Abstract
Objective: Previous studies have shown that Astragalus polysaccharide (APS) can be applied to anti-cancer. However, the mechanism by which APS mediate this effect is unclear. In the present study, APS-mediated NSCLC cell apoptosis was investigated through the regulation of the notch signaling pathway. Methods: The cell viability was detected by the CCK8 assay. The mRNA and protein expression of notch1/3 and tumor suppressors were analyzed by RT-PCR and western blotting, respectively. Results: The mRNA and protein of notch1 and notch3 were significantly up-regulated in tumor tissues as compared to non-tumor adjacent tissues. Treatment of human NSCLC cells with APS induced cell death in a dose-and time-dependent manner by using CCK8 assay. The mRNA and protein expression of notch1 and notch3 were significantly lower in NSCLC cells with APS treatment than that in control group. Moreover, western blotting analysis showed that treatment of H460 cells with APS significantly increased the pro-apoptotic Bax and caspase 8 levels, decreased the anti-apoptotic Bcl-2 level. Furthermore, p53, p21 and p16 were obviously up-regulated by APS treatment in H460 cell. Conclusions: This study demonstrated that APS-treated could inhibit proliferation and promote cell apoptosis, at least partially, through suppressing the expression of notch1 and notch3 and up-regulating the expression of tumor suppressors in H460 NSCLC cell lines.
Keywords: Astragalus polysaccharide, non-small cell lung cancer, notch, tumor suppressors
Introduction
Lung cancer, including small cell lung carcinoma (SCLC) and non-small cell lung carcinoma (NSCLC), is the most common cause of cancer-related deaths in worldwide [1]. NSCLC accounts for approximately 85% of all lung cancer cases, with a high rate of relapse and failure of therapy [2]. Thus, the better and updated drug targets are essential to be exploited. A previous study suggests that Notch signaling plays a crucial role in the transformation and neoplastic proliferation of human malignancy [3]. Aberrant expression of Notch has been reported in breast cancer [4], lung cancer [5], acute myeloid leukemia [6], prostate cancer [7,8]. Some recent studies show that Notch signaling is activated in human non-small cell lung cancer [2,9-11]. Intriguingly, high notch activity induces radiation resistance and chemotherapy resistance in non-small cell lung cancer [10,12]. In clinical study, activation of Notch signaling by either Notch1 up-regulation or Numb down-regulation is observed in 30% of primary human NSCLCs [13]. Notch3 also appears to be a key player in NSCLC, with over-expressed in 40% of NSCLC tumors [14]. Elevated the expressions levels of notch1/3 and their ligand are found to be associated with tumor progression and predicted poor prognosis in NSCLC, suggesting a promising biomarker for NSCLC [9]. In experimental study, notch signaling inhibitors can attenuate cell growth and induce apoptosis of lung cancer cell lines [15,16].
The dry roots of Astragalus membranaceus, which is also known as Huang Qi in China and belongs to the Fabaceae family, have long been used as an important component of many herbal prescriptions in traditional Chinese medicine [17,18]. Astragalus polysaccharide (APS), the extract from Astragalus membranaceus, exerts strong anti-tumor [19] and effectively alleviates inflammation-induced artery endothelium cell injury [20] and atherosclerosis [21] and insulin resistance [22]. In H22 hepatocarcinoma transplanted BALB/c mice, APS could effectively inhibit the solid tumor growth and exert anti-cancer activity in vivo, which at least partly via improving immune responses of host organism [23,24]. Recent studies have shown that APS combined treatment promotes the efficacy of chemotherapeutic drug in NSCLC patients, stabilizes and improves performance status, and reduces chemotherapy toxicity [25]. However, the underlying signaling mechanisms accounting for APS-induced NSCLC cell apoptosis are still not well characterized.
In this study, we intended to investigate the effect of APS on NSCLC cell lines proliferation in vitro. The results showed that APS induced cell death through the suppression of notch1/3 signaling. These data suggested that APS might be an effective adjuvant therapy drug for patients with NSCLC.
Materials and methods
Tissue samples
Human lung carcinoma samples were obtained with written informed consent from Department of Respiratory Medicine of Changhai Hospital Attached to Second Military Medical University. The study was approved by the Ethics Committee of Department of Respiratory Medicine of Changhai Hospital Attached to and Second Military Medical University. 20 tumor samples and 20 cases of non-tumor adjacent tissues were collected between 02/2013 and 6/2014.
Cell culture
The Human H460 NSCLC cells were obtained from the Chinese Academy of Sciences (Institute of Shanghai Cell Biology and Chinese Type Culture Collection, China) and maintained in DMEM (Dulbecco’s modified Eagle’s medium; Invitrogen) and supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan, UT), 100 units/ml penicillin, and 100 mg/ml streptomycin (Invitrogen) at 37°C in a humidified, 5% CO2, 95% air atmosphere. The medium was replenished every day.
