Abstract
Interleukin-2 (IL-2) is an important member of the cytokines that play critical roles in carcinogenesis. Many studies have investigated the association between IL-2 rs2069762 polymorphism and cancer risk; however, the results remain controversial. The aim of this study is to assess the correlation between IL-2 rs2069762 polymorphism and cancer risk. All eligible case-control studies accorded with criteria published up to March 30, 2015 were identified by searching Embase and PubMed databases. Association between IL-2 rs2069762 polymorphism and cancer risk was assessed by crude odds ratios (ORs) with 95% confidence intervals (CIs), respectively. Ten case-control studies from nine publications with 3095 cases and 4480 controls were included. Overall, IL-2 rs2069762 polymorphism was not associated with cancer risk in five genetic models (G vs. T: OR = 1.07, 95% CI = 0.95-1.21, P = 0.278; GG vs. TT: OR = 1.16, 95% CI = 0.86-1.57, P = 0.317; GG + TG vs. TT: OR = 1.09, 95% CI = 0.93-1.28, P = 0.273; GG vs. TT + TG: OR = 1.11, 95% CI = 0.85-1.44, P = 0.451; TG vs. TT: OR = 1.08, 95% CI = 0.92-1.28, P = 0.339, respectively). Similar results were also obtained after stratified by ethnicity and cancer type. This meta-analysis indicates that IL-2 rs2069762 T>G polymorphism is not associated with cancer risk. And the same conclusion is drawn after stratified by cancer type and ethnicity.
Keywords: IL-2, polymorphism, cancer susceptibility, meta-analysis
Introduction
Cancer is one of the leading causes of death in economically both developed and developing countries; the burden of cancer is estimated to grow worldwide on account of the aging and growth of the population, particularly in developing countries [1]. According to 2012 global cancer statistics, approximately 14.1 million new cases and 8.2 million deaths of cancer occurred in 2012 worldwide [1]. Cancer is attributed to many factors. Inflammation has been considered as a driving factor in pathogenesis of many cancers [2,3]. Chronic inflammation may lead to oxidative stress and help to bring about tumor promotion and progression [4]. Cytokines production generated by immune/inflammatory cells is one of the major tumor-promoting mechanisms [5]. Cytokines are soluble glycoproteins or proteins that are necessary to the function of immunocytes and play vital roles in the inflammatory response [4].
As a potent immunoregulatory cytokine participating in cell-mediated immune response, interleukin-2 (IL-2) is generated by T cells when they are stimulated by mitogens, or interplay with major histocompatibility complexes in antigen-presenting cells and act as one of the T-cell growth factors [6]. IL-2 participates in the regulation of functional activities and proliferation of T cell and natural killer (NK) cell [6]. It also increases NK cell cytolytic activity and promotes immunoglobulin production by B cells [7,8]. Moreover, IL-2 regulates the expansion and apoptosis of activated T cells and is involved in the regulatory T cells development [9]. In regard to its correlation to cancer, IL-2 has been estimated to play a crucial role in antitumor immunity [10]. Many clinical studies have indicated that IL-2 gene transfected into cancer cells can enhance both nonspecific and specific antitumor immune responses [11]. What’s more, recombinant IL-2 has been proved to be a promising agent for activation of immune response against different cancers [12]. These results indicated that the IL-2 gene could be a candidate for cancer gene treatment or therapy.
The IL-2 gene is located in chromosome 4q26-q27 in humans, which includes five exons and five introns. IL-2 genetic polymorphisms may influence IL-2 production or protein expression and thus might modulate cancer risk. IL-2 rs2069762 (-330 T>G) polymorphism in the promoter region of IL-2 gene lies near an important transcription factor which is nuclear factor of activated T cells. Thus, this site may affect the activity of transcription factors and then influence the transcription and translation standards of IL-2 [13]. This single nucleotide polymorphism (SNP) acts as a desired marker for genetic study since it has two common alleles. Recently, it was estimated that IL-2 polymorphisms have been related to the susceptibility to several cancers, such as bladder cancer [14], nasopharyngeal carcinoma [15], head and neck cancer [16] and Non-Hodgkin Lymphoma [17]. In this study, we aimed to estimate more precisely the association between IL-2 rs2069762 T>G polymorphism and cancer risk by combining all related publications up to date.
