Abstract
Objective: To investigate the potential therapeutic effects of adenovirus expressing IFN-λ1 and IFN-λ2 (Ad/hIFN-λ) in treating squamous cell carcinoma of the oral tongue (SCCOT) and to explore the underlying mechanisms. Methods: Two SCCOT cell lines HSC-3 and Tca8113 were adopted as study objects. Cell Counting Kit-8 (CCK-8) cell proliferation and viability assay was performed to evaluate the antiproliferative effects of Ad/hIFN-λ and IFN-λ treatments at different dosages. Flow cytometry (FCM) was performed to investigate the apoptosis rate induced by Ad/hIFN-λ. In vivo study was performed through evaluating tumorigenicity and tumor volume on BALB/c nu/nu mice inoculated with HSC-3 cells with or without infection of Ad/hIFN-λ. qPCR was used to screen important apoptosis related genes expression and western blot (WB) was performed to verify the results. WB was also used to test the phosphorylation of STATs protein in the JAK/STAT signaling pathways. Results: Our results indicated an obvious antiproliferative effect of Ad/hIFN-λ in vitro on infected HSC-3 and Tca8113 cells. The antiproliferative effects started to appear at 48 h (day 2) after infection. IFN-λs alone treating HSC-3 and Tca8113 cells also showed a dose-dependent inhibitory manner. Though the antiproliferative effects did not show on 24 h (day 1), early apoptosis rate already increased significantly in cells infected with Ad/hIFN-λ (P<0.05) detected by FCM. The underlying mechanisms of antiproliferative activity rely on the IFN-λ signaling by phosphorylation of STATs protein. Expression of Bax, Bcl-2 and Caspase-3 were promoted by Ad/hIFN-λ leading to higher apoptosis rate. Upper stream of p21 and Rb dephosphorylation explained the Caspase-3 activation. Animal study showed that HSC-3 cells infected with Ad/hIFN-λ significantly promoted the survival rate and decreased mean tumor volume comparing to HSC-3 cells group. Conclusion: Ad/hIFN-λ injection had obvious antiproliferative effects on HSC-3 and Tca8113 cells. Ad/hIFN-λ induced apoptosis in SCCOT cells through increasing Bcl-2, Bax and Caspase-3 expression. Ad/hIFN-λ is a potential therapeutic strategy in treating oral tongue carcinoma.
Keywords: Adenovirus, IFN-λ, oral tongue carcinoma, JAK/STAT, apoptosis
Introduction
Interferons (IFNs) are crucial cytokines secreted from host cells in response to various pathological conditions like viruses, bacteria or tumor. Typically, IFNs are classified into three distinct types: Type I, Type II, and Type III. The recognition is mainly based on the type of receptors, and also distinct biological features and structures. The essential role of type I IFNs is their antiviral activity in almost all cell types upon viral infection [1]. In contrast, type II IFNs expression is restricted to immune cells like NK cells and T cells stimulating cell-mediated immune responses [1]. All IFNs share common effects in resisting viral infection and modulating immune system yet each subtype of IFNs has its own particular feature.
Interferon-λs (IFN-λs) are new members of the old INF family. Belonging to type III IFN, three subtypes of IFN-λs were identified named IFN-λ1 (IL-29), IFN-λ2 (IL-28A), and IFN-λ3 (IL-28B) [2-4]. Different from the receptors of type I and type II IFNs, IFN-λs interact and signal through receptor composed of IL-10R2 (CRF2-4) and IFN-λR1 (CRF2-12) [5]. Based on their structure, IFN-λs are more like the IL-10 family, which is also the reason of their sharing receptors with IL-10, IL-22 and IL-26 [3,6]. Since its discovery, IFN-λs were found to have antiproliferative actions and antitumor activities [6-8]
Squamous cell carcinoma of the oral tongue (SCCOT) is one type of head and neck squamous cell carcinomas and remains one of the top 10 leading cancers in the US [9]. Despite the largely improved surgical and medical management, patients with recurrences remain the big difficulty in treating this disease. Besides, the side effect of chemotherapy and radiotherapy is another thorny problem during the treatment of SCCOT. In this regard, novel therapies are urgently required to improve the prognosis and patients’ quality of life. Gene therapies with adenoviruses (Ad) are potential therapeutic strategies in treating malignant tumor. Recombinant INF-α has already been clinically tested and for its therapeutic potentiality in treating various malignancies [10]. The recently discovered IFN-λs showed excellent antitumor activities, researchers have already noticed its potential application in treating malignancies [11-13]. Based on the remarkable antitumor effects of IFN-λs, yet no research has been done of its role in SCCOT. It was proposed that IFN-λs could play important parts in SCCOT.
In this study, we investigated the transduction efficacy of our Ad vector Ad/hIFN-λ1 and Ad/hIFN-λ2 with human SCCOT cell lines (HSC-3 and Tca 8113). The antitumor and antiproliferative activities of IFN-λ were tested from both in vitro and in vivo perspectives. Both vectors were shown suppressive in the growth of SCCOT cells and also tumor volume revealing its possible clinical value.
