Abstract
Purpose: To figure out the association between plasma Hcy status and type 1 diabetes mellitus (T1DM). Methods: We searched the PubMed Web of Science, and The Cochrane Library to identify eligible studies. The Newcastle-Ottawa Quality Assessment Scale was used to assess the quality of selected studies. All analyses were performed using the STATA, version 12 software. Results: 15 studies were included in this investigation. Our meta-analysis indicated that plasma Hcy concentrations in T1DM patients without any complications were normal compared with healthy people [13 studies, SMD: -0.08, 95% confidence interval (CI): -0.44 to 0.28, P=0.67]. However, a significant elevation of plasma Hcy concentrations was observed in T1DM patients with only diabetic retinopathy (DR) (5 studies, SMD: 0.34, 95% CI: 0.13 to 0.55, P=0.002), only diabetic nephropathy (DN) (4 studies, SMD: 0.76, 95% CI: 0.18 to 1.33, P=0.01) and both the two complications (3 studies, SMD: 1.05, 95% CI: 0.03 to 2.07, P=0.043) compared with T1DM patients without any complications. Conclusions: Homocysteine levels elevate in T1DM patients with DR and DN, but don’t elevate in T1DM without any complications.
Keywords: Homocysteine, type 1 diabetes mellitus, diabetic nephropathy, diabetic retinopathy, meta-analysis
Introduction
Type 1 diabetes mellitus (T1DM) is an autoimmune disease manifesting as the destruction of pancreatic β-cells and the onset of hyperglycemia [1], resulting from a complex interaction between host genetics, immune system and environmental factors [2]. Diabetes-related microvascular complications, such as nephropathy, retinopathy are life-threatening complications in patients with T1DM [3]. Diabetic nephropathy (DN) is characterized by persistent albuminuria, elevation of arterial blood pressure and a decline in glomerular filtration rate (GFR) [4]. Diabetic retinopathy (DR) is the fourth most common cause of vision loss in adults [5], the main changes encompass thickening of the basement membrane, loss of pericytes and proliferation of mesangium [6].
Homocysteine (Hcy), a sulfhydryl-containing amino acid, has been reported to be elevated in patients with type 2 diabetes mellitus (T2DM) and its vascular complication [7,8]. However, plasma Hcy levels have been found to be lower [9,10], normal [11,12] or higher [13,14] in patients with T1DM compared with healthy subjects in conflicting studies. Moreover, most previous cross-sectional studies in patients with T1DM indicated positive associations between Hcy and DN [15,16], but not for DR [17,18], although some did [15,19].
Considering all those conflicting reports, meta-analysis may be an appropriate way to summarize current available data to provide more robust evidences than the individual study.
Materials and methods
Search strategy
All the studies that investigated plasma Hcy status and the association between Hcy and the risk of vascular complications in patients with T1DM were considered in this meta-analysis. A comprehensive literature search was performed for original studies published up to June, 2015 using PubMed, Web of Science databases and The Cochrane Library. No restriction was imposed on search language. The search terms used were as follows: “type 1 diabetes”, “T1DM”, “diabetic retinopathy”, “diabetic nephropathy”, “homocysteine” and “Hcy”. References of retrieved articles were also reviewed for fear of neglecting additional published reports not included in PubMed, Web of Science databases and The Cochrane Library.
Study selection
First screening was based on titles and abstracts in searching studies, any studies lacking information regarding plasma Hcy levels in patients with T1DM was rejected. Editorials, abstracts, and review articles were also excluded. Then, second screening was based on the full texts of interested studies. Inclusion criteria for study selection were as follows: 1) cross-sectional, case-control, prospective or cohort study; 2) case: T1DM without any complications, T1DM with DR or DN, T1DM with both DR and DN; 3) control: healthy people; 4) data of interest (plasma Hcy concentration) in both controls and cases presented as continuous (mean value and SD).
Quality scale
The results of quality assessment using the Newcastle-Ottawa Quality Assessment Scale were in Table 1. The quality scores of included studies ranged from 5 to 7 (low quality: 1-3, median quality: 4-6, high quality: 7-9).
Table 1.