Materials and methods
Identification of eligible studies
We searched relative articles and reviews up to March 30, 2015 in two electronic databases, Embase and PubMed, using key words ‘IL-2 or interleukin-2’, ‘polymorphism or SNP’, and ‘cancer or carcinoma or malignance’. Only English papers were adopted in our study. References of relative studies and reviews were manually retrieved for additional studies. Studies included in our meta-analysis conformed to the following criteria: (1) evaluation of the IL-2 polymorphism and cancer risk, (2) case-control study and (3) control genotype distributions according with Hardy-Weinberg equilibrium (HWE). Excluded criteria were: (1) overlapping data, (2) not case-control studies and (3) review publications, comments and letters.
Data extraction
According to the standard criteria, data from the published studies were extracted independently by three authors (Y. Wang, Y. Shu and H. Jiang) to populate the requisite information. For each study, the following details were collected: publication data, the first author’s surname, country of origin, year of publication, cancer type, ethnicity of subjects, sample size, source of controls, genotype frequency in controls and cases and HWE in controls. In regard to conflicting evaluations, they were solved among all authors by further discussion.
Statistical analysis
We tested HWE of genotype frequencies in control populations using the X2 test. The strength of the association between IL-2 rs2069762 T>G polymorphism and cancer risk was measured by Odds ratios (ORs) with 95% confidence intervals (CIs) in allele model (G vs. T), homozygous model (GG vs. TT), dominant model (GG + TG vs. TT), recessive model (GG vs. TG + TT), and additive model (TG vs. TT). Z test was used to examine the statistical significance of the pooled ORs. I2 test and Q-test were used to quantify the effect of heterogeneity. The random effects model (DerSimonian Laird method) was used when a significant Q-test (P<0.1) or I2>50%, otherwise, the fixed effects model (Mantel-Haenszel method) was carried out [18]. To identify the source of heterogeneity, we performed subgroup analysis by ethnicity, cancer type, source of controls and number of cases. Sensitivity was examined by omitting each study in turn to estimate the stability of results. The publication bias was evaluated by Begger’s funnel plots and Egger’s linear regression test (P<0.1) [19]. Between-study heterogeneities and variations were estimated by Galbraith radial plot, and P<0.05 was considered to be statistically significant [20]. All P values were two-sided and statistical analyses were calculated by Stata Version 12.0 software (Stata Corp, College Station, TX).
Results
Characteristics of studies
After searching based on the eligible criteria, initially a total of 542 potentially relevant publications were yielded. The detailed study extraction processing was shown in Figure 1. In the article reported by Savage et al., esophageal cancer and gastric cancer were separately studied, thus each of them was regarded as an individual study. Finally, a total of 10 case-control studies from 9 articles were included in our meta-analysis.
Figure 1.
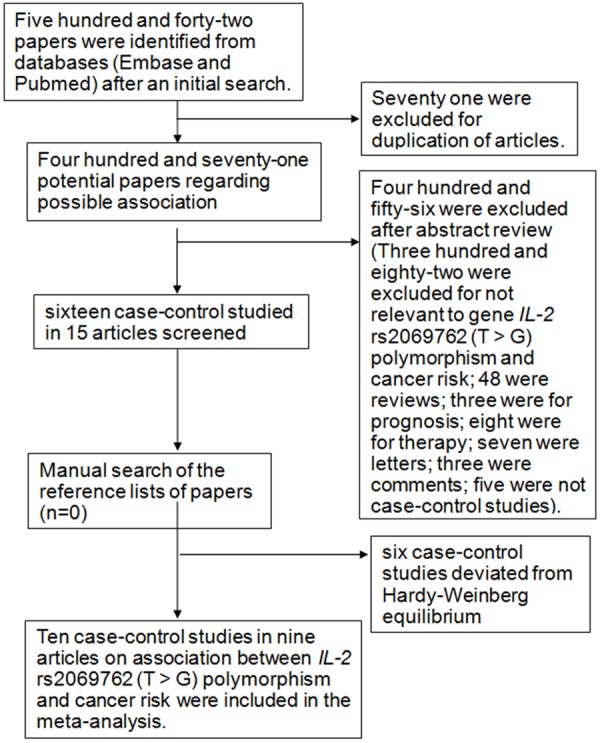
Flow diagram of articles selection procedures for IL-2 rs2069762 polymorphism and cancer risk meta-analysis.