Materials and methods
Cell lines
Oral squamous carcinoma cell line of human (HSC-3), tongue squamous cell carcinoma cell line (Tca8113) and human embryonic kidney 293 cells (HEK293) were purchased from Shanghai Type Culture Collection (Shanghai, China).
Animals
6 to 8 week old male BALB/c nude mice were purchased from Shanghai Experimental Animal Center (Shanghai, China). Experimental procedures were approved and performed according to guidelines of laboratory animal care and use. All efforts were made to reduce the number of animals tested and their suffering.
Adenovirus vector generation and infection
HSC-3 and Tca8113 cells were infected with Ad/hIFN-λ1 and Ad/hIFN-λ2 (Genechem life technologies, Shanghai) for stable expressing of IFN-λ1 and IFN-λ2. HEK293 cells were used to test infection efficiency. Infected cells were selected in medium containing 1 mg/mL puromycin after three rounds of infection (6 hours each) in the presence of polybrene (Sigma-Aldrich).
CCK-8 cell proliferation and viability assay
HSC-3 and Tca8113 cells were seeded (2×103 per well) into 96-well plates and were cultured overnight. Culture medium was removed the next day and fresh medium was added together with Ad/hIFN-λ (Ad/hIFN-λ1 and Ad/hIFN-λ2) infection. Cell proliferation and viability were evaluated on day 1, 3 and 5 by Cell Counting Kit-8 (CCK8, Dojindo, Japan) reagent according to the manufacturers’ instructions. The absorbency of cells was measured using a 96-well plate reader at 450 nm.
Flow cytometry cell apoptosis assay
Ad/hIFN-λ induced apoptosis of HSC-3 and Tca8113 cells was detected by FCM with Annexin V-FITC Apoptosis Detection Kit (KeyGEN) following to the manufacturer’s instructions. The apoptosis rate was assayed by using FACSCalibur Flow Cytometry (BD, USA) at 488 nm.
Enzyme-linked immunosorbent assay (ELISA)
Levels of murine IFN-λ1 and IFN-λ2 in culture supernatants were assayed by ELISA according to the manufacturers’ instructions (R&D Systems). OD values were measured in an ELISA plate reader at a wavelength of 450 nm.
qPCR
Total RNA was isolated using Trizol reagent (Life Technologies). Reverse transcriptase and oligo’dT primer were used to prepare cDNA from 1 μg of RNA according the manufacturer’s instructions (Takara, Japan). Two microlitres of each cDNA was then used for PCR amplification using primers for Bax, Bcl-2, Bcl-6, Caspase-3, Caspase-9 and Caspase-8. The detailed information of primers were shown in Table 1.
Table 1.
Primer sequences for qPCR
| primers | Forward | Reverse | Tm (°C) |
|---|---|---|---|
| Bax | 5’-GCGGCATTACCAACAT-3’ | 5’-CTGGAAGCACCAACGA-3’ | 59 |
| Bcl-2 | 5’-ACCCGAAGCGGACATT-3’ | 5’-GGCATCTCCCTGAACG-3’ | 61 |
| Bcl-6 | 5’-AGATGGAGATTTCTGATGGTCCTC-3’ | 5’-CTTGCTTAGTTTCTTGTCTGGTGG-3’ | 62 |
| Caspase-3 | 5’-TACCCACCTCAGACAACAGCACC-3’ | 5’-ATCCCCAATCAGAAAACCAGCAC-3’ | 60 |
| Caspase-8 | 5’-AGCAAAGAAGACAGGGAG-3’ | 5’-CAGCGTCAAACAAAGG-3’ | 62 |
| Caspase-9 | 5’-CGGTCAAAGGAGGTAG-3’ | 5’-GGAGCCATAGTCCAATA-3’ | 61 |
| β-actin | 5’-TCCCTGTATGCCTCTG-3’ | 5’-ATGTCACGCACGATTT-3’ | 61 |
Western blot
Cells were lysed in prepared buffer containing 10 mM Tris, pH 7.2, 150 mM NaCl, 5 mM EDTA, 0.1% SDS, 0.5% Triton X-100, and 1% deoxycholic acid. For Western blots, 30 μg of protein samples were subjected to SDS-PAGE followed by transfer onto PVDF membranes. After blocking in 5% BSA in PBS, membranes were incubated with antibodies against Bcl-2 (1:1000), Caspase-3 (1:1000), Bax (1:1000), STAT1 (1:1000), STAT2 (1:1000), STAT3 (1:1000), p-STAT1 (1:1000), p-STAT2 (1:1000), p-STAT3 (1:1000) p21 (1:1000), p-Rb (1:1000) and β-actin (1:1000) overnight at 4°C followed by 1 h-incubation with secondary antibody (1:2000). Blots against β-actin served as loading control.
