. Furthermore, the current study has demonstrated that EMR-induced damage of the testis tissue was ameliorated by the aqueous extract of leaf MO. This effect is attributed to its anti-oxidant activity and free radical scavenging activity.
PCNA expressed in spermatogonia and early-phase primary spermatocytes in all stages of the seminiferous tubules [41]. The decrease of PCNA in testicular germ cells indicates the reduction of proliferative activity and spermatogenesis. In the present study, PCNA-positive cells were strongly detected in spermatogonia and early-stage spermatocytes of control rats. The reduction in these cells is attributed to the fact that the differentiation of spermatogonia is an especially vulnerable step in the spermatogenic process. However, the signal density of positive cells was significantly lower in the EMR exposure group than in the control animals’ group. Also, a rise in the expression of PCNA in testis tissues was in the MOE-treated EMR group. Futhermore, in the EMR exposed rats, the PCNA index was decreased [42]. This observation is in agreement with Weiss and Landauer study [43]. As the MOE is rich with anti-oxidants, these anti-oxidants may interfere with the initial mediation of apoptosis by ROS [44], as well as later membrane lipid peroxidation, which is a characteristic of radiation-induced apoptosis [45]. Central issues involve whether radiation-induced apoptosis can be promoted by some anti-oxidants in tumors but not in normal tissue, and when it is useful to protect normal cells apoptosis against radiation. Protection against damaged cells apoptosis is also relevant to chemoprevention strategies in populations exposed to environmental radiation, as well as in radiotherapy planning [43].
Conclusion
This study indicates that EMR attenuated-related testicular dysfunction and histopathological change. MOE renewed the activities of the anti-oxidant enzymes, downregulated the levels of ROS and increased PCNA activity in the testis of EMR exposed. MO consequently improved EMR-induced oxidative DNA damage and apoptosis in the testes of rats. The long-term exposure to mobile phone radiation can cause hypospermatogenesis and maturation arrest of spermatozoa in the testes of rats. However, Moringa Oleifera leaf extract had a protective effect against the infertility-induced by EMR which was manifested by the improvement in sperm parameters of rats treated with EMR and Moringa Oleifera leaf extract.
Acknowledgements
The authors thanks Deanship of Scientific Research, Princess Nourah bint Abdulrahman University, Saudi Arabia, for the financial support for complete this study.
Disclosure of conflict of interest
None.
References
- 1.Sharma P, Parmar J, Verma P, Goyal P. Radiation induced oxidative stress and its toxicity in testes of mice and their prevention by Tinospora cordifolia extract. Journal of Reproductive Health and Medicine. 2015 [Google Scholar]
- 2.Pacey AA. Male fertility and infertility. Obstetrics, Gynaecology & Reproductive Medicine. 2009;19:42–7. [Google Scholar]
- 3.Aitken R, De Iuliis G. On the possible origins of DNA damage in human spermatozoa. Mol Hum Reprod. 2010;16:3–13. doi: 10.1093/molehr/gap059. [DOI] [PubMed] [Google Scholar]
- 4.Zhou JX, Ding GR, Zhang J, Zhou YC, Zhang YJ, Guo GZ. Detrimental effect of electromagnetic pulse exposure on permeability of in vitro blood-brain-barrier model. Biomed Environ Sci. 2013;26:128–37. doi: 10.3967/0895-3988.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Jauchem JR. Effects of low-level radio-frequency (3kHz to 300GHz) energy on human cardiovascular, reproductive, immune, and other systems: a review of the recent literature. Int J Hyg Environ Health. 2008;211:1–29. doi: 10.1016/j.ijheh.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Merhi ZO. Challenging cell phone impact on reproduction: a review. J Assist Reprod Genet. 2012;29:293–7. doi: 10.1007/s10815-012-9722-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kundi M. The controversy about a possible relationship between mobile phone use and cancer. Cien Saude Colet. 2010;15:2415–30. doi: 10.1590/s1413-81232010000500016. [DOI] [PubMed] [Google Scholar]