Characteristics of studies included in this meta-analysis
| Control | Case 1 | Case 2 | Case 3 | Case 4 | Quality score | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||||||||||
| Study | Year | Country | n | Age (Y) | Hcy (μM) | n | Age (Y) | Hcy (μM) | n | Age (Y) | Hcy (μM) | n | Age (Y) | Hcy (μM) | n | Age (Y) | Hcy (μM) | |
| Hultberg et al | 1991 | Sweden | 46 | 52 | 11.0±3.4 | 25 | 45 | 10.7±4.3 | 18 | 40.6 | 12.1±5.5 | No | No | No | 12 | No | 20.1±4.5 | 5 |
| Agardh et al | 1994 | Sweden | No | No | No | 9 | 34.6 | 7.6±2.0 | No | No | No | 10 | 37 | 8.0±1.6 | 15 | No | 9.8±3.9 | 5 |
| Targher et al | 2000 | Italy | 30 | 33 | 10.3±2.2 | 60 | 32 | 12.5±4.8 | No | No | No | No | No | No | No | No | No | 6 |
| Vaccaro et al | 2000 | Italy | 44 | 44.2 | 9.3±3.6 | 27 | 43.3 | 6.9±3.1 | 5 | 40.7 | 8.8±2.6 | 9 | 47 | 8.9±2.7 | No | No | No | 6 |
| Mutus et al | 2001 | Italy | 34 | 46 | 9.4±2.0 | 28 | 38 | 9.9±1.8 | No | No | No | No | No | No | No | No | No | 5 |
| Abdel et al | 2001 | Egypt | 15 | 13.21 | 11.10±2.56 | 15 | 13.7 | 20.10±3.24 | No | No | No | 15 | 15.7 | 30.16±8.05 | No | No | No | 6 |
| Saeed et al | 2003 | England | 28 | 11.9 | 6.6±1.7 | 16 | 13.4 | 5.7±2.1 | No | No | No | No | No | No | No | No | No | 5 |
| García-Unzueta et al | 2005 | Spain | 64 | 32 | 6.9±2.4 | 117 | No | 5.7±2.1 | 24 | No | 5.7±1.4 | No | No | No | 14 | No | 6.7±3.4 | 6 |
| Atabek et al | 2006 | Turkey | 27 | 10.9 | 5.7±2.2 | 27 | 11.3 | 5.6±2.9 | No | No | No | No | No | No | No | No | No | 5 |
| Janickova et al | 2007 | Czech | 13 | 30.8 | 7.61±3.71 | 13 | 25.8 | 8.29±3.71 | No | No | No | 20 | 34.2 | 10.39±4.43 | No | No | No | 6 |
| Jehlicka et al | 2009 | Czech | 30 | 15.1 | 8.6±3.86 | 30 | 14.6 | 5.42±1.9 | No | No | No | No | No | No | No | No | No | 6 |
| Harrington et al | 2010 | Australia | 32 | 14.2 | 9.0±2.6 | 66 | 14.1 | 7.0±2.4 | No | No | No | No | No | No | No | No | No | 6 |
| Giannattasio et al | 2010 | Italy | 123 | 14.2 | 8.3±2.5 | 41 | 20 | 7.3±2.7 | No | No | No | No | No | No | No | No | No | 5 |
| Babar et al | 2011 | American | 15 | 7.6 | 3.9±1.55 | 21 | 8.3 | 4.3±1.37 | No | No | No | No | No | No | No | No | No | 6 |
| Bulum et al | 2014 | Croatia | No | No | No | 85 | 42 | 9.3±2.3 | 78 | 49 | 10.4±2.9 | No | No | No | No | No | No | 7 |
No = undescribed, Control = healthy people, Case 1 = type 1 diabetes without any complications, Case 2 = type 1 diabetic retinopathy, Case 3 = type 1 diabetic nephropathy with microabuminuria, Case 4 = type 1 diabetic retinopathy and nephropathy, Hcy = homocysteine mean ± SD (μM).
Data extraction
The data elements of interest were extracted by two investigators from each study independently and another senior researcher reviewed all items for completeness and accuracy. Information was recorded as follows: first author’s surname, year of publication, subjects’ country, participant number, definition and characteristics of cases and controls, plasma Hcy concentration in all groups.
Statistical analyses
We used standard mean deviation (SMD) as effect measure to assess the differences in Hcy status among healthy people, T1DM patients without vascular complications, T1DM patients with DR/DN and T1DM patients with both DR and DN. Heterogeneity of SMDs was quantified using the I-square (I2) value. I2>50% was considered to represent significant heterogeneity [20]. Given with heterogeneity, SMDs were calculated using random-effects model. If there was no heterogeneity, fixed-effects model was applied. Potential publication bias was assessed by Begg’s and Egger’s test. All analyses were performed using STATA 12.0 (Stata Corp, College Station, TX, USA).
Results
Literature search
We initially retrieved 196 articles from PubMed, Web of Science databases and The Cochrane Library. After duplicates removed, 78 articles were excluded by reviewing titles and abstracts, mainly because they were reviews/editorials or irrelevant to topics. Then 39 full-text articles were excluded because of some detail reasons showed in Figure 1. Finally, 15 studies [15,21-34] were included in our meta-analysis.
Figure 1.
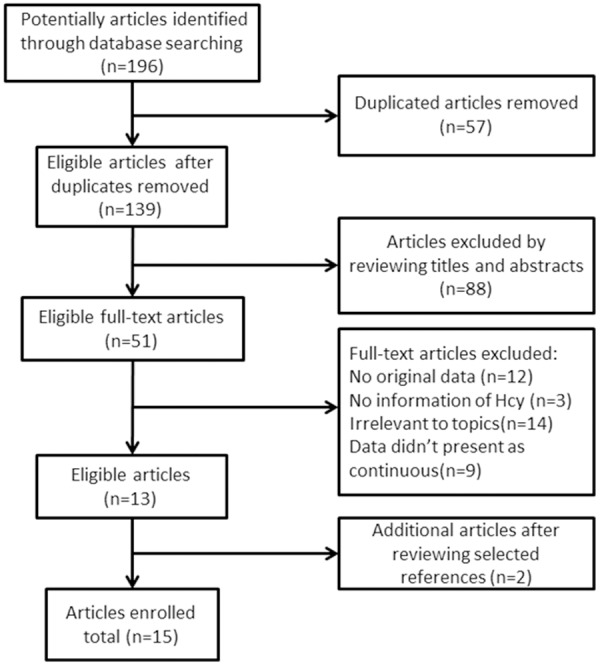
Search strategy for meta-analysis.