Cell viability detection by CCK8
H460 cells (1.0 × 105/well) were plated and treated in 96-well plates (three wells per group) with APS (0-30 mg/mL) for 24 or 48 h, respectively. 10 μL of CCK8 (Dojindo, Kumamoto, Japan) was added to the cells, and the viability of the cells was measured at 490 nm using an ELISA reader (BioTek, Winooski, VT, USA) according to the manufacturer’s instructions.
Over-expression and small interfering RNA
For the transfection of the H460 NSCLC cell lines, lentiviral vectors harboring notch1 and notch3 were constructed, and the H460 NSCLC cells were infected. Briefly, the H460 NSCLC cells were cultured in McCoy’s 5α medium containing 10% FBS and when they reached the exponential growth phase, 1.0 × 105 cells per well were plated in 96 plates. Next, 300 μl complete culture medium, containing recombinant lentiviruses, control lentiviruses or McCoy’s 5α medium (all containing 6 μg/ml polybrene; Sigma) was added into the plates when the cells reached 50-60% confluence. Two days later, the virus-containing medium was replaced with fresh complete medium.
The small interfering (si) RNAs for human notch1 or notch3 were obtained from Dharmacon (Lafayette, USA). The small interfering with the following primers: notch1, Forward 5’-GAGUGUGUGUGCGACAUGCACAUCA-3’ and Reverse 5’-ACUAGAGGAUCUGAGUCACUGUCUG-3’; notch3, Forward 5’-CCUUACACUCGUCUGAGCAAGGU-3’ and Reverse 5’-UGCUCAAGGACAUAGGAGUUAGC-3’. The siRNA oligonucleotides (at a final concentration of 100 nM) were transfected into H460 NSCLC cells using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions.
Reverse transcription-polymerase chain reaction (RT-PCR)
The H460 NSCLC cells RNA extraction was performed according to the TRIzol manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA). RNA integrity was verified by agarose gel electrophoresis. Synthesis of cDNAs was performed by reverse transcription reactions with 2 μg of total RNA using moloney murine leukemia virus reverse transcriptase (Invitrogen) with oligo dT (15) primers (Fermentas) as described by the manufacturer. The first strand cDNAs served as the template for the regular polymerase chain reaction (PCR) performed using a DNA Engine (ABI 7300). PCR with the following primers: notch1, forward 5’-CTTAGATGTGCTGAGCGCGTCAATGTGTC-3’, reverse 5’-GCGCGATCCTTGATAACCTGCGGAT-3’; notch3, forward 5’-TGCTAATTAGTTCGGCTAT-3’, reverse 5’-TATTCGCGACGATGGTTAG-3’; GAPGH, forward 5’-GGTGGAGGTCGGGAGTCAACGGA-3’, reverse 5’-GAGGGATCTCGCTCCTGGAGGA-3’. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal control was used to normalize the data to determine the relative expression of the target genes. The reaction conditions were set according to the kit instructions.
Western blotting
The tumor tissues and H460 cells were homogenized and extracted in NP-40 buffer, followed by 5-10 min boiling and centrifugation to obtain the supernatant. Samples containing 100 μg of protein were separated on 10% SDS-PAGE gel, transferred to nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA, USA). After saturation with 5% (w/v) non-fat dry milk in TBS and 0.1% (w/v) Tween 20 (TBST), the membranes were incubated with the following antibodies: notch1, notch3, caspase 8, Bcl-2, BAX, p16, p21 and p53 (Santa Cruz Biotechnology, CA, USA), at dilutions ranging from 1:500 to 1:2,000 at 4°C over-night. After three washes with TBST, membranes were incubated with secondary immunoglobulins (Igs) conjugated to IRDye 800CW Infrared Dye (LI-COR), including donkey anti-goat IgG and donkey anti-mouse IgG at a dilution of 1:10,000-1:20,000. After 1 hour incubation at 37°C, membranes were washed three times with TBST. Blots were visualized by the Odyssey Infrared Imaging System (LI-COR Biotechnology). Signals were densitometrically assessed (Odyssey Application Software version 3.0) and normalized to the β-actin signals to correct for unequal loading using the mouse monoclonal anti-β-actin antibody (Bioworld Technology, USA).
Statistical analysis
The data from these experiments were reported as mean ± standard errors of mean (SEM) for each group. All statistical analyses were performed by using PRISM version 4.0 (GraphPad). Inter-group differences were analyzed by one-way ANOVA, and followed by Tukey’s multiple comparison test as a post test to compare the group means if overall P < 0.05. Differences with P value of < 0.05 were considered statistically significant.