- 8.Baan R, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Islami F, Galichet L, Straif K WHO International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of radiofrequency electromagnetic fields. Lancet Oncol. 2011;12:624–6. doi: 10.1016/s1470-2045(11)70147-4. [DOI] [PubMed] [Google Scholar]
- 9.Upadhyay AK, Kumar K, Kumar A, Mishra HS. Tinospora cordifolia (Willd. ) Hook. f. and Thoms. (Guduchi)-validation of the Ayurvedic pharm acology through experimental and clinical studies. Int J Ayurveda Res. 2010;1:112–21. doi: 10.4103/0974-7788.64405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kesari KK, Kumar S, Nirala J, Siddiqui MH, Behari J. Biophysical evaluation of radiofrequency electromagnetic field effects on male reproductive pattern. Cell Biochem Biophys. 2013;65:85–96. doi: 10.1007/s12013-012-9414-6. [DOI] [PubMed] [Google Scholar]
- 11.Khalafalla MM, Abdellatef E, Dafalla HM, Nassrallah AA, Aboul-Enein KM, Lightfoot DA, et al. Active principle from Moringa oleifera Lam leaves effective against two leukemias and a hepatocarcinoma. Afr J Biotechnol. 2010;9:8467–71. [Google Scholar]
- 12.Anwar F, Latif S, Ashraf M, Gilani AH. Moringa oleifera: a food plant with multiple medicinal uses. Phytother Res. 2007;21:17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- 13.Verma AR, Vijayakumar M, Mathela CS, Rao CV. In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem Toxicol. 2009;47:2196–201. doi: 10.1016/j.fct.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Tiloke C, Phulukdaree A, Chuturgoon AA. The antiproliferative effect of Moringa oleifera crude aqueous leaf extract on cancerous human alveolar epithelial cells. BMC Complement Altern Med. 2013;13:226. doi: 10.1186/1472-6882-13-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fakurazi S, Hairuszah I, Nanthini U. Moringa oleifera Lam prevents acetaminophen induced liver injury through restoration of glutathione level. Food Chem Toxicol. 2008;46:2611–5. doi: 10.1016/j.fct.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Mansour HH, El Azeem MGA, Ismael NE. Protective Effect of Moringa oleifera on γ-Radiation-Induced Hepatotoxicity and Nephrotoxicity in Rats. American Journal of Phytomedicine and Clinical Therapeutics. 2014;2:495–508. [Google Scholar]
- 17.Jaiswal D, Rai PK, Mehta S, Chatterji S, Shukla S, Rai DK, Sharma G, Sharma B, Khair S, Watal G. Role of Moringa oleifera in regulation of diabetes-induced oxidative stress. Asian Pac J Trop Med. 2013;6:426–32. doi: 10.1016/S1995-7645(13)60068-1. [DOI] [PubMed] [Google Scholar]
- 18.El-Bediwi AB, Saad M, El-Kott AF, Eid E. Influence of electromagnetic radiation produced by mobile phone on some biophysical blood properties in rats. Cell Biochem Biophys. 2013;65:297–300. doi: 10.1007/s12013-012-9432-4. [DOI] [PubMed] [Google Scholar]
- 19.Prabu P, Biravi K, Jarisa S, Sreepriya S, Panchapakesan S. Biochemical, Pathomorphological and Semen Characteristics Analysis in Male Wistar Rats Treated with Withania somnifera Root Extract. Asian J Chem. 2014;26:3700–4. [Google Scholar]
- 20.Bergmeyer HU. Methods of enzymatic analysis. Elsevier; 2012. [Google Scholar]
- 21.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 22.Draper H, Hadley M. Malondialdehyde determination as index of lipid Peroxidation. Methods Enzymol. 1990;186:421–31. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 23.An YH, Moreira PL, Kang QK, Gruber HE. Handbook of Histology Methods for Bone and Cartilage. Springer; 2003. Principles of embedding and common protocols; pp. 185–97. [Google Scholar]
- 24.Cohen MB, Waldman FM, Carroll PR, Kerschmann R, Chew K, Mayall BH. Comparison of five histopathologic methods to assess cellular proliferation in transitional cell carcinoma of the urinary bladder. Hum Pathol. 1993;24:772–8. doi: 10.1016/0046-8177(93)90015-9. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal A, Singh A, Hamada A, Kesari K. Cell phones and male infertility: a review of recent innovations in technology and consequences. Int Braz J Urol. 2011;37:432–54. doi: 10.1590/s1677-55382011000400002. [DOI] [PubMed] [Google Scholar]
- 26.International Commission on Non-Ionizing Radiation Protection. Guidelines for limiting exposure to time-varying electric and magnetic fields (1 Hz to 100 kHz) Health Phys. 2010;99:818–36. doi: 10.1097/HP.0b013e3181f06c86. [DOI] [PubMed] [Google Scholar]