Study characteristics
The characteristics of the 15 enrolled studies are shown in Table 1. There were 12 case-control studies [15,21-23,26-30,32-34], 2 prospective studies [24,29] and 1 post hoc analysis study [31]. The mean age of T1DM patients ranged from 8.3 to 45 years which were generally matched in healthy controls and other cases. The sizes of studies ranged from 34 to 219.
Meta-analysis of plasma Hcy levels
Firstly, we compared plasma Hcy concentrations between healthy controls and T1DM patients without any complications and found that plasma Hcy levels in T1DM patients without any complications were similar to that in healthy controls [13 studies, SMD: -0.08, 95% confidence interval (CI): -0.44 to 0.28, P=0.67, as shown in Figure 2]. Significant heterogeneity was observed among studies (I2=84.9%, P=0). Begg’s test (P=0.127) and Egger’s test (P=0.045) indicated the existence of publication bias.
The sources of heterogeneity were then investigated by meta-regression, which showed that the significant heterogeneity could not be explained by the factors such as publication year, studies’ region, mean age of healthy controls and T1DM patients without any complications, study designs and Hcy detection method-high performance liquid chromatography (HPLC) or not. Duration of T1DM was not included in meta-regression due to lack of information in some studies.
6 studies [23,24,27,29-31] were excluded by using sensitivity analysis because they were appearing to be outliers with others. Among these, there were one small-sample studies [29], one study [30] with T1DM patients significantly younger than healthy controls. After exclusion, a meta-analysis of other 7 studies indicated that the main results remained unchanged, a significant elevation or reduction of plasma Hcy levels was not observed in T1DM patients without any complication compared with healthy controls (SMD: -0.23, CI: -0.48 to 0.02, P=0.066). There was no significant heterogeneity among studies (I2=33.8%, P=0.17). No evidence of publication bias was noted (Begg, P=0.133; Egger, P=0.222).
Plasma Hcy levels were higher in T1DM patients with only DR than in T1DM patients without any complications (5 studies, SMD: 0.34, 95% CI: 0.13 to 0.55, P=0.002, as shown in Figure 3). No significant heterogeneity was observed among studies (I2=0%, P=0.442). No evidence of publication bias was noted (Begg, P=1.000; Egger, P=0.770). T1DM patients with only DN demonstrated significantly higher levels of plasma Hcy than T1DM patients without any complications (4 studies, SMD: 0.76, 95% CI: 0.18 to 1.33, P=0.01, as shown in Figure 4). Significant heterogeneity was observed among studies (I2=51.5%, P=0.103). Omission of any single study didn’t significantly influence the overall SMD. No evidence of publication bias was noted (Begg, P=1.000; Egger, P=0.887). Also, plasma Hcy levels were significantly higher in T1DM patients with both DR and DN than in T1DM patients without any complications (3 studies, SMD: 1.05, 95% CI: 0.03 to 2.07, P=0.043, as shown in Figure 5). Significant heterogeneity was observed among studies (I2=82.1%, P=0.004). Omission of any single study didn’t significantly influence the overall SMD. No evidence of publication bias was noted (Begg, P=0.296; Egger, P=0.512).
Figure 3.
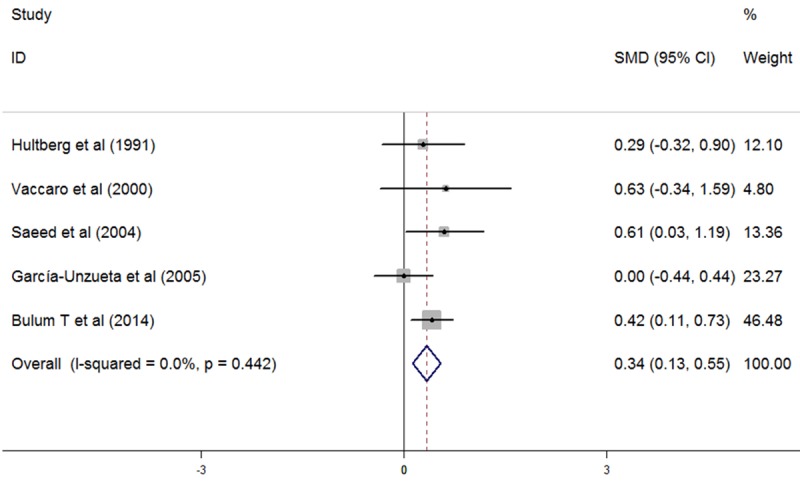
Comparasion of plasma homocysteine level between T1DM patients with only DR and T1DM patients without any complications.
Figure 4.