Animal experiments and survival analysis
HSC-cells (1×106), Tca8113 cells (1×106) and mixed HSC-cells and Tca8113 cells (1×106) with or without Ad/hIFN-λ (Ad/hIFN-λ1 and Ad/hIFN-λ2) infection were injected subcutaneously into BALB/c nude mice (8-week-old). Tumor volume growth curve were figured up according to formula (V=a2 · b · 0.52). Tumor inhibition rates were figured up in reference with previous study: tumor inhibition rate = (average tumor volume in CT group minus average tumor volume in Ad/hIFN-λ group)/mean tumor volume in CT group · 100% [13]. Mice were transfected with Ad/hIFN-λ (Ad/hIFN-λ1 and Ad/hIFN-λ2) when tumors size reached 10-15 mm in diameter. In situ injections were performed with 200 μL PBS as blank group, Ad-null as control group and Ad/hIFN-λ1, Ad/hIFN-λ2 with 3×108 pfu. Tumor size was measured at days 0, day 7, day 14 and day 21. Animals were sacrificed after 21 days for tumor dissection.
Statistical analysis
All data were analyzed by SPSS (ver. 13.0) software and the results were showed by mean ± SD. Student’s t-test and two-way analysis of variance (ANOVA) were used to assess statistical significance, with P≤0.05 being regarded as significant.
Results
IFN-λ expression in human SCCOT cell lines after delivery of Ad/hIFN-λ
HSC-3, Tca 8113 and HEK293 cells were infected with Ad/hIFN-λ (Ad/hIFN-λ1 and Ad/hIFN-λ2) at multiplicity of infection (MOI) of 3 or 30 for 60 min. Cells were then cultured for 3 days followed by analysis of GFP-positive cells percentage by FCM. From the results we can see that HSC-3 cells showed slightly better transduction efficacy without statistical significance (Figure 1A, 1B). Secretions of IFN-λ1 and IFN-λ2 of the two cell types were examined through ELISA. Consistent with the results of FCM, secretion of IFN-λ1 and IFN-λ2 were higher in infected HSC-3 cells still with on significance (Figure 1C). It is worthy to be noticed that IFN-λ2 secreted from both cells were much more than IFN-λ1. This result was consistent with a previous study [11], yet the underlying mechanism is still unknown.
Figure 1.
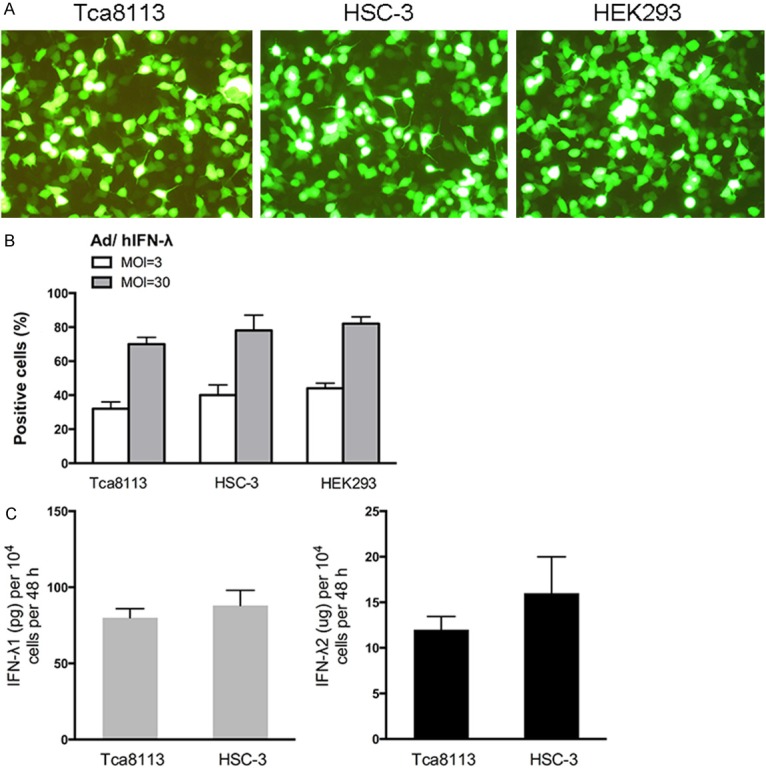
IFN-λ expression in human SCCOT cell lines after delivery of Ad/hIFN-λ. A. Representative images of Ad/hIFN-λ (GFP labeled) infection in Tca8113 cells, HSC-3 cells and HEK293 cells. B. Flow cytometrical analysis of GFP positive Tca8113 cells, HSC-3 cells and HEK293 cells with different MOIs. C. Secreted amounts of IFN-λ1 and IFN-λ2 from cells infected with Ad/hIFN-λ1 or Ad/hIFN-λ2 at 1000 MOI were tested with an ELISA. (n=3).