- 27.Erogul O, Oztas E, Yildirim I, Kir T, Aydur E, Komesli G, Irkilata HC, Irmak MK, Peker AF. Effects of electromagnetic radiation from a cellular phone on human sperm motility: an in vitro study. Arch Med Res. 2006;37:840–3. doi: 10.1016/j.arcmed.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Vignera S, Condorelli RA, Vicari E, D’Agata R, Calogero AE. Effects of the exposure to mobile phones on male reproduction: a review of the literature. J Androl. 2012;33:350–6. doi: 10.2164/jandrol.111.014373. [DOI] [PubMed] [Google Scholar]
- 29.Akunna GG, Ogunmodede OS, Saalu CL, Ogunlade B, Bello AJ, Salawu EO. Ameliorative effect of Moringa oleifera (drumstick) leaf extracts on chromium-induced testicular toxicity in rat testes. World J Life Sci Med Res. 2012;2:20. [Google Scholar]
- 30.Maida A, Farooq A, Raziya N, Umer R, Kazi T, Nadeem M. Mineral composition of Moringa oleifera leaves and pods from different regions of Punjab, Pakistan. Asian Journal of Plant Sciences. 2005 [Google Scholar]
- 31.Jung A, Schill W. [Male infertility. Current life style could be responsible for infertility] . MMW Fortschr Med. 2000;142:31–3. [PubMed] [Google Scholar]
- 32.Wang SM, Wang DW, Peng RY, Gao YB, Yang Y, Hu WH, Chen HY, Zhang YR, Gao Y. [Effect of electromagnetic pulse irradiation on structure and function of Leydig cells in mice] . Zhonghua Nan Ke Xue. 2003;9:327–30. [PubMed] [Google Scholar]
- 33.Ozguner M, Koyu A, Cesur G, Ural M, Ozguner F, Gokcimen A, Delibas N. Biological and morphological effects on the reproductive organ of rats after exposure to electromagnetic field. Saudi Med J. 2005;26:405–10. [PubMed] [Google Scholar]
- 34.Salama N, Kishimoto T, Kanayama Ho. Retracted: Effects of exposure to a mobile phone on testicular function and structure in adult rabbit. Int J Androl. 2010;33:88–94. doi: 10.1111/j.1365-2605.2008.00940.x. [DOI] [PubMed] [Google Scholar]
- 35.Agarwal A, Deepinder F, Sharma RK, Ranga G, Li J. Effect of cell phone usage on semen analysis in men attending infertility clinic: an observational study. Fertil Steril. 2008;89:124–8. doi: 10.1016/j.fertnstert.2007.01.166. [DOI] [PubMed] [Google Scholar]
- 36.Ahmadpoor P, Eftekhar E, Nourooz-Zadeh J, Servat H, Makhdoomi K, Ghafari A. Glutathione, glutathione-related enzymes, and total antioxidant capacity in patients on maintenance dialysis. Iran J Kidney Dis. 2009;3:22–7. [PubMed] [Google Scholar]
- 37.Lawler JM, Song W. Specificity of antioxidant enzyme inhibition in skeletal muscle to reactive nitrogen species donors. Biochem Biophys Res Commun. 2002;294:1093–100. doi: 10.1016/S0006-291X(02)00602-2. [DOI] [PubMed] [Google Scholar]
- 38.Marzook EA, El Moneim AEA, Elhadary AA. Protective role of sesame oil against mobile base station-induced oxidative stress. Journal of Radiation Research and Applied Sciences. 2014;7:1–6. [Google Scholar]
- 39.Saunders R, Kowalczuk C. Effects of 2.45 GHz Microwave Radiation and Heat on Mouse Spermatogenic Epithelium. Int J Radiat Biol Relat Stud Phys Chem Med. 1981;40:623–32. doi: 10.1080/09553008114551611. [DOI] [PubMed] [Google Scholar]
- 40.Saunders R, Kowalczuk C. The Effect of Acute Far Field Exposure at 2. 45 GHz on the Mouse Testis. Int J Radiat Biol Relat Stud Phys Chem Med. 1981;39:587–96. doi: 10.1080/09553008114550711. [DOI] [PubMed] [Google Scholar]
- 41.Kang MJ, Kim MK, Terhune A, Park JK, Kim YH, Koh GY. Cytoplasmic localization of cyclin D3 in seminiferous tubules during testicular development. Exp Cell Res. 1997;234:27–36. doi: 10.1006/excr.1997.3590. [DOI] [PubMed] [Google Scholar]
- 42.Li HY, Zhang H, Miao GY, Yi X, Chao S, Di CX, Liu Y, Liu YY, Zhang X, Ma XF, Xu S, Gan L, Zhou X. Simulated microgravity conditions and carbon ion irradiation induce spermatogenic cell apoptosis and sperm DNA damage. Biomed Environ Sci. 2013;26:726–34. doi: 10.3967/0895-3988.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003;189:1–20. doi: 10.1016/s0300-483x(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 44.Salganik RI. The benefits and hazards of antioxidants: controlling apoptosis and other protective mechanisms in cancer patients and the human population. J Am Coll Nutr. 2001;20:464S–72S. doi: 10.1080/07315724.2001.10719185. [DOI] [PubMed] [Google Scholar]
- 45.McCLAIN DE, Kalinich JF, Ramakrishnan N. Trolox inhibits apoptosis in irradiated MOLT-4 lymphocytes. FASEB J. 1995;9:1345–54. doi: 10.1096/fasebj.9.13.7557025. [DOI] [PubMed] [Google Scholar]