A total of 3095 cases and 4480 controls were involved in our meta-analysis (Table 1). Among these studies, three discussed the association between the IL-2 rs2069762 T>G polymorphism and gastric cancer, two discussed the association between the IL-2 rs2069762 T>G polymorphism and hepatocellular carcinoma, and five discussed the association between the IL-2 rs2069762 T>G polymorphism and other cancers (lymphoma, nasal pharyngeal cancer, esophageal cancer, melanoma, skin cancer). According to ethnicity, two studies discussed Caucasians and 8 studies discussed Asians. According to control source, eight studies were hospital-based and two were population-based. According to the number of cases, they were <500 in seven studies and ≥500 in three studies. The distribution of IL-2 rs2069762 T>G polymorphism and allele among controls and patients is listed in Table 2.
Table 1.
Characteristics of individual studies included in the meta-analysis
| Study | Year | Vountry | Ethnicity | Cancer type | No. of cases/control | Souce of control | Genotype method |
|---|---|---|---|---|---|---|---|
| Peng et al. | 2014 | China | Asians | Hepatocellular Carcinoma | 107/105 | hospital based | PCR-RFLP |
| Bei et al. | 2014 | China | Asians | Hepatocellular Carcinoma | 720/784 | hospital based | TaqMan |
| Song et al. | 2012 | Korea | Asians | Lymphoma | 40/254 | hospital based | OPA |
| Wei et al. | 2010 | China | Asians | Nasal Pharyngeal Cancer | 180/200 | hospital based | PCR-RFLP |
| Ko et al. | 2009 | Korea | Asians | Gastric cancer | 84/336 | population based | Snapshot |
| Wu et al. | 2009 | China | Asians | Gastric cancer | 1045/1100 | population based | PCR-RFLP |
| Savage et al. | 2004 | China | Asians | Esophageal cancer | 130/454 | hospital based | Snapshot |
| Savage et al. | 2004 | China | Asians | Gastric cancer | 91/454 | hospital based | Snapshot |
| Howell et al. | 2003 | Britain | Caucasians | Melanoma | 169/261 | hospital based | PCR-ARMS |
| Rizzato1 et al. | 2011 | Hungary | Caucasians | Skin cancer | 529/532 | hospital based | KASPar SNP genotyping |
PCR-RFLP: polymerase chain reaction-restriction fragment length polymorphism; OPA: GoldenGate™ oligonucleotide pool assay; PCR-ARMS: Amplification Refractory Mutation System-Polymerase Chain Reaction.
Table 2.
Distribution of IL-2 rs2069762 polymorphism genotype and allele among cancer patients and controls
| Study | Year | Case | Control | Case | Control | HWE | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| TT | TG | GG | TT | TG | GG | G | T | G | T | |||
| Peng et al. | 2014 | 47 | 54 | 6 | 42 | 54 | 9 | 66 | 148 | 72 | 138 | YES |
| Bei et al. | 2014 | 292 | 333 | 95 | 311 | 373 | 100 | 523 | 917 | 573 | 995 | YES |
| Song et al. | 2012 | 7 | 11 | 5 | 87 | 54 | 7 | 21 | 25 | 68 | 228 | YES |
| Wei et al. | 2010 | 46 | 106 | 28 | 81 | 102 | 17 | 162 | 198 | 136 | 264 | YES |
| Ko et al. | 2009 | 34 | 44 | 3 | 156 | 132 | 43 | 50 | 112 | 218 | 444 | YES |
| Wu et al. | 2009 | 491 | 441 | 94 | 516 | 480 | 87 | 629 | 1423 | 654 | 1512 | YES |
| Savage et al. | 2004 | 33 | 43 | 35 | 109 | 174 | 96 | 113 | 109 | 366 | 392 | YES |
| Savage et al. | 2004 | 20 | 47 | 16 | 109 | 174 | 96 | 79 | 87 | 366 | 392 | YES |
| Howell et al. | 2003 | 79 | 44 | 14 | 86 | 61 | 13 | 72 | 202 | 87 | 233 | YES |
| Rizzato et al. | 2011 | 215 | 223 | 58 | 221 | 221 | 60 | 339 | 653 | 341 | 663 | YES |
Quantitative synthesis
Overall, we found no evidence of association between IL-2 rs2069762 T>G polymorphism and cancer risk (G vs. T: OR = 1.07, 95% CI = 0.95-1.21, P = 0.278; GG vs. TT: OR = 1.16, 95% CI = 0.86-1.57, P = 0.317; GG + TG vs. TT: OR = 1.09, 95% CI = 0.93-1.28, P = 0.273; GG vs. TT + TG: OR = 1.11, 95% CI = 0.85-1.44, P = 0.451; TG vs. TT: OR = 1.08, 95% CI = 0.92-1.28, P = 0.339, Table 3 and Figure 2). In the stratified analyses, there was no significant relationship for different cancer, similarly, there was no significant relationship for Asians nor Caucasians (Table 3).