Antiproliferative effects of Ad/hIFN-λ infection
We next tested the effects of IFN-λ and Ad/h IFN-λ (Ad/hIFN-λ1 and Ad/hIFN-λ2) on proliferation and cell viability of SCCOT cells. IFN-λ1 and IFN-λ2 alone were first administered to HSC-3 and Tca 8113 cells. IFN-λ was induced to the culture media of HSC-3 and Tca 8113 cells respectively with proper concentrations. At 24 h after IFN-λ induction, the antiproliferative effects of IFN-λ was still not obvious (Figure 2A, 2C). The results showed that IFN-λ started to have significant antiproliferative activity at 48h especially when the concentration reached to 100 ng/mL (P<0.01) (Figure 2B, 2D). We then infected HSC-3 and Tca 8113 cells with constructed Ad/hIFN-λ (Ad/hIFN-λ1 and Ad/hIFN-λ2). Cell number was counted at day 0, 1, 3 and 5 to evaluate the total trend. Results revealed that both Ad/hIFN-λ1 and Ad/hIFN-λ2 have strong antiproliferative effects on the two SCCOT cell lines (Figure 2E, 2F). The effect started to become obvious on day 3.
Figure 2.
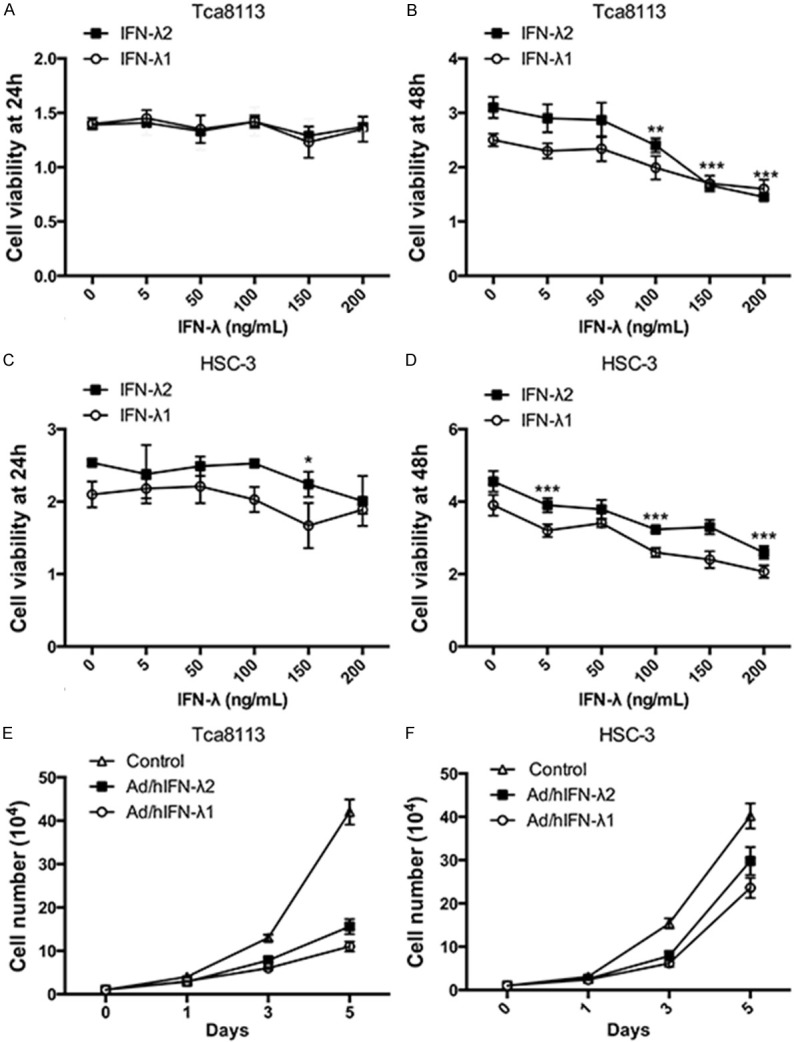
Antiproliferative effects of Ad/hIFN-λ infection. A. Cell viability of Tca8113 cells after treatments of IFN-λ1 and IFN-λ2 at different dosages (0, 5 ng/mL, 50 ng/mL, 100 ng/mL, 150 ng/mL, 200 ng/mL) for 24 h was examined. B. Cell viability of Tca8113 cells after treatments of IFN-λ1 and IFN-λ2 at different dosages for 48 h was examined. C. Cell viability of HSC-3 cells after treatments of IFN-λ1 and IFN-λ2 at different dosages for 24 h was examined. D. Cell viability of HSC-3 cells after treatments of IFN-λ1 and IFN-λ2 at different dosages for 48 h was examined. E. Tca8113 cells were treated with Ad/hIFN-λ1 and Ad/hIFN-λ2 and cultured for 5 days. Live cells were counted at day 1, 3 and 5. F. HSC-3 cells were treated with Ad/hIFN-λ1 and Ad/hIFN-λ2 and cultured for 5 days. Live cells were counted at day 1, 3 and 5. Data in the figures represent average ± SD. (n=3) *P<0.05, **P<0.01, ***P<0.001 based on one way ANOVA.