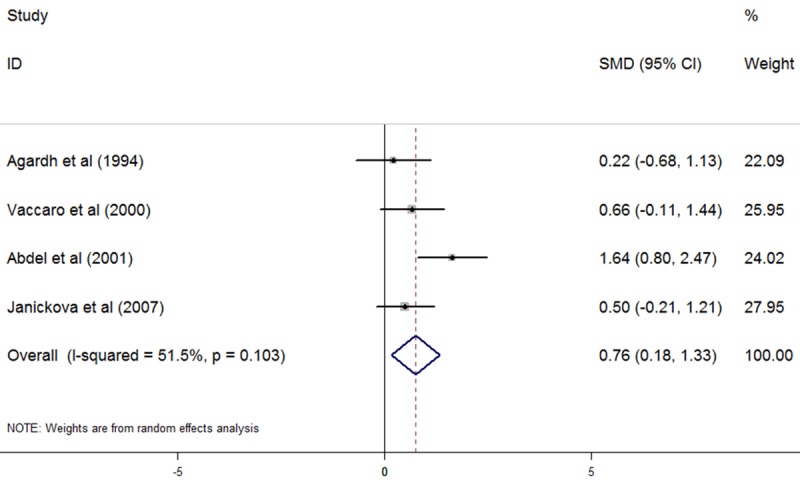
Comparasion of plasma homocysteine level between T1DM patients with only DN and T1DM patients without any complications.
Figure 5.
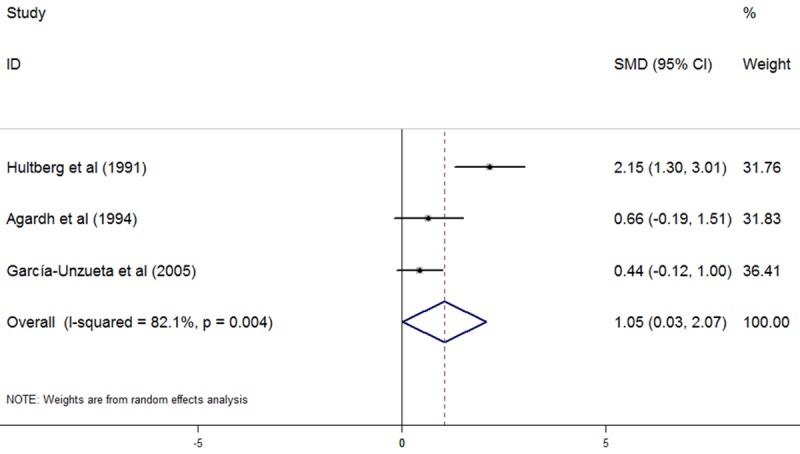
Comparasion of plasma homocysteine level between T1DM patients with both DR and DN and T1DM patients without any complications.
Figure 2.
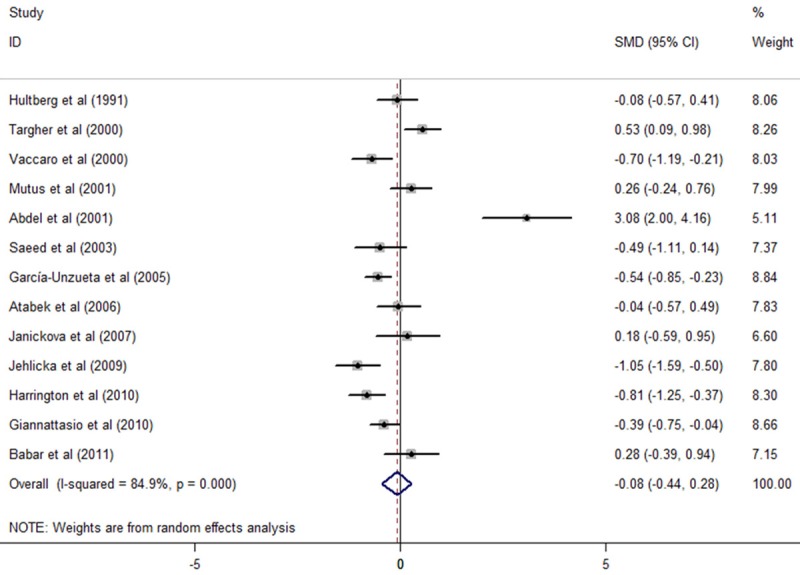
Comparasion of plasma homocysteine level between T1DM patients without any complications and healthy people.
Discussion
The association between Hcy and T1DM has been paid increasing attention. Our meta-analysis indicated that plasma Hcy concentrations in T1DM patients without any complications were normal compared with healthy people. Nevertheless, significant elevations of plasma Hcy concentrations were observed in T1DM patients with only DR, only DN and both the two complications compared with T1DM patients without any complications.
In vivo, the main source for synthetizing Hcy is methionine, obtained from diet. As key intermediaries in Hcy synthesis, S-adenosyl-methionine and S-adenosyl-homocysteine generate in the process called demethylation [35]. Kidney is a major issue for removal and metabolism of Hcy [36], which is independently associated with GFR and albuminuria [37]. The deteriorating renal function may reduce the renal clearance of Hcy resulting in elevated plasma Hcy concentration. Thus, proper renal function is crucial to Hcy metabolism [38]. Our meta-analysis indicated that there were no significant differences in Hcy concentration between healthy people and T1DM patients without any complications, implying that Hcy may not an independently risk factor in the progression of T1DM. However, in the early stages of T1DM and in T1DM patients with proper renal function, hyperfiltration may cause lower plasma Hcy [39]. We may suggest that it’s too early to negate the effect of Hcy on the progression of T1DM, since hyperfiltration may cover the changes of plasma Hcy status in T1DM patients without any complications. Therefore, more studies excluded the effect of hyperfiltration in patients with T1DM should be carried out in the future.