Results
The expression of notch1 and notch3 in tumor tissues from NSCLC patients
RT-PCR and western blotting assays were performed to detect the expression of notch1 and notch3 in the tumor tissues from NSCLC patients. As shown in Figure 1, the relative expression levels of notch1 and notch3 mRNA (Figure 1A and 1C) and protein (Figure 1B and 1C) in tumor tissues were significantly higher than those in corresponding non-tumor adjacent tissues (P < 0.05). These data indicated that up-regulation of notch1 and notch3 might play critical roles in the development of NSCLC.
Figure 1.
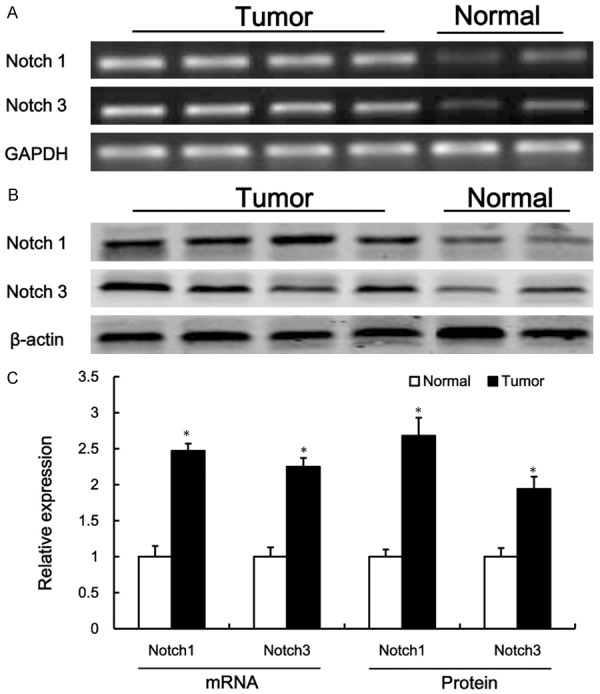
The expression of notch1 and notch3 in the tumor tissues from NSCLC patients. Semi-quantitative reverse transcription polymerase chain reaction (RT-PCR) (A) and western blotting (B) were used to detect the expression of notch1 and notch3 in the tumor tissues from NSCLC. Densitometric quantification for RT-PCR and western blotting (C). Values are expressed as mean ± SEM, n = 10 in each group. *P < 0.05, versus normal control group.
APS inhibits NSCLC cell growth
H460 NSCLC cell viability was measured when cells were exposed to various concentrations of APS (0-30 mg/mL) for 24 and 48 h. The growth of cells was inhibited with APS (Figure 2A). The viabilities of NSCLC cells treated with APS were significantly lower than those of control group. As shown the growth curve in Figure 2A, the concentrations at which APS inhibited cell growth by 50% (IC50) were 15 mg/mL and 5 mg/mL at 24 h and 48 h, respectively. Treatment of NSCLC cells with APS induced cell growth inhibition in a dose-dependent manner by using CCK8 assay (Figure 2A). To evaluate the time-dependent effect of APS on the cell viability, the cells were exposed to 5 mg/mL APS for various times. The results showed that the cell viability was significantly decreased after 12 h of APS treatment, although a slight up-regulation of cell proliferation was observed at 6 h (Figure 2B).
Figure 2.
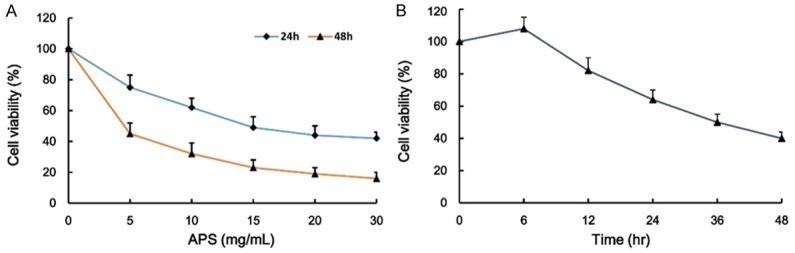
The effect of APS on the cell viability of H460 NSCLC cell. The NSCLC cells were incubated with various concentrations of APS (0-30 mg/mL) for 24 h and 48 h, and the cell viability was examined by CCK8 assay (A). The H460 NSCLC cells were incubated with APS (5 mg/mL) for 0, 6, 12, 24, 36 and 48 h, and the cell viability was examined by CCK8 assay (B). Values are expressed as mean ± SEM, n = 3 in each group.
The effect of APS on the protein expression of notch1 and notch3
In an attempt to explore the influence of notch1 and notch3 on human NSCLC cells when they are prescribed to increase risk of a variety of cancers. To determine whether APS induced apoptosis by triggering the notch apoptotic pathway, we measured the change in the mRNA and protein expression of notch1 and notch3 in H460 cell lines. The current study suggested that notch1 and notch3 were associated with proliferation of NSCLC cells. The mRNA (Figure 3A and 3B) and protein (Figure 3C and 3D) expression of notch1 and notch3 were significantly lower in NSCLC cells with APS treatment than that in un-treatment group. Treatment of H460 cells with increasing doses of APS and increasing durations significantly decreased the mRNA and protein expression of notch1 and notch3 (Figure 3). Therefore, our data suggest that suppression the expression of notch1 and notch3 was involved in APS-mediated cell death.