Apoptosis induction by Ad/hIFN-λ infection
We further investigated the effects of Ad/hIFN-λ (Ad/hIFN-λ1 and Ad/hIFN-λ2) infection on apoptosis induction of SCCOT cells in vitro. HSC-3 and Tca8113 cells were infected with Ad/hIFN-λ1 infection, Ad/hIFN-λ2 infection or Ad/hIFN-λ infection. Cells were incubated for 24 h before the obvious antiproliferative effects occurred as previously described. At day 1 (24 h), FCM was performed to evaluated the early and late apoptosis rate of each cell (Figure 3A). The results showed that both Ad/hIFN-λ1 and Ad/hIFN-λ2 infection significantly increased the early cell apoptosis rate (P<0.001). Cells infected with both Ad/hIFN-λ1 and Ad/hIFN-λ2 showed the highest early apoptosis rate compare to single infection (Figure 3B). As for late apoptosis rate, although the trend was not as obvious as that of the early apoptosis rate, it was also clear that Ad/hIFN-λ infection increased late apoptosis rate (P<0.05). On the molecular level, qPCR was first performed for screening of several important apoptosis related genes (Figure 3C). The results indicated that Caspase-3, Bax and Bcl-2 were significantly overexpressed by Ad/hIFN-λ infection, while Caspase-8, Caspase-9 and Bcl-6 expression were not affected. We then performed western blot to verify the results on protein level. The results remained consistent with our qPCR results (Figure 3D).
Figure 3.
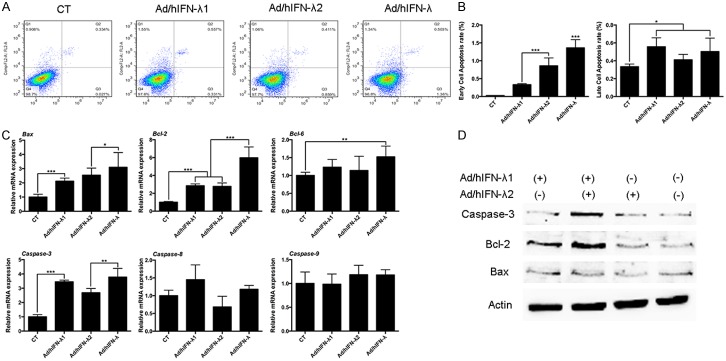
Apoptosis induction by Ad/hIFN-λ infection. A. FCM analysis of early and late cell apoptosis infected with Ad/hIFN-λ1, Ad/hIFN-λ2 or both. B. Analysis of early and late cell apoptosis rate. C. Relative mRNA expression levels of Bax, Bcl-2, Bcl-6, Caspase-3, Caspase-8 and Caspase-9 in cells infected with Ad/hIFN-λ1, Ad/hIFN-λ2 or both. D. Representative western blot images of Bcl-2, Bax, Caspase-3 and β-actin in cells infected with Ad/hIFN-λ1, Ad/hIFN-λ2 or both. Data in the figures represent average ± SD. (n=3) *P<0.05, **P<0.01, ***P<0.001 based on one way ANOVA.
Antitumor effects of Ad/hIFN-λ infection in vivo
We then explored the potential antitumor effects of Ad/hIFN-λ (Ad/hIFN-λ1 and Ad/hIFN-λ2) in vivo by evaluating tumorigenicity of HSC-3 cells with or without Ad/hIFN-λ (Ad/hIFN-λ1 and Ad/hIFN-λ2) infection. Cells were inoculated subcutaneously in nude mice and observed for the following 50 days. The results revealed that most of the mice injected with Ad/hIFN-λ infected HSC-3 cells remained alive until no mice survived in the HSC-3 injection group (Figure 4A). It is worthy to notice that HSC-3 cells infected with both Ad/hIFN-λ1 and Ad/hIFN-λ2 gave the highest survival rate among the four groups. Tumors were not even developed in the Ad/hIFN-λ group. Tumor volume was evaluated after the animals were sacrificed. Consistent with the survival study, tumor growth was significantly retarded in virus infection group compare to HSC-3 group. HSC-3 cells infected with Ad/hIFN-λ (Ad/hIFN-λ1 and Ad/hIFN-λ2) showed smallest volume (Figure 4B).
Figure 4.
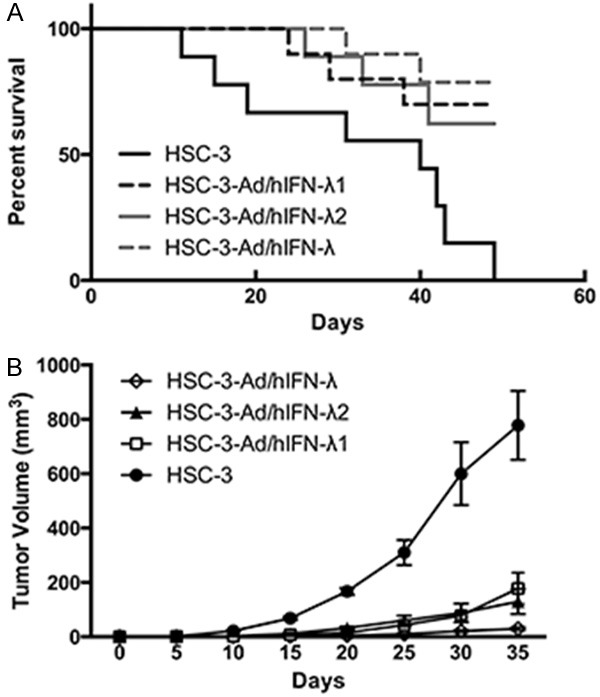
Antitumor effects of Ad/hIFN-λ infection in vivo. Uninfected HSC-3 cells, Ad/hIFN-λ1 infected HSC-3 cells, Ad/hIFN-λ2 infected cells, Ad/hIFN-λ (Ad/hIFN-λ1 and Ad/hIFN-λ2) infected HSC-3 cells were injected subcutaneous into BALB/c nude mice (n=10). A. Percentage of survival mice. B. Average tumor volumes in each group. Data in the figures represent average ± SD.