The toxic effect of Hcy has been identified by a number of studies. Hcy is a pro-inflammatory amino acid and actives inflammatory transcriptional signal pathways [40]. Hyper homocysteinemia induces endoplasmic reticulum stress through activating mitogen-activated protein kinase, promotes proinflammatory cytokine production via activating JNK and facilitates macrophage infiltration [41,42]. In vitro studies have demonstrated that Hcy auto-oxidation formed reactive oxygen species (ROS), and reduced the production of glutathione peroxidase [43]. Elevated plasma Hcy represents an oxidative stress, elicits an increase in glutathione peroxidase activity [44]. Hcy can also induce endothelial dysfunction via increased ROS production and impaired nitric oxide’s bioactivity [45]. Hcy involves in the initiation and progression of vascular diseases via inducing the expression and secretion of inflammatory cytokines, such as interleukin-8 and monocyte chemoattractant protein-1 (MCP-1) [46].
Impaired renal function leads to hyperhomocysteinemia, elevated hcy status can also produce toxic effect on kidney [47]. Diet-induced chronic hyperhomocysteinemia could promote vascular remodeling and induce tubulointerstitial injury in the kidney [48]. Elevated Hcy concentration induces glomerular injury via ceramide-redox signaling pathway [49]. Another study has confirmed that Hcy induced MCP-1 expression in the kidney by activating NF-κB, which contributed to renal injury in hyperhomocysteinemic rat [50]. The results of our meta-analysis also showed that a significant higher plasma Hcy concentration was observed in T1DM patients with DN, compared with T1DM patients without any complications, suggesting a close association between Hcy and renal function.
In recent years, increasing studies have identified the toxic effect of hyperhomocysteinemia on retina. Hyperhomocysteinemia is not only an independent risk factor for macular edema [51]and retinal venous occlusion [52], but also a catalyst in retinopathy of prematurity [53] and age-related macular degeneration [54]. Moreover, elevated Hcy concentration has been measured in the blood plasma, vitreous body and aqueous humor of patients with proliferative diabetic retinopathy [55]. Hcy produces superoxide from NADPH oxidase resulting in impaired endothelium-dependent NO-mediated dilation in the retinal arterioles, which facilitates the development of retinal vascular diseases [56]. Our meta-analysis showed that plasma Hcy concentration is higher in T1DM patients with only DR than in T1DM patients without any complications, implying that pathological retina could cause elevated Hcy status without the involvement of impaired renal function. Nevertheless, the specific mechanisms relating Hcy status and retinopathy in T1DM are not clear. One study included in this meta-analysis demonstrated that compared with T1DM patients without any complications, T1DM patients with only DR accompanied with slightly increased (non-significant and normal) urinary albumin excretion (UAE) and significantly reduced, but normal estimated GFR, which may provide an explanation to elevated Hcy [34].
Although our investigation enrolled relatively high-quality studies which shared similar baseline characteristics, there were several limitations existing in this meta-analysis. First, detection methods of Hcy concentration varied among enrolled studies, which may influence the accuracy of Hcy concentration. Second, substantial heterogeneity was observed across studies in several meta-analyses, which might affect the outcomes, although a random effects model was used. Finally, we needed more studies included in our meta-analysis.
Based on our results, we may conclude that in T1DM patients without any complications, plasma Hcy concentrations keep in normal range. While as the disease progresses, T1DM microvascular complications, such as DR and DN, may accompany with elevated plasma Hcy concentrations.
Disclosure of conflict of interest
None.
References
- 1.Watkins RA, Evans-Molina C, Blum JS, Dimeglio LA. Established and emerging biomarkers for the prediction of type 1 diabetes: a systematic review. Transl Res. 2014;164:110–21. doi: 10.1016/j.trsl.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson SJ, Morgan NG, Foulis AK. Pancreatic pathology in type 1 diabetes mellitus. Endocr Pathol. 2014;25:80–92. doi: 10.1007/s12022-014-9297-8. [DOI] [PubMed] [Google Scholar]
- 3.Frohlich-Reiterer EE, Borkenstein MH. [Microvascular and macrovascular complications in children and adolescents with type 1 diabetes mellitus] . Wien Med Wochenschr. 2010;160:414–418. doi: 10.1007/s10354-010-0816-z. [DOI] [PubMed] [Google Scholar]