Table 3.
Synthesis of results of the meta-analysis from different comparative gene models in the total and subgroup analysis
| No. of study | G vs. T | GG vs. TT | GG + TG vs. TT | GG vs. TG + TT | TG vs. TT | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||||||||
| OR (95% CI) | P | P (Q-test) | OR (95% CI) | P | P (Q-test) | OR (95% CI) | P | P (Q-test) | OR (95% CI) | P | P (Q-test) | OR (95% CI) | P | P (Q-test) | ||
| Total | 10 | 1.07 (0.95-1.21) | 0.278 | 0.019 | 1.16 (0.86-1.57) | 0.317 | 0.009 | 1.09 (0.93-1.28) | 0.273 | 0.047 | 1.11 (0.85-1.44) | 0.451 | 0.016 | 1.08 (0.92-1.28) | 0.339 | 0.060 |
| Ethnicity | ||||||||||||||||
| Asians | 8 | 1.10 (0.94-1.29) | 0.226 | 0.007 | 1.21 (0.82-1.78) | 0.343 | 0.003 | 1.16 (0.94-1.42) | 0.170 | 0.023 | 1.11 (0.79-1.56) | 0.531 | 0.006 | 1.15 (0.93-1.42) | 0.198 | 0.035 |
| Caucasians | 2 | 1.00 (0.85-1.18) | 0.980 | 0.789 | 1.03 (0.71-1.48) | 0.886 | 0.722 | 0.99 (0.79-1.23) | 0.893 | 0.486 | 1.03 (0.73-1.45) | 0.874 | 0.537 | 0.97 (0.77-1.23) | 0.829 | 0.330 |
| Cancer type | ||||||||||||||||
| Hepatocellular carci | 2 | 0.97 (0.85-1.12) | 0.704 | 0.505 | 0.97 (0.71-1.32) | 0.848 | 0.371 | 0.95 (0.78-1.15) | 0.594 | 0.676 | 1.00 (0.75-1.34) | 0.991 | 0.383 | 0.94 (0.77-1.16) | 0.572 | 0.840 |
| Gastric cancer | 3 | 1.00 (0.89-1.13) | 0.943 | 0.827 | 1.00 (0.76-1.32) | 0.992 | 0.135 | 1.03 (0.89-1.21) | 0.669 | 0.532 | 0.72 (0.37-1.42) | 0.348 | 0.027 | 1.20 (0.85-1.68) | 0.305 | 0.116 |
| Other cancer | 5 | 1.26 (0.96-1.66) | 0.094 | 0.004 | 1.70 (0.96-3.01) | 0.069 | 0.007 | 1.26 (0.87-1.83) | 0.220 | 0.009 | 1.49 (0.98-2.26) | 0.060 | 0.059 | 1.15 (0.81-1.63) | 0.446 | 0.029 |
| Source of Control | ||||||||||||||||
| Hospital based | 8 | 1.11 (0.94-1.31) | 0.218 | 0.008 | 1.27 (0.89-1.82) | 0.185 | 0.012 | 1.13 (0.90-1.41) | 0.288 | 0.023 | 1.18 (0.88-1.57) | 0.271 | 0.044 | 1.09 (0.88-1.34) | 0.440 | 0.062 |
| Population based | 2 | 1.01 (0.89-1.14) | 0.892 | 0.560 | 0.70 (0.21-2.35) | 0.558 | 0.049 | 1.02 (0.86-1.19) | 0.853 | 0.413 | 0.61 (0.14-2.66) | 0.511 | 0.016 | 1.14 (0.74-1.76) | 0.551 | 0.092 |
| Number of cases | ||||||||||||||||
| <500 | 7 | 1.15 (0.90-1.46) | 0.260 | 0.007 | 1.28 (0.70-2.32) | 0.423 | 0.002 | 1.24 (0.91-1.67) | 0.172 | 0.030 | 1.13 (0.67-1.92) | 0.639 | 0.003 | 1.23 (0.90-1.67) | 0.194 | 0.045 |
| ≥500 | 3 | 1.01 (0.92-1.10) | 0.852 | 0.953 | 1.05 (0.86-1.28) | 0.603 | 0.838 | 0.99 (0.88-1.11) | 0.870 | 0.927 | 1.07 (0.88-1.28) | 0.507 | 0.781 | 0.98 (0.86-1.10) | 0.694 | 0.873 |
Figure 2.