Mechanisms of Ad/hIFN-λ antitumor effects in vitro
Previous results indicated the obvious antiproliferative, apoptosis inductive and antitumor acitivity of Ad/hIFN-λ (Ad/hIFN-λ1 and Ad/hIFN-λ2). We further explored the underlying mechanism in vitro trying to reveal the antiproliferative mechanism. We investigated the well-studied JAK/STAT signaling activated by IFN-λ and particularly focused on the phosphorylation of STAT1, STAT2 and STAT3. The results showed that phosphorylation of the STATs occurred early after infection (20 min) and almost disappeared 2 hours later (Figure 5A). Among all the three STATs, STAT1 showed the most obvious change. We proved that Ad/hIFN-λ1 induce apoptosis through up regulating Bcl-2, Bax and Caspase-3. To further explore the underlying mechanism, upper stream p-Rb and p21 expression were tested through western blot. We found that phosphorylation of Rb was inhibited while expression of p21 was increased (Figure 5B). This result explained the following change of caspase-3 activity.
Figure 5.
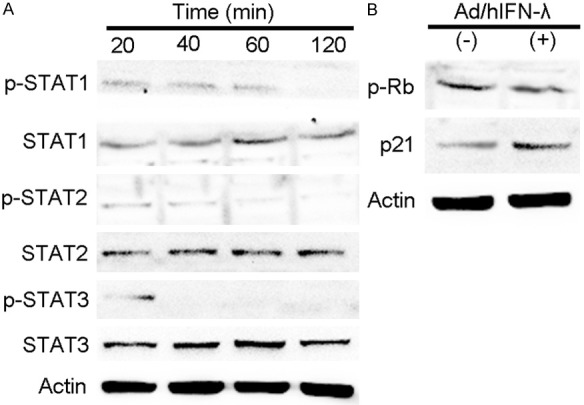
Mechanisms of Ad/hIFN-λ antitumor effects in vitro. HSC-3 cells were infected with Ad/hIFN-λ (Ad/hIFN-λ1 and Ad/hIFN-λ2) in previous. A. Representative western blot images of STAT1, pSTAT1, STAT2, pSTAT2, STAT3 pSTAT3, and β-actin. B. Representative western blot images of pRb, p21 and β-actin.
Discussion
In our study, we focused on the antitumor effect of IFN-λ in vitro and in vivo through constructing Ad/hIFN-λ (Ad/hIFN-λ1 and Ad/hIFN-λ2). Infection of Ad/hIFN-λ (Ad/hIFN-λ1 and Ad/hIFN-λ2) into SCCOT cell lines HSC-3 and Tca8113 cells in vitro showed remarkable antiproliferative and apoptosis inductive activity. In vivo study of tumorigenecity and survival evaluation verified our assumption that Ad/hIFN-λ has antitumor effects. Compare to other IFNs, IFN-λs have more biological functions and are considered to be one of the most potential therapeutic strategies in treating cancer [6]. The antitumor effects of IFN-λs in vivo have been reported yet far from well learned [14]. More and more studies emerged regarding the potential antitumor effects of IFN-λs [8,15,16]. One group recently reported the epigenetic reprogramming of the type III interferon treatment in tumor suggesting that histone deacetylase (HDAC) inhibitor could improve IFN-λs sensitivity through elevating the expression of its receptors [17]. However, the detailed underlying mechanism of its antiproliferative, antitumor and apoptosis inductive activity remains largely unknown. In this regard, we constructed Ad/hIFN-λ (Ad/hIFN-λ1 and Ad/hIFN-λ2) to study its influence on SCCOT cells and its possible application in treating SCCOT. As far as we know, this is the first study considering IFN-λ effects on SCCOT cells both in vitro and in vivo.