- 4.Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164–176. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- 5.Barr CC. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive insulin therapy, by The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N. Engl. J. Med 342:381-9, 2000. Surv Ophthalmol. 2001;45:459–460. doi: 10.1016/s0039-6257(01)00187-4. [DOI] [PubMed] [Google Scholar]
- 6.Zorena K, Raczynska D, Raczynska K. Biomarkers in diabetic retinopathy and the therapeutic implications. Mediators Inflamm. 2013;2013:193604. doi: 10.1155/2013/193604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mtiraoui N, Ezzidi I, Chaieb M, Marmouche H, Aouni Z, Chaieb A, Mahjoub T, Vaxillaire M, Almawi WY. MTHFR C677T and A1298C gene polymorphisms and hyperhomocysteinemia as risk factors of diabetic nephropathy in type 2 diabetes patients. Diabetes Res Clin Pract. 2007;75:99–106. doi: 10.1016/j.diabres.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Sharaf SM, Gawish HH, Elsherbiny EM. Methylenetetrahydrofolate Reductase (Mthfr C677t) Gene Polymorphism Effect on Development of Diabetic Nephropathy in Egyptien Patients with Type 2 Diabetes Mellitus. Life Science Journal-Acta Zhengzhou University Overseas Edition. 2012;9:874–880. [Google Scholar]
- 9.Heilman K, Zilmer M, Zilmer K, Kool P, Tillmann V. Elevated plasma adiponectin and decreased plasma homocysteine and asymmetric dimethylarginine in children with type 1 diabetes. Scand J Clin Lab Invest. 2009;69:85–91. doi: 10.1080/00365510802419454. [DOI] [PubMed] [Google Scholar]
- 10.Wiltshire E, Thomas DW, Baghurst P, Couper J. Reduced total plasma homocyst(e) ine in children and adolescents with type 1 diabetes. J Pediatr. 2001;138:888–893. doi: 10.1067/mpd.2001.113708. [DOI] [PubMed] [Google Scholar]
- 11.Meloni GF, Tonolo GC, Zuppi C, Zappacosta B, Musumeci S. Hyper-homocysteinemia is not a main feature of juvenile uncomplicated type 1 diabetes. J Atheroscler Thromb. 2005;12:14–19. doi: 10.5551/jat.12.14. [DOI] [PubMed] [Google Scholar]
- 12.Huemer M, Simma B, Mayr D, Muhl A, Rami B, Schober E, Ulmer H, Zanier U, Bodamer OA. Low levels of asymmetric dimethylarginine in children with diabetes mellitus type I compared with healthy children. J Pediatr. 2011;158:602–606.e601. doi: 10.1016/j.jpeds.2010.09.058. [DOI] [PubMed] [Google Scholar]
- 13.Glowinska B, Urban M, Peczynska J, Florys B, Szydlowska E. Elevated concentrations of homocysteine in children and adolescents with arterial hypertension accompanying Type 1 diabetes. Med Sci Monit. 2001;7:1242–1249. [PubMed] [Google Scholar]
- 14.Dinleyici EC, Kirel B, Alatas O, Muslumanoglu H, Kilic Z, Dogruel N. Plasma total homocysteine levels in children with type 1 diabetes: relationship with vitamin status, methylene tetrahydrofolate reductase genotype, disease parameters and coronary risk factors. J Trop Pediatr. 2006;52:260–266. doi: 10.1093/tropej/fmk001. [DOI] [PubMed] [Google Scholar]
- 15.Vaccaro O, Perna AF, Mancini FP, Iovine C, Cuomo V, Sacco M, Tufano A, Rivellese AA, Ingrosso D, Riccardi G. Plasma homocysteine and microvascular complications in type 1 diabetes. Nutr Metab Cardiovasc Dis. 2000;10:297–304. [PubMed] [Google Scholar]
- 16.Matteucci E, Rossi L, Mariani S, Fagnani F, Quilici S, Cinapri V, Giampietro O. Blood levels of total homocysteine in patients with type 1 diabetes (with no complications, diabetic nephropathy and/or retinopathy) and in their non-diabetic relatives. Nutr Metab Cardiovasc Dis. 2002;12:184–189. [PubMed] [Google Scholar]
- 17.Agardh E, Hultberg B, Agardh CD. Severe retinopathy in type 1 diabetic patients is not related to the level of plasma homocysteine. Scand J Clin Lab Invest. 2000;60:169–174. doi: 10.1080/003655100750044811. [DOI] [PubMed] [Google Scholar]
- 18.Saeed BO, Nixon SJ, White AJ, Summerfield GP, Skillen AW, Weaver JU. Fasting homocysteine levels in adults with type 1 diabetes and retinopathy. Clin Chim Acta. 2004;341:27–32. doi: 10.1016/j.cccn.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 19.Buysschaert M, Jamart J, Dramais AS, Wallemacq P, Hermans MP. Micro- and macrovascular complications and hyperhomocysteinaemia in type 1 diabetic patients. Diabetes Metab. 2001;27:655–659. [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babar GS, Zidan H, Widlansky ME, Das E, Hoffmann RG, Daoud M, Alemzadeh R. Impaired endothelial function in preadolescent children with type 1 diabetes. Diabetes Care. 2011;34:681–685. doi: 10.2337/dc10-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giannattasio A, Calevo MG, Minniti G, Gianotti D, Cotellessa M, Napoli F, Lorini R, d’Annunzio G. Folic acid, vitamin B12, and homocysteine levels during fasting and after methionine load in patients with Type 1 diabetes mellitus. J Endocrinol Invest. 2010;33:297–299. doi: 10.1007/BF03346589. [DOI] [PubMed] [Google Scholar]