Figure 3.
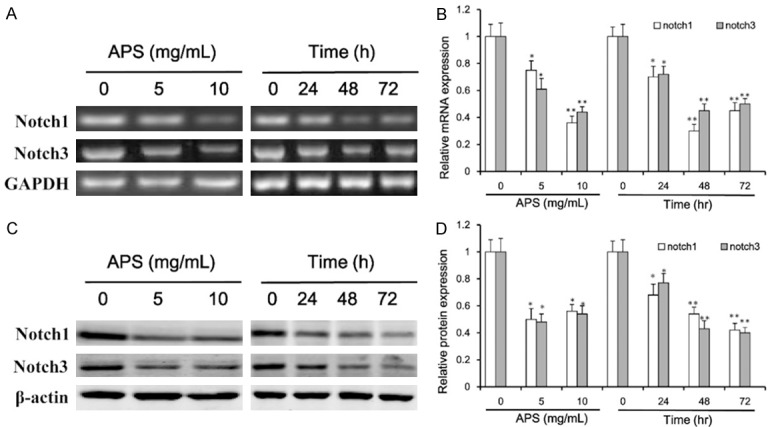
Effect of APS on the mRNA and protein expression of notch1 and notch3. The H460 cells were incubated with APS (0, 5 or 10 mg/mL) for 48 h, and the cells exposed to APS (5 mg/mL) for 0, 24, 48 and 72 h, the expression levels of notch1 and notch3 were determined by RT-PCR (A) and western blotting (C). mRNA (B) and protein (D) bands were quantified in H460 cells. Values are expressed as mean ± SEM, n = 3 in each group. *P < 0.05, **P < 0.01 versus control group.
The effect of APS on the protein expression of apoptosis-related regulators and tumor suppressors
The CCK8 assay results showed that APS could induce apoptosis in H460 NSCLC cell. The apoptotic response was further investigated by measuring apoptosis-related proteins expression. Western blotting analysis showed that treatment of H460 cells with APS significantly increased the pro-apoptotic Bax and caspase 8 level, decreased the anti-apoptotic Bcl-2 level (Figure 4A and 4B). Moreover, significant changes in the protein levels of tumor suppressors were observed in H460 cells with APS-treated. As shown in Figure 4C and 4D, p53, p21 and p16 were obviously up-regulated by APS treatment. These results indicated that APS might induce NSCLC cell death through activation tumor suppressors signaling pathway.
Figure 4.
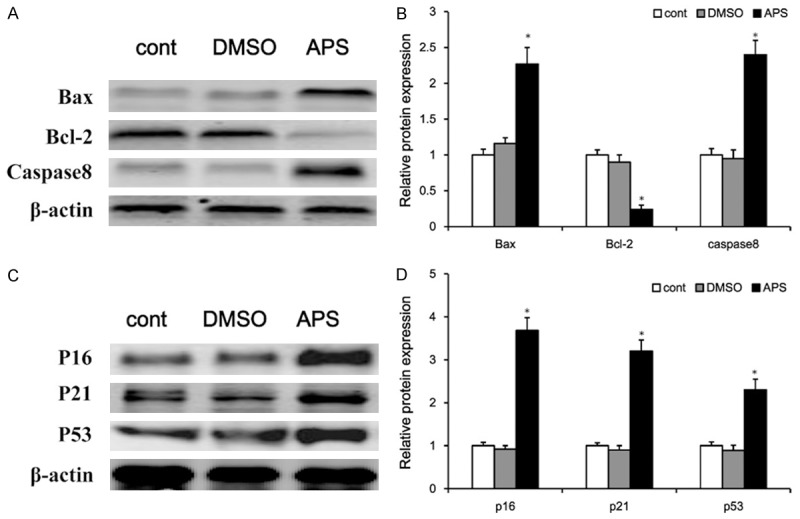
The effect of APS on the protein expression of apoptosis-related regulators and tumor suppressors. The H460 cells were incubated with DMSO or APS (5 mg/mL) for 48 h, the expression levels of Bax, Caspase8 and Bcl-2 were determined by western blotting (A), and protein bands were quantified in H460 cell (B). The H460 cells were incubated with DMSO or APS (5 mg/mL) for 48 h, the expression levels of p53, p21 and p16 were determined by western blotting (C), and protein bands were quantified in H460 cell (D), normalized to β-actin protein bands. Values are expressed as mean ± SEM, n = 3 in each group. *P < 0.05 versus control group.