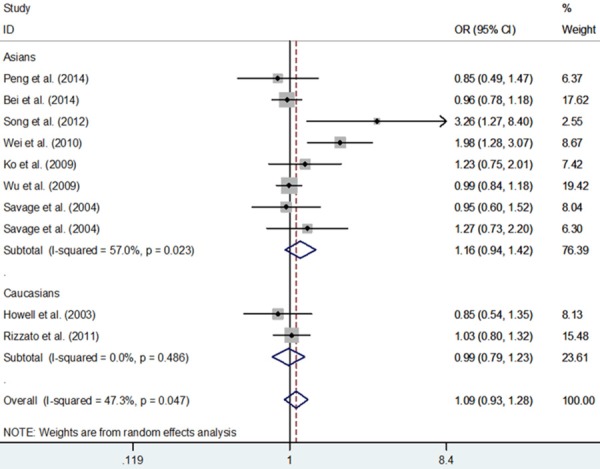
Meta-analysis with a random-effects model for the association between IL-2 rs2069762 polymorphism and cancer risk (GG + TG vs. TT).
Bias diagnostics
Egger’s test and Begg’s funnel plot were used to assess the publication bias, and no evidence of bias was found (G vs. T: Begg’s test P = 0.592, Egger’s test P = 0.279; GG vs. TT: Begg’s test P = 1.000, Egger’s test P = 0.633; GG + TG vs. TT: Begg’s test P = 0.210, Egger’s test P = 0.124; GG vs. TT + TG: Begg’s test P = 1.000, Egger’s test P = 0.973; TG vs. TT: Begg’s test P = 0.283, Egger’s test P = 0.136, Figure 3).
Figure 3.
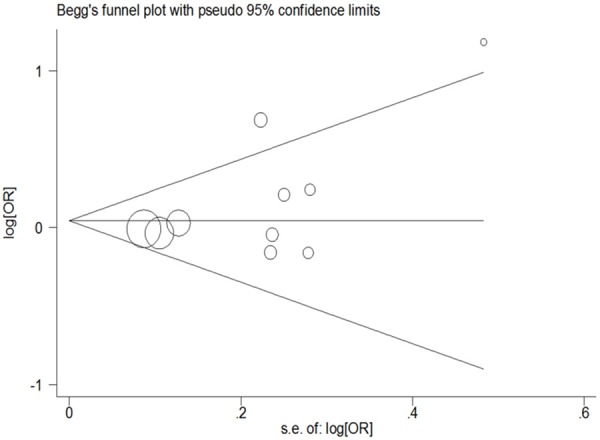
Funnel plot analysis to detect publication bias for the IL-2 rs2069762 polymorphism in the involved ten data sets (random-effects estimates, GG + TG vs. TT compare genetic model).
Sensitivity analyses
We performed one-way sensitivity analyses by sequential omission of individual study each time. The analysis results indicated that no individual study significantly influenced the pooled ORs (Figure 4, data not shown), indicating the statistically robust results.
Figure 4.
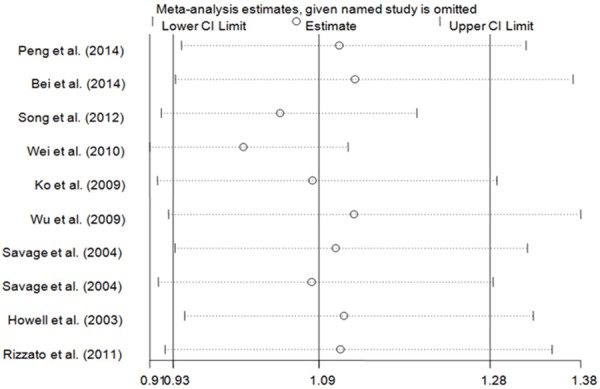
Sensitivity analysis of the effect of GG + TG vs. TT in overall cancer meta-analysis (random-effects assess).