- 23.Harrington J, Pena AS, Gent R, Hirte C, Couper J. Aortic intima media thickness is an early marker of atherosclerosis in children with type 1 diabetes mellitus. J Pediatr. 2010;156:237–241. doi: 10.1016/j.jpeds.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 24.Jehlicka P, Stozicky F, Mayer O Jr, Varvarovska J, Racek J, Trefil L, Siala K. Asymmetric dimethylarginine and the effect of folate substitution in children with familial hypercholesterolemia and diabetes mellitus type 1. Physiol Res. 2009;58:179–184. doi: 10.33549/physiolres.931418. [DOI] [PubMed] [Google Scholar]
- 25.Janickova Zdarska D, Zavadova E, Kvapil M. The effect of ramipril therapy on cytokines and parameters of incipient diabetic nephropathy in patients with type 1 diabetes mellitus. J Int Med Res. 2007;35:374–383. doi: 10.1177/147323000703500312. [DOI] [PubMed] [Google Scholar]
- 26.Atabek ME, Pirgon O, Karagozoglu E. Plasma homocysteine levels in children and adolescents with type 1 diabetes. Indian Pediatr. 2006;43:401–407. [PubMed] [Google Scholar]
- 27.Garcia-Unzueta MT, Berrazueta JR, Pesquera C, Obaya S, Fernandez MD, Sedano C, Amado JA. Levels of plasma total adrenomedullin are related with two acute phase inflammatory reactants (fibrinogen and sialic acid) but not with markers of endothelial dysfunction in Type 1 diabetes Adrenomedullin and vascular risk factors in Type 1 DM. J Diabetes Complications. 2005;19:147–154. doi: 10.1016/j.jdiacomp.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Saeed BO, Banerjee K, Nixon SJ, Brown K. Plasma homocysteine concentrations in patients with Type 1 diabetes. Diabet Med. 2003;20:867–868. doi: 10.1046/j.1464-5491.2003.01031.x. [DOI] [PubMed] [Google Scholar]
- 29.Abdel Aziz MT, Fouad HH, Mohsen GA, Mansour M, Abdel Ghaffar S. TNF-alpha and homocysteine levels in type 1 diabetes mellitus. East Mediterr Health J. 2001;7:679–688. [PubMed] [Google Scholar]
- 30.Mutus B, Rabini RA, Staffolani R, Ricciotti R, Fumelli P, Moretti N, Martarelli D, Mazzanti L. Homocysteine-induced inhibition of nitric oxide production in platelets: a study on healthy and diabetic subjects. Diabetologia. 2001;44:979–982. doi: 10.1007/s001250100581. [DOI] [PubMed] [Google Scholar]
- 31.Targher G, Bertolini L, Zenari L, Cacciatori V, Muggeo M, Faccini G, Zoppini G. Cigarette smoking and plasma total homocysteine levels in young adults with type 1 diabetes. Diabetes Care. 2000;23:524–528. doi: 10.2337/diacare.23.4.524. [DOI] [PubMed] [Google Scholar]
- 32.Agardh CD, Agardh E, Andersson A, Hultberg B. Lack of association between plasma homocysteine levels and microangiopathy in type 1 diabetes mellitus. Scand J Clin Lab Invest. 1994;54:637–641. doi: 10.3109/00365519409087544. [DOI] [PubMed] [Google Scholar]
- 33.Hultberg B, Agardh E, Andersson A, Brattstrom L, Isaksson A, Israelsson B, Agardh CD. Increased levels of plasma homocysteine are associated with nephropathy, but not severe retinopathy in type 1 diabetes mellitus. Scand J Clin Lab Invest. 1991;51:277–282. doi: 10.3109/00365519109091615. [DOI] [PubMed] [Google Scholar]
- 34.Bulum T, Blaslov K, Duvnjak L. Plasma Homocysteine is Associated with Retinopathy in Type 1 Diabetic Patients in the Absence of Nephropathy. Semin Ophthalmol. 2014 doi: 10.3109/08820538.2014.912338. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Veeranki S, Tyagi SC. Defective homocysteine metabolism: potential implications for skeletal muscle malfunction. Int J Mol Sci. 2013;14:15074–15091. doi: 10.3390/ijms140715074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.House JD, Brosnan ME, Brosnan JT. Renal uptake and excretion of homocysteine in rats with acute hyperhomocysteinemia. Kidney Int. 1998;54:1601–1607. doi: 10.1046/j.1523-1755.1998.00144.x. [DOI] [PubMed] [Google Scholar]
- 37.Soedamah-Muthu SS, Chaturvedi N, Teerlink T, Idzior-Walus B, Fuller JH, Stehouwer CD. Plasma homocysteine and microvascular and macrovascular complications in type 1 diabetes: a cross-sectional nested case-control study. J Intern Med. 2005;258:450–459. doi: 10.1111/j.1365-2796.2005.01560.x. [DOI] [PubMed] [Google Scholar]
- 38.Ruan L, Chen W, Srinivasan SR, Xu J, Toprak A, Berenson GS. Plasma homocysteine is adversely associated with glomerular filtration rate in asymptomatic black and white young adults: the Bogalusa heart study. Eur J Epidemiol. 2009;24:315–319. doi: 10.1007/s10654-009-9340-0. [DOI] [PubMed] [Google Scholar]