Notch1 and notch3 gain-of-function or loss-of-function in H460 cell lines
Consistent with previous studies, knockdown of notch1 or notch3 inhibited H460 cell proliferation. The inhibiting effect of H460 cell proliferation was significantly promoted in the combined treatment group compared with single treatment group (Figure 5A). In contrast, notch1 or notch3 over-expression resulted in a restored cell proliferation (Figure 5B). Intriguingly, APS could inhibit the proliferation of notch1 and notch3 gain-of-function cell in a dose-dependent manner (Figure 5C). These results confirmed that APS inhibited proliferation and promoted cell apoptosis, at least partially, through suppression the expression of notch1 and notch3.
Figure 5.
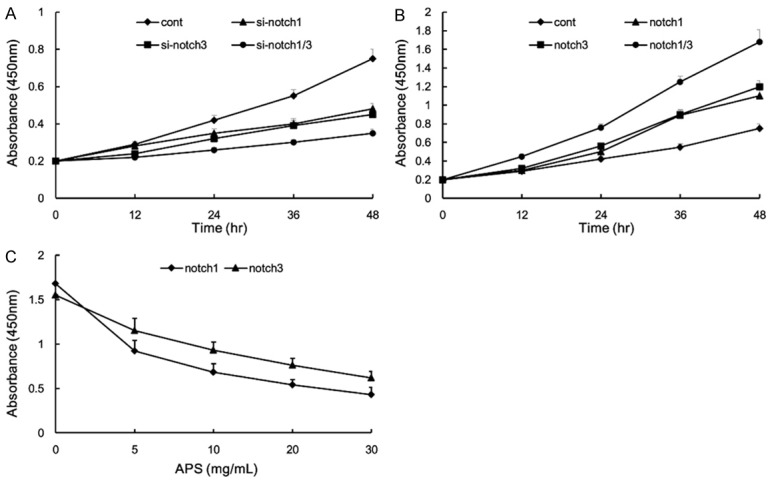
Notch1 and notch3 gain-of-function or loss-of-function in H460 cell lines. H460 cells were treated with notch1-siRNA and notch3-siRNA, and the numbers of cells per well were measured by the absorbance (450 nm) (A). H460 cells were overexpressed with notch1 and notch3, and the numbers of cells per well were measured by the absorbance (450 nm) (B). Notch1 and notch3 gain-of-function in H460 cells were incubated with various concentration APS for 48 h, and the numbers of cells per well were measured by the absorbance (450 nm) (C). Values are expressed as mean ± SEM, n = 3 in each group.
Discussion
The role of notch signaling in oncogenesis remains unclear, due to the complex nature of notch signaling and that can have both tumor promoting and inhibiting functions in the same cancer cell lines, and it can play different roles in early and late stages of cancer development [1,26,27]. Our present study provides an extensive evaluation of notch1 and notch3 expression in human NSCLC, to elucidate its role in cell proliferation and apoptosis.
We found that the components of Notch signaling pathway were activated in lung tumor samples. Gain-of-function notch1/3 resulted in increasing cell proliferation, while its loss-of-function caused inhibition of NSCLC cell growth. Recently, notch1 and notch3 widely studied and reported to aberrantly express in malignant tumors [28-30]. It is unexpectedly that the level of notch-1 protein is down-regulated in lung adenocarcinoma cells or tissues [31]. Notch1 and notch3 are considered as a highly controversial gene because of its complex biological functions. Intriguingly, several studies reported opposite effect of notch signaling on cellular apoptosis. Knockdown notch1 resulted in increasing cell proliferation, while its induction caused inhibition of SCLC cell growth [26]. Clinical research shows that notch3 has high expression in 67 of 131 cases of NSCLC (51.1%), which is significantly higher than in adjacent noncancerous lung tissues. Moreover, notch3 over-expression is correlated with metastasis and poor prognosis [11]. Importantly, the apoptotic activity of notch1 depends on cell type. Notch1 enhances apoptosis in H69AR and SBC-3 cells, but such induction of apoptosis couldn’t effectively counteract the mitogenic response to inhibiting notch1 in these cells [26]. Notch signaling pathway participates in Wnt signaling-induced cell apoptosis. CHIR99021, a Wnt signaling agonist, up-regulates the protein expression of Notch3 and promotes cell proliferation, which suggest that Notch3 signaling can be activated by inhibiting Wnt signaling and a functional link between Wnt and Notch signaling pathways exists in NSCLC [2]. In the present study, we demonstrated that the protein expression of notch1 and notch3 was up-regulated in NSCLC tissues and H460 cell lines, APS-treated could suppress the expression of notch1 and notch3 and induce cell apoptosis in vitro. APS has been most widely studied, mainly on their immuno-potentiating properties [32,33]. Evidence supporting the use of APS in NSCLC is sparse. In the present work, APS-induced cell death in NSCLC cell has been reported firstly.