Test of heterogeneity
The results manifested there were significant heterogeneities among the studies included. Because ethnicity and cancer type could affect the results of meta-analyses, we carried out subgroup analyses by cancer type and ethnicity (Table 3). The results suggested that Asian population subgroup and other cancer type may contribute to the heterogeneity. As shown in Table 3, heterogeneity was significant in five models comparison. Galbraith radial plot also was used to analyze the heterogeneity in dominant model comparison (Figure 5). According to the forest plot, we identified that the studies of Wei et al. [15] and Song et al. [21] might mainly contribute to heterogeneity. Further stratified meta-analysis suggested that more prominent heterogeneity was associated with studies of small number of cases (<500 cases) and hospital-based (Table 3).
Figure 5.
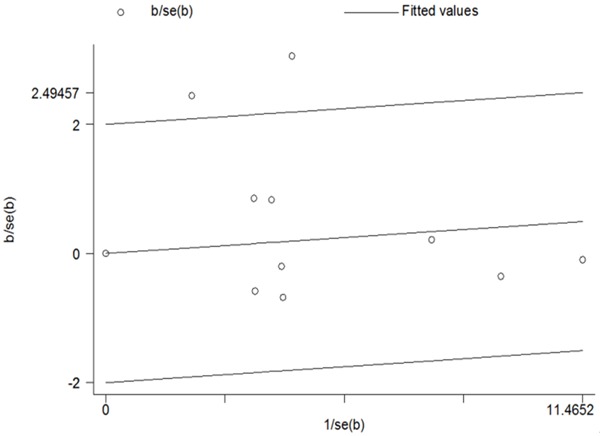
Meta-analysis heterogeneity by Galbraith radial plot (GG + TG vs. TT compare genetic model).
Discussion
The results showed that IL-2 rs2069762 T>G polymorphism may not be correlated with cancer risk. Analogous results were found after stratified by cancer type and ethnicity. This is the first meta-analysis including a total of 3095 cases and 4480 controls from 10 case-control studies examining the association of IL-2 rs2069762 T>G polymorphism with cancer risk.
With the increase of gene association studies, it is even more encouraged to synthesize available data to solve persistent difficulties in obtaining replicable and robust results. Most common gene defects usually make small-to-moderate contributions to malignance risk. So far, many studies had concentrated on the association between IL-2 rs2069762 T>G polymorphism and cancer risk, but the results were still vague. Several individual studies have provided positive signals of IL-2 gene rs2069762 T>G polymorphism with cancer, such as hepatocellular carcinoma [22], breast cancer [23], bladder cancer [14], contrastingly, as illustrated in our meta-analysis among 3095 cases and 4480 controls, there was null association, even in different ethnicity and cancer type. In consideration of a modest sum of studies included in our meta-analysis and most of small sample size, there is a suggestion of possible beneficial, reinforcing additional large studies to overthrow or confirm these findings. It is also possible that the latent role of this polymorphism is masked or diluted by other gene-environment or gene-gene interactions. Carcinogenesis is a multifactorial process involving interaction between genetic and environmental factors. In this study, we only summarized one single polymorphism and did not take environmental factors, such as tobacco and alcohol consumption, into account.
In the subgroup analysis of ethnicity, no obvious association was found among Caucasians and Asians, which was consistent with the overall data. Variations on various genes may alter cancer susceptibility among different ethnicities [24,25]. Thus, Variations of IL-2 rs2069762 T>G polymorphism may exert different influences on cancer risk among different races. Nevertheless, the results of this study suggested that the polymorphism variations may exert little influence on cancer susceptibility. In this meta-analysis, only two studies about Caucasians were obtained. The insufficient number may give rise to limited statistical power to assess race effect of gene. Therefore, the results should be interpreted carefully. More investigations considering Caucasians are needed to deblur the possible effects of ethnic variations of this SNP on cancer risk.