The present data showed that Ad/hIFN-λ (Ad/hIFN-λ1 and Ad/hIFN-λ2) had strong antiproliferative effects on HSC-3 and Tca8113 cells. CCK-8 cell viability assay indicated the toxicity of both IFN-λ and Ad/hIFN-λ and showed that the toxicity effects occurred at a time point of 48 h (day 2) after treatment. Since type III IFNs shares many similarities with type I IFNs both functionally, structurally and receptor-oriented. Both of the two types of IFNs activate the JAK/STAT pathway [3]. The signaling via their receptors results in the tyrosine phosphorylation of STAT1, STAT2, STAT3 and STAT5 [7]. Activation of the JAK/STAT pathway is closely related to its effects of antiviral and antiproliferative activity. Previous studies already showed this clear relationship between activation of the JAK/STAT pathway and its antiproliferative effects [18,19]. In our study, we also showed that Ad/hIFN-λ increased phosphorylation of STAT1, STAT2 and STAT3. It is interesting to notice that one group believed that the JAK/STAT pathway is not enough to maintain the antiproliferative activity especially in IFN-resistant carcinoma cell lines, which is melanoma in their case [20]. Taking this into consideration, we also focused on the apoptosis induced by Ad/hIFN-λ.
Previous studies already confirmed that antitumor effect of type III IFNs in murine models [14,21]. One of the most important potency is the apoptosis induction activity of IFN-λs. Li Q et al. reported that IFN-λs induce cell arrest on G1 phase and apoptosis [22]. Similar to our study, they discovered that the G1 phase arrest occurred together with the up-regulation of p21 and also dephosphorylation of retinoblastoma (Rb). Apoptosis was then occurred symbolized by cleavage of caspase-3 and PARP [22]. Among the apoptosis related genes we tested (Figure 3C), Bcl2, Bax and Caspase-3 were found to have significant fold change alteration. Slightly different with the research results from Li W’s group, we screened only caspase-3 elevation and no caspase-9 and caspase-8 change was detected. In Li W et al’s report, Caspase-3, caspase-9 and caspase-8 were both activated [23].
The antitumor activity IFN-λs is a more complex behavior than merely modulating specific cellular activities like proliferation and apoptosis. IFN-λs functions in a synergic manner with NK cells and other immune cells like B cells and macrophages. Q Li et al. exhibited that IFN-λs could activate NK cells with obvious over expression of DX5 and CD69 [11]. IFN-λ treatments can also activate NK cells manifested as IFN-γ release. A very recent study showed that macrophages produce IL-12 under IFN-λs induction thus inducing IFN-γ in NK cells [24]. Angiogenesis is a crucial event in tumor growth and metastasis. Another study recently reported that type III IFNs signaling also function in Myeloid-derived suppressor cells (MDSCs) mediated angiogenesis [25]. The possible mechanism lies on the stimulation of STAT3 and the following subsequent angiogenesis factors induction. Further studies still need to be down evaluating the angiogenesis effects of IFN-λs together with antitumor activity.
In conclusion, this study for the first time revealed the antitumor effects of Ad/hIFN-λ (Ad/hIFN-λ1 and Ad/hIFN-λ2) in treating SCCOT. We presented that Ad/hIFN-λ is potential in its clinical application towards treating oral squamous carcinoma.
Disclosure of conflict of interest
None.
References
- 1.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 2.Donnelly RP, Kotenko SV. Interferon-lambda: a new addition to an old family. J Interferon Cytokine Res. 2010;30:555–564. doi: 10.1089/jir.2010.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 4.Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J, Ostrander C, Dong D, Shin J, Presnell S, Fox B, Haldeman B, Cooper E, Taft D, Gilbert T, Grant FJ, Tackett M, Krivan W, McKnight G, Clegg C, Foster D, Klucher KM. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4:63–68. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]
- 5.Lasfar A, Abushahba W, Balan M, Cohen-Solal KA. Interferon lambda: a new sword in cancer immunotherapy. Clin Dev Immunol. 2011;2011:349575. doi: 10.1155/2011/349575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steen HC, Gamero AM. Interferon-lambda as a potential therapeutic agent in cancer treatment. J Interferon Cytokine Res. 2010;30:597–602. doi: 10.1089/jir.2010.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumoutier L, Tounsi A, Michiels T, Sommereyns C, Kotenko SV, Renauld JC. Role of the interleukin (IL)-28 receptor tyrosine residues for antiviral and antiproliferative activity of IL-29/interferon-lambda 1: similarities with type I interferon signaling. J Biol Chem. 2004;279:32269–32274. doi: 10.1074/jbc.M404789200. [DOI] [PubMed] [Google Scholar]