In addition, we observed the expressions of apoptosis-related regulators and tumor suppressors. The results showed that APS-treated could up-regulate Bax and caspase3 expression and down-regulate Bcl-2 expression. Apoptosis-related proteins expression which regulated by APS could induce H460 cell apoptosis. Furthermore, human NSCLC cell exposure to APS could up-regulate the expression of p16, p21 and p53. Previous study shows that APS can induce synovial apoptosis, and APS treatment accompanies by increased staining for pro-apoptotic Bax protein and decreased staining for anti-apoptotic Bcl-2 protein in adjuvant-induced arthritis rats [34]. In basal-like breast cancer cell line MDA-MB-468, the anti-proliferation mechanisms of APS may be related to its effects of up-regulating the expressions of p53 [35]. P53 transcriptional function is considered as a marker of large-scale differently expressed genes and involved in cell cycle arrest, which is main causes of cell apoptosis [36]. In the A549 NSCLC cells, liquiritin, isoliquiritin and isoliquirigenin induce apoptotic cell death through up-regulating p53 and p21 [37]. New clinical research shows that activation of p53/p21 expression associates with poor prognosis in non-small cell lung cancer patients [38]. These studies indicate that p21/p53 signaling may possess a potential efficiency in the development of lung carcinoma.
To the best of our knowledge, this was the first study to demonstrate that APS induced cell apoptosis in H460 NSCLC cell lines, the underlying molecular mechanisms, at least partially, through activation p21/p53 and suppression notch signaling. In view of the results of this experiment, it seemed reasonable to highlight the possibility of APS in the clinical treatment of lung carcinoma.
Disclosure of conflict of interest
None.
References
- 1.Wael H, Yoshida R, Kudoh S, Hasegawa K, Niimori-Kita K, Ito T. Notch1 signaling controls cell proliferation, apoptosis and differentiation in lung carcinoma. Lung Cancer. 2014;85:131–140. doi: 10.1016/j.lungcan.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Li C, Zhang S, Lu Y, Zhang Y, Wang E, Cui Z. The roles of Notch3 on the cell proliferation and apoptosis induced by CHIR99021 in NSCLC cell lines: a functional link between Wnt and Notch signaling pathways. PLoS One. 2013;8:e84659. doi: 10.1371/journal.pone.0084659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohnuki H, Jiang K, Wang D, Salvucci O, Kwak H, Sanchez-Martin D, Maric D, Tosato G. Tumor-infiltrating myeloid cells activate Dll4/ Notch/TGF-beta signaling to drive malignant progression. Cancer Res. 2014;74:2038–2049. doi: 10.1158/0008-5472.CAN-13-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villa JC, Chiu D, Brandes AH, Escorcia FE, Villa CH, Maguire WF, Hu CJ, de Stanchina E, Simon MC, Sisodia SS, Scheinberg DA, Li YM. Nontranscriptional Role of Hif-1alpha in Activation of gamma-secretase and notch signaling in breast cancer. Cell Rep. 2014;8:1077–1092. doi: 10.1016/j.celrep.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Li D, Liu H, Xu H, Zheng H, Qian F, Li W, Zhao C, Wang Z, Wang X. Notch-1 signaling facilitates survivin expression in human nonsmall cell lung cancer cells. Cancer Biol Ther. 2011;11:14–21. doi: 10.4161/cbt.11.1.13730. [DOI] [PubMed] [Google Scholar]
- 6.Chen PM, Yen CC, Wang WS, Lin YJ, Chu CJ, Chiou TJ, Liu JH, Yang MH. Down-regulation of Notch-1 expression decreases PU. 1-mediated myeloid differentiation signaling in acute myeloid leukemia. Int J Oncol. 2008;32:1335–1341. doi: 10.3892/ijo_32_6_1335. [DOI] [PubMed] [Google Scholar]
- 7.Kashat M, Azzouz L, Sarkar SH, Kong D, Li Y, Sarkar FH. Inactivation of AR and Notch-1 signaling by miR-34a attenuates prostate cancer aggressiveness. Am J Transl Res. 2012;4:432–442. [PMC free article] [PubMed] [Google Scholar]