In the subgroup analysis of cancer type, we also found no association between IL-2 rs2069762 T>G polymorphism and cancer. Perhaps IL-2 rs2069762 T>G polymorphism had disparate influence on different type of cancers with different environmental and exposures carcinogenic mechanisms. Also, for some cancer types, there were only several studies and some studies had limited sample sizes. Thus, our study may be underpowered to detect meaningful association. Therefore, more well-designed and large-scale studies are needed to confirm the association.
In this meta-analysis, we used Galbraith radial plot analysis to test heterogeneity. Heterogeneity between studies for the overall data were evidently found in the five genetic models; therefore, random-effect models were utilized. Then subgroup analysis was carried out to explore the sources of heterogeneity. In the subgroup analyses, evident heterogeneity was also observed in the subgroup analysis on other cancer type, Asians, hospital-based controls and small number of cases. From the forest plot in dominant genetic model, we identified that the studies of Wei et al. [15] and Song et al. [21] as the outliers might mainly contribute to heterogeneity. As regard to publication bias, we did not detect an obvious publication bias in this meta-analysis, indicating the reliability of our results.
Several limitations of this meta-analysis should be addressed. Firstly, among the studied included in this meta-analysis, most of them concerned Asians and only two concerned Caucasians. Data regarding Caucasians were not sufficient. Secondly, subgroup analyses on histological types, age, gender, and other factors (such as smoke and alcohol consumption) were not performed in this study because relevant data were not available in the primary articles. Thirdly, among the included studies, other IL-2 polymorphisms were studied. However, the number of them was so small that we could not perform the corresponding analysis. More investigations with strict matching criteria and larger sample size in view of multiple factors are needed to confirm or refute our findings.
In conclusion, this meta-analysis indicates that the IL-2 rs2069762 T>G polymorphism is not associated with cancer risk. And the same conclusion is drawn after stratified by cancer type and ethnicity. Therefore, for practical reasons, more well-designed and large-scale studies are needed in future to validate or refute the results of this preliminary study.
Acknowledgements
This study was supported by Jiangsu University Clinical medicine science and technology development fund (JLY20120004) and Jiangsu Province Natural Science Foundation (BK2010333, BK2011481).
Abbreviations
- CI
confidence interval
- OR
odds ratio
- IL-2
Interleukin-2
- HWE
Hardy-Weinberg equilibrium
- SNP
single nucleotide polymorphism
- NK
natural killer.
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemai A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schottenfeld D, Beebe-Dimmer J. Chronic inflammation: a common and important factor in the pathogenesis of neoplasia. CA Cancer J Clin. 2006;56:69–83. doi: 10.3322/canjclin.56.2.69. [DOI] [PubMed] [Google Scholar]
- 4.Rizzato C, Canzian F, Rudnai P, Gurzau E, Stein A, Koppova K, Hemminki K, Kumar R, Campa D. Interaction between functional polymorphic variants in cytokine genes, established risk factors and susceptibility to basal cell carcinoma of skin. Carcinogenesis. 2011;32:1849–1854. doi: 10.1093/carcin/bgr197. [DOI] [PubMed] [Google Scholar]
- 5.Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8:887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 6.Seder RA, Paul WE. Acquisition of lymphokineproducing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 7.Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 9.D’Souza WN, Lefrancois L. IL-2 is not required for the initiation of CD8 T cell cycling but sustains expansion. J Immunol. 2003;171:5727–5735. doi: 10.4049/jimmunol.171.11.5727. [DOI] [PubMed] [Google Scholar]