- 8.Numasaki M, Tagawa M, Iwata F, Suzuki T, Nakamura A, Okada M, Iwakura Y, Aiba S, Yamaya M. IL-28 elicits antitumor responses against murine fibrosarcoma. J Immunol. 2007;178:5086–5098. doi: 10.4049/jimmunol.178.8.5086. [DOI] [PubMed] [Google Scholar]
- 9.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 10.Parmar S, Platanias LC. Interferons: mechanisms of action and clinical applications. Curr Opin Oncol. 2003;15:431–439. doi: 10.1097/00001622-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Li Q, Kawamura K, Okamoto S, Fujie H, Numasaki M, Namba M, Nagata M, Shimada H, Kobayashi H, Tagawa M. Adenovirusesmediated transduction of human oesophageal carcinoma cells with the interferon-lambda genes produced anti-tumour effects. Br J Cancer. 2011;105:1302–1312. doi: 10.1038/bjc.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Gao Q, Yuan X, Zhou M, Peng X, Liu X, Zheng X, Xu D, Li M. Adenovirus expressing IFN-lambda1 (IL-29) attenuates allergic airway inflammation and airway hyperreactivity in experimental asthma. Int Immunopharmacol. 2014;21:156–162. doi: 10.1016/j.intimp.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Yan Y, Zhang J, Liu Y, Zhu T, Yuan L, Ge Y, Ding H, Bu X. Inhibition of lung adenocarcinoma transfected with interleukin 28A recombinant adenovirus (Ad-mIFN-lambda2) in vivo. Cancer Biother Radiopharm. 2013;28:124–130. doi: 10.1089/cbr.2012.1247. [DOI] [PubMed] [Google Scholar]
- 14.Sato A, Ohtsuki M, Hata M, Kobayashi E, Murakami T. Antitumor activity of IFN-lambda in murine tumor models. J Immunol. 2006;176:7686–7694. doi: 10.4049/jimmunol.176.12.7686. [DOI] [PubMed] [Google Scholar]
- 15.Swider A, Siegel R, Eskdale J, Gallagher G. Regulation of interferon lambda-1 (IFNL1/IFNlambda1/ IL-29) expression in human colon epithelial cells. Cytokine. 2014;65:17–23. doi: 10.1016/j.cyto.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Wongthida P, Diaz RM, Galivo F, Kottke T, Thompson J, Pulido J, Pavelko K, Pease L, Melcher A, Vile R. Type III IFN interleukin-28 mediates the antitumor efficacy of oncolytic virus VSV in immune-competent mouse models of cancer. Cancer Res. 2010;70:4539–4549. doi: 10.1158/0008-5472.CAN-09-4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding S, Khoury-Hanold W, Iwasaki A, Robek MD. Epigenetic reprogramming of the type III interferon response potentiates antiviral activity and suppresses tumor growth. PLoS Biol. 2014;12:e1001758. doi: 10.1371/journal.pbio.1001758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamshou M, Van Damme EJ, Vandenborre G, Ghesquiere B, Trooskens G, Gevaert K, Smagghe G. GalNAc/Gal-binding Rhizoctonia solani agglutinin has antiproliferative activity in Drosophila melanogaster S2 cells via MAPK and JAK/STAT signaling. PLoS One. 2012;7:e33680. doi: 10.1371/journal.pone.0033680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thoennissen NH, Iwanski GB, Doan NB, Okamoto R, Lin P, Abbassi S, Song JH, Yin D, Toh M, Xie WD, Said JW, Koeffler HP. Cucurbitacin B induces apoptosis by inhibition of the JAK/STAT pathway and potentiates antiproliferative effects of gemcitabine on pancreatic cancer cells. Cancer Res. 2009;69:5876–5884. doi: 10.1158/0008-5472.CAN-09-0536. [DOI] [PubMed] [Google Scholar]
- 20.Jackson DP, Watling D, Rogers NC, Banks RE, Kerr IM, Selby PJ, Patel PM. The JAK/STAT pathway is not sufficient to sustain the antiproliferative response in an interferon-resistant human melanoma cell line. Melanoma Res. 2003;13:219–229. doi: 10.1097/00008390-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Abushahba W, Balan M, Castaneda I, Yuan Y, Reuhl K, Raveche E, de la Torre A, Lasfar A, Kotenko SV. Antitumor activity of type I and type III interferons in BNL hepatoma model. Cancer Immunol Immunother. 2010;59:1059–1071. doi: 10.1007/s00262-010-0831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Q, Kawamura K, Ma G, Iwata F, Numasaki M, Suzuki N, Shimada H, Tagawa M. Interferonlambda induces G1 phase arrest or apoptosis in oesophageal carcinoma cells and produces anti-tumour effects in combination with anticancer agents. Eur J Cancer. 2010;46:180–190. doi: 10.1016/j.ejca.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Li W, Lewis-Antes A, Huang J, Balan M, Kotenko SV. Regulation of apoptosis by type III interferons. Cell Prolif. 2008;41:960–979. doi: 10.1111/j.1365-2184.2008.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Groen RA, Boltjes A, Hou J, Liu BS, McPhee F, Friborg J, Janssen HL, Boonstra A. IFNlambda-mediated IL-12 production in macrophages induces IFN-gamma production in human NK cells. Eur J Immunol. 2015;45:250–259. doi: 10.1002/eji.201444903. [DOI] [PubMed] [Google Scholar]
- 25.Mucha J, Majchrzak K, Taciak B, Hellmen E, Krol M. MDSCs mediate angiogenesis and predispose canine mammary tumor cells for metastasis via IL-28/IL-28RA (IFN-lambda) signaling. PLoS One. 2014;9:e103249. doi: 10.1371/journal.pone.0103249. [DOI] [PMC free article] [PubMed] [Google Scholar]