- 8.Carvalho FL, Simons BW, Eberhart CG, Berman DM. Notch signaling in prostate cancer: a moving target. Prostate. 2014;74:933–945. doi: 10.1002/pros.22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan X, Wu H, Han N, Xu H, Chu Q, Yu S, Chen Y, Wu K. Notch signaling and EMT in nonsmall cell lung cancer: biological significance and therapeutic application. J Hematol Oncol. 2014;7:87. doi: 10.1186/s13045-014-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie M, He CS, Wei SH, Zhang L. Notch-1 contributes to epidermal growth factor receptor tyrosine kinase inhibitor acquired resistance in non-small cell lung cancer in vitro and in vivo. Eur J Cancer. 2013;49:3559–3572. doi: 10.1016/j.ejca.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Ye YZ, Zhang ZH, Fan XY, Xu XL, Chen ML, Chang BW, Zhang YB. Notch3 overexpression associates with poor prognosis in human non-small-cell lung cancer. Med Oncol. 2013;30:595. doi: 10.1007/s12032-013-0595-7. [DOI] [PubMed] [Google Scholar]
- 12.Theys J, Yahyanejad S, Habets R, Span P, Dubois L, Paesmans K, Kattenbeld B, Cleutjens J, Groot AJ, Schuurbiers OC, Lambin P, Bussink J, Vooijs M. High NOTCH activity induces radiation resistance in non small cell lung cancer. Radiother Oncol. 2013;108:440–445. doi: 10.1016/j.radonc.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westhoff B, Colaluca IN, D’Ario G, Donzelli M, Tosoni D, Volorio S, Pelosi G, Spaggiari L, Mazzarol G, Viale G, Pece S, Di Fiore PP. Alterations of the Notch pathway in lung cancer. Proc Natl Acad Sci U S A. 2009;106:22293–22298. doi: 10.1073/pnas.0907781106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dang TP, Gazdar AF, Virmani AK, Sepetavec T, Hande KR, Minna JD, Roberts JR, Carbone DP. Chromosome 19 translocation, overexpression of Notch3 and human lung cancer. J Natl Cancer Inst. 2000;92:1355–1357. doi: 10.1093/jnci/92.16.1355. [DOI] [PubMed] [Google Scholar]
- 15.Haruki N, Kawaguchi KS, Eichenberger S, Massion PP, Olson S, Gonzalez A, Carbone DP, Dang TP. Dominant-negative Notch3 receptor inhibits mitogen-activated protein kinase pathway and the growth of human lung cancers. Cancer Res. 2005;65:3555–3561. doi: 10.1158/0008-5472.CAN-04-3132. [DOI] [PubMed] [Google Scholar]
- 16.Konishi J, Kawaguchi KS, Vo H, Haruki N, Gonzalez A, Carbone DP, Dang TP. Gammasecretase inhibitor prevents Notch3 activation and reduces proliferation in human lung cancers. Cancer Res. 2007;67:8051–8057. doi: 10.1158/0008-5472.CAN-07-1022. [DOI] [PubMed] [Google Scholar]
- 17.Fu J, Wang Z, Huang L, Zheng S, Wang D, Chen S, Zhang H, Yang S. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi) Phytother Res. 2014;28:1275–1283. doi: 10.1002/ptr.5188. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Liu F, Yang Y, Li D, Lv J, Ou Y, Sun F, Chen J, Shi Y, Xia P. Astragalus polysaccharide ameliorates ionizing radiation-induced oxidative stress in mice. Int J Biol Macromol. 2014;68:209–214. doi: 10.1016/j.ijbiomac.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Tian QE, De Li H, Yan M, Cai HL, Tan QY, Zhang WY. Effects of Astragalus polysaccharides on P-glycoprotein efflux pump function and protein expression in H22 hepatoma cells in vitro. BMC Complement Altern Med. 2012;12:94. doi: 10.1186/1472-6882-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang BQ, Hu SJ, Qiu LH, Zhu JH, Xie XJ, Sun J, Zhu ZH, Xia Q, Bian K. Effects of Astragalus membranaceus and its main components on the acute phase endothelial dysfunction induced by homocysteine. Vascul Pharmacol. 2007;46:278–285. doi: 10.1016/j.vph.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Jin M, Zhao K, Huang Q, Shang P. Structural features and biological activities of the polysaccharides from Astragalus membranaceus. Int J Biol Macromol. 2014;64:257–266. doi: 10.1016/j.ijbiomac.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Liu H, Bai J, Weng X, Wang T, Li M. Amelioration of insulin resistance in rat cells by Astragalus polysaccharides and associated mechanisms. Exp Ther Med. 2014;7:1599–1604. doi: 10.3892/etm.2014.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang B, Xiao B, Sun T. Antitumor and immunomodulatory activity of Astragalus membranaceus polysaccharides in H22 tumorbearing mice. Int J Biol Macromol. 2013;62:287–290. doi: 10.1016/j.ijbiomac.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Tian QE, Li HD, Yan M, Cai HL, Tan QY, Zhang WY. Astragalus polysaccharides can regulate cytokine and P-glycoprotein expression in H22 tumor-bearing mice. World J Gastroenterol. 2012;18:7079–7086. doi: 10.3748/wjg.v18.i47.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo L, Bai SP, Zhao L, Wang XH. Astragalus polysaccharide injection integrated with vinorelbine and cisplatin for patients with advanced non-small cell lung cancer: effects on quality of life and survival. Med Oncol. 2012;29:1656–1662. doi: 10.1007/s12032-011-0068-9. [DOI] [PubMed] [Google Scholar]