- 10.Chou SH, Shetty AV, Geng Y, Xu L, Munirathinam G, Pipathsouk A, Tan I, Morris T, Wang B, Chen A, Zheng G. Palmitate-derivatized human IL-2: a potential anticancer immunotherapeutic of low systemic toxicity. Cancer Immunol Immunother. 2013;62:597–603. doi: 10.1007/s00262-012-1364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okamoto T, Tsuburaya A, Yanoma S, Yoshikawa T, Cho H, Takanashi Y, Noguchi Y. Inhibition of peritoneal metastasis in an animal gastric cancer model by interferon-gamma and interleukin-2. Anticancer Res. 2003;23:149–153. [PubMed] [Google Scholar]
- 12.Jarmalaite S, Andrekute R, Scesnaite A, Suziedelis K, Husgafvel-Pursiainen K, Jankevicius F. Promoter hypermethylation in tumour suppressor genes and response to interleukin-2 treatment in bladder cancer: a pilot study. J Cancer Res Clin Oncol. 2010;136:847–854. doi: 10.1007/s00432-009-0725-y. [DOI] [PubMed] [Google Scholar]
- 13.Crabtree GR, Clipstone NA. Signal transmission between the plasma membrane and nucleus of T lymphocytes. Annu Rev Biochem. 1994;63:1045–1083. doi: 10.1146/annurev.bi.63.070194.005145. [DOI] [PubMed] [Google Scholar]
- 14.Shen Y, Liu Y, Liu S, Zhang A. The association between -330T/G polymorphism of interleukin 2 gene and bladder cancer. DNA Cell Biol. 2012;31:983–987. doi: 10.1089/dna.2011.1476. [DOI] [PubMed] [Google Scholar]
- 15.Wei YS, Lan Y, Zhang L, Wang JC. Association of the interleukin-2 polymorphisms with interleukin-2 serum levels and risk of nasopharyngeal carcinoma. DNA Cell Biol. 2010;29:363–368. doi: 10.1089/dna.2010.1019. [DOI] [PubMed] [Google Scholar]
- 16.Lopez RV, Zago MA, Eluf-Neto J, Curado MP, Daudt AW, da Silva-Junior WA, Zanette DL, Levi JE, de Carvalho MB, Kowalski LP, Abrahão M, de Góis-Filho JF, Boffetta P, Wünsch-Filho V. Education, tobacco smoking, alcohol consumption, and IL-2 and IL-6 gene polymorphisms in the survival of head and neck cancer. Braz J Med Biol Res. 2011;44:1006–1012. doi: 10.1590/s0100-879x2011007500097. [DOI] [PubMed] [Google Scholar]
- 17.Song H, Chen L, Cha Z, Bai J. Interleukin 2 gene polymorphisms are associated with non-Hodgkin lymphoma. DNA Cell Biol. 2012;31:1279–1284. doi: 10.1089/dna.2011.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang W, Qiu H, Ding H, Sun B, Wang L, Yin J, Gu H. Association between the STK15 F31I polymorphism and cancer susceptibility: a metaanalysis involving 43,626 subjects. PLoS One. 2013;8:e82790. doi: 10.1371/journal.pone.0082790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–680. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 21.Song N, Han S, Lee KM, Choi JY, Park SK, Jeon S, Lee Y, Ahn HS, Shin HY, Kang HJ, Koo HH, Seo JJ, Choi JE, Kang D. Genetic variants in interleukin-2 and risk of lymphoma among children in Korea. Asian Pac J Cancer Prev. 2012;13:621–623. doi: 10.7314/apjcp.2012.13.2.621. [DOI] [PubMed] [Google Scholar]
- 22.Saxena R, Chawla YK, Verma I, Kaur J. Effect of IL-12B, IL-2, TGF-beta1, and IL-4 polymorphism and expression on hepatitis B progression. J Interferon Cytokine Res. 2014;34:117–128. doi: 10.1089/jir.2013.0043. [DOI] [PubMed] [Google Scholar]
- 23.Hu XB, Ouyang LZ, Tang LL. Interleukin-2 gene polymorphisms and prognosis of breast cancer. Genet Test Mol Biomarkers. 2013;17:453–457. doi: 10.1089/gtmb.2012.0494. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt HM, Ha DM, Taylor EF, Kovach Z, Goh KL, Fock KM, Barrett JH, Forman D, Mitchell H. Variation in human genetic polymorphisms, their association with Helicobacter pylori acquisition and gastric cancer in a multi-ethnic country. J Gastroenterol Hepatol. 2011;26:1725–1732. doi: 10.1111/j.1440-1746.2011.06799.x. [DOI] [PubMed] [Google Scholar]
- 25.Solus JF, Arietta BJ, Harris JR, Sexton DP, Steward JQ, McMunn C, Ihrie P, Mehall JM, Edwards TL, Dawson EP. Genetic variation in eleven phase I drug metabolism genes in an ethnically diverse population. Pharmacogenomics. 2004;5:895–31. doi: 10.1517/14622416.5.7.895. [DOI] [PubMed] [Google Scholar]