- 39.Wollesen F, Brattstrom L, Refsum H, Ueland PM, Berglund L, Berne C. Plasma total homocysteine and cysteine in relation to glomerular filtration rate in diabetes mellitus. Kidney Int. 1999;55:1028–1035. doi: 10.1046/j.1523-1755.1999.0550031028.x. [DOI] [PubMed] [Google Scholar]
- 40.Meng S, Ciment S, Jan M, Tran T, Pham H, Cueto R, Yang XF, Wang H. Homocysteine induces inflammatory transcriptional signaling in monocytes. Front Biosci (Landmark Ed) 2013;18:685–695. doi: 10.2741/4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ingram AJ, Krepinsky JC, James L, Austin RC, Tang D, Salapatek AM, Thai K, Scholey JW. Activation of mesangial cell MAPK in response to homocysteine. Kidney Int. 2004;66:733–745. doi: 10.1111/j.1523-1755.2004.00795.x. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Zhang H, Jiang C, Xu M, Pang Y, Feng J, Xiang X, Kong W, Xu G, Wang X. Hyperhomocysteinemia promotes insulin resistance by inducing endoplasmic reticulum stress in adipose tissue. J Biol Chem. 2013;288:9583–9592. doi: 10.1074/jbc.M112.431627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayden MR, Tyagi SC. Homocysteine and reactive oxygen species in metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: the pleiotropic effects of folate supplementation. Nutr J. 2004;3:4. doi: 10.1186/1475-2891-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moat SJ, Bonham JR, Cragg RA, Powers HJ. Elevated plasma homocysteine elicits an increase in antioxidant enzyme activity. Free Radic Res. 2000;32:171–179. doi: 10.1080/10715760000300171. [DOI] [PubMed] [Google Scholar]
- 45.Weiss N. Mechanisms of increased vascular oxidant stress in hyperhomocys-teinemia and its impact on endothelial function. Curr Drug Metab. 2005;6:27–36. doi: 10.2174/1389200052997357. [DOI] [PubMed] [Google Scholar]
- 46.Poddar R, Sivasubramanian N, DiBello PM, Robinson K, Jacobsen DW. Homocysteine induces expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human aortic endothelial cells: implications for vascular disease. Circulation. 2001;103:2717–2723. doi: 10.1161/01.cir.103.22.2717. [DOI] [PubMed] [Google Scholar]
- 47.Bridges CC, Zalups RK. Homocysteine, system b0,+ and the renal epithelial transport and toxicity of inorganic mercury. Am J Pathol. 2004;165:1385–1394. doi: 10.1016/S0002-9440(10)63396-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumagai H, Katoh S, Hirosawa K, Kimura M, Hishida A, Ikegaya N. Renal tubulointerstitial injury in weanling rats with hyperhomocysteinemia. Kidney Int. 2002;62:1219–1228. doi: 10.1111/j.1523-1755.2002.kid558.x. [DOI] [PubMed] [Google Scholar]
- 49.Yi F, Zhang AY, Li N, Muh RW, Fillet M, Renert AF, Li PL. Inhibition of ceramide-redox signaling pathway blocks glomerular injury in hyperhomocysteinemic rats. Kidney Int. 2006;70:88–96. doi: 10.1038/sj.ki.5001517. [DOI] [PubMed] [Google Scholar]
- 50.Hwang SY, Woo CW, Au-Yeung KK, Siow YL, Zhu TY, O K. Homocysteine stimulates monocyte chemoattractant protein-1 expression in the kidney via nuclear factor-kappaB activation. Am J Physiol Renal Physiol. 2008;294:F236–244. doi: 10.1152/ajprenal.00331.2007. [DOI] [PubMed] [Google Scholar]
- 51.Aydin E, Demir HD, Ozyurt H, Etikan I. Association of plasma homocysteine and macular edema in type 2 diabetes mellitus. Eur J Ophthalmol. 2008;18:226–232. doi: 10.1177/112067210801800210. [DOI] [PubMed] [Google Scholar]
- 52.Lahiri KD, Dutta J, Datta H, Das HN. Hyperhomocysteinemia, as an independent risk factor for retinal venous occlusion in an Indian population. Indian J Clin Biochem. 2013;28:61–64. doi: 10.1007/s12291-012-0238-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarici AM, Yetik H, Akar S, Arvas S. The association between serum homocysteine levels and retinopathy of prematurity. J Int Med Res. 2012;40:1912–1918. doi: 10.1177/030006051204000532. [DOI] [PubMed] [Google Scholar]
- 54.Gopinath B, Flood VM, Rochtchina E, Wang JJ, Mitchell P. Homocysteine, folate, vitamin B-12, and 10-y incidence of age-related macular degeneration. Am J Clin Nutr. 2013;98:129–135. doi: 10.3945/ajcn.112.057091. [DOI] [PubMed] [Google Scholar]
- 55.Lim CP, Loo AV, Khaw KW, Sthaneshwar P, Khang TF, Hassan M, Subrayan V. Plasma, aqueous and vitreous homocysteine levels in proliferative diabetic retinopathy. Br J Ophthalmol. 2012;96:704–707. doi: 10.1136/bjophthalmol-2011-301044. [DOI] [PubMed] [Google Scholar]
- 56.Omae T, Nagaoka T, Tanano I, Yoshida A. Homocysteine inhibition of endothelium-dependent nitric oxide-mediated dilation of porcine retinal arterioles via enhanced superoxide production. Invest Ophthalmol Vis Sci. 2013;54:2288–2295. doi: 10.1167/iovs.12-11082. [DOI] [PubMed] [Google Scholar]