- 26.Hassan WA, Yoshida R, Kudoh S, Hasegawa K, Niimori-Kita K, Ito T. Notch1 controls cell invasion and metastasis in small cell lung carcinoma cell lines. Lung Cancer. 2014;86:304–310. doi: 10.1016/j.lungcan.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Guo J, He L, Yuan P, Wang P, Lu Y, Tong F, Wang Y, Yin Y, Tian J, Sun J. ADAM10 overexpression in human non-small cell lung cancer correlates with cell migration and invasion through the activation of the Notch1 signaling pathway. Oncol Rep. 2012;28:1709–1718. doi: 10.3892/or.2012.2003. [DOI] [PubMed] [Google Scholar]
- 28.Krikelis D, Kotoula V, Bobos M, Fountzilas E, Markou K, Karasmanis I, Angouridakis N, Vlachtsis K, Kalogeras KT, Nikolaou A, Fountzilas G. Protein and mRNA expression of notch pathway components in operable tumors of patients with laryngeal cancer. Anticancer Res. 2014;34:6495–6503. [PubMed] [Google Scholar]
- 29.Dell’albani P, Rodolico M, Pellitteri R, Tricarichi E, Torrisi SA, D’Antoni S, Zappia M, Albanese V, Caltabiano R, Platania N, Aronica E, Catania MV. Differential patterns of NOTCH1-4 receptor expression are markers of glioma cell differentiation. Neuro Oncol. 2014;16:204–216. doi: 10.1093/neuonc/not168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasto A, Serafin V, Pilotto G, Lago C, Bellio C, Trusolino L, Bertotti A, Hoey T, Plateroti M, Esposito G, Pinazza M, Agostini M, Nitti D, Amadori A, Indraccolo S. NOTCH3 signaling regulates MUSASHI-1 expression in metastatic colorectal cancer cells. Cancer Res. 2014;74:2106–2118. doi: 10.1158/0008-5472.CAN-13-2022. [DOI] [PubMed] [Google Scholar]
- 31.Huang J, Song H, Liu B, Yu B, Wang R, Chen L. Expression of Notch-1 and its clinical significance in different histological subtypes of human lung adenocarcinoma. J Exp Clin Cancer Res. 2013;32:84. doi: 10.1186/1756-9966-32-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abuelsaad AS. Supplementation with Astragalus polysaccharides alters Aeromonas-induced tissue-specific cellular immune response. Microb Pathog. 2014;66:48–56. doi: 10.1016/j.micpath.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Zhao X, Liang M, Yang P, Guo F, Pan D, Huang X, Li Y, Wu C, Qu T, Zhu R. Taishan Pinus massoniana pollen polysaccharides promote immune responses of recombinant Bordetella avium ompA in BALB/c mice. Int Immunopharmacol. 2013;17:793–798. doi: 10.1016/j.intimp.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Jiang JB, Qiu JD, Yang LH, He JP, Smith GW, Li HQ. Therapeutic effects of astragalus polysaccharides on inflammation and synovial apoptosis in rats with adjuvant-induced arthritis. Int J Rheum Dis. 2010;13:396–405. doi: 10.1111/j.1756-185X.2010.01555.x. [DOI] [PubMed] [Google Scholar]
- 35.Ye MN, Chen HF, Zhou RJ, Liao MJ. [Effects of Astragalus polysaccharide on proliferation and Akt phosphorylation of the basal-like breast cancer cell line] . Zhong Xi Yi Jie He Xue Bao. 2011;9:1339–1346. doi: 10.3736/jcim20111210. [DOI] [PubMed] [Google Scholar]
- 36.Shan X, Fu YS, Aziz F, Wang XQ, Yan Q, Liu JW. Ginsenoside Rg3 inhibits melanoma cell proliferation through down-regulation of histone deacetylase 3 (HDAC3) and increase of p53 acetylation. PLoS One. 2014;9:e115401. doi: 10.1371/journal.pone.0115401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Y, Ho WS. Combination of liquiritin, isoliquiritin and isoliquirigenin induce apoptotic cell death through upregulating p53 and p21 in the A549 non-small cell lung cancer cells. Oncol Rep. 2014;31:298–304. doi: 10.3892/or.2013.2849. [DOI] [PubMed] [Google Scholar]
- 38.Xie D, Lan L, Huang K, Chen L, Xu C, Wang R, Shi Y, Wu X, Wang L, Liu Y, Lu B. Association of p53/p21 expression and cigarette smoking with tumor progression and poor prognosis in non-small cell lung cancer patients. Oncol Rep. 2014;32:2517–2526. doi: 10.3892/or.2014.3538. [DOI] [PubMed] [Google Scholar]


