Abstract
Introduction: The porcine model is generally accepted for the development and testing of new forms oftherapy including ablation of atrial fibrillation (AF). However, the challenging left atrial (LA) and pulmonary vein (PV) anatomy enables only limited percutaneous catheter-based PV access. Results: Here we present I) an alternative percutaneous transapical access, which enables easy and safe retrograde transmitral LA and PV mapping and ablation; II) early experience of LA mapping and successful circumferential PV isolation with novel mapping system (RhythmiaTM) and new generation of ablation catheter equipped with micro electrodes (IntellaTip MiFi). Conclusion: Although the experience with the transapical approach is limited, the initial results are promising as this may offer an alternative approach for tasting new technologies and translational research.
Keywords: Porcine model, ablation, atrial fibrillation, translational research
Introduction
Recently, a new rapid high-resolution electroanatomical cardiac mapping system (RhythmiaTM Mapping System, Boston Scientific Inc., Natick, MA, USA) has been introduced. The first preclinical experience described by Nakagawa et al. [1] has illustrated accurate and quick identification of right atrial geometry and complex activation patterns in 10 canines.
The pig heart is an accepted model for the development and testing of new forms for atrial fibrillation (AF) therapies [2]. However, the challenging left atrial (LA) and pulmonary vein (PV) anatomy enables only limited percutaneous catheter-based PV access via a transseptal route and limits the potential in this experimental setting [3].
In this report, we present two novel findings: (1) we propose a percutaneous transapical access technique in which a fluoroscopically guided puncture of the left ventricular apex (LVA) enables easy and safe retrograde transmitral LA and PV mapping and ablation; (2) we describe our experience of LA mapping and successful circumferential antral PV isolation in a porcine model using the RhythmiaTM mapping system in 3 animals.
Methods
Experimental setting
Three consecutive female pigs weighing 45 to 77 kg were anesthetized and mechanically ventilated. The right carotid artery was cannulated for monitoring arterial pressure, the left femoral artery and the right femoral vein were cannulated with 8 Fr sheaths for catheter placement. A 6 Fr 8-pole catheter was positioned in the coronary sinus (CS) under fluoroscopic guidance. A second 6 Fr 8-electrode catheter was retrogradely positioned in LVA for anatomical guidance and backup pacing.
A 6 Fr sheath was positioned through the right jugular vein in the atrial orifice of the superior vena cava for drug administration. Intravenous infusion of amiodarone (450 mg bolus followed by 90 mg/hour) was initiated from the beginning of the procedure to avoid mechanically induced ventricular tachycardia or fibrillation. In case of amiodarone-induced pressure drop the flow rate was reduced and/or continued infusion of noradrenalin (200-1000 mg/h) was started.
Transapical approach
The precise location of the puncture site within the subxyphoidal region and the needle orientation (30 and 45°) was guided fluoroscopically in right lateral (comparable to human RAO 30°) and anterior-posterior view (comparable to human LAO 60°). A commercially available 4F subxyphoidal puncture needle (Pajunk, Tuohy 120 mm) and guide wire were used.
After entering the pericardial space the LVA was punctured and LV chamber cannulated and a contrast injection was used to confirm the correct position (Video S1). The guide wire was placed into the left ventricular outflow tract assuring safe and atraumatic introduction of a 18-25 cm long, 9 Fr sheath (Video S2). A conventional sheath (Terumo, Radiofucus Introducer II, 9 Fr) was used in the first experiment. However, because of the substantial thickness of the apical myocardium (up to 2 cm) and resulting partial crush of the sheath an armored 9 Fr sheath (Arrow, Super Arrow-Flex, 24 mm) was used in the other 2 cases.
For retrograde transmitral access a steerable catheter was first introduce to the left ventricular outflow tract and then deflected and retracted to the posterior region of the ventricle in order to shape a loop to cross the mitral valve and enter the LA which was followed by advancement of the sheath. The position was than confirmed by the LA and PV angiogram (Figure 1) followed by LA (case 1) and PV mapping and ablation (cases 2 and 3).
Figure 1.
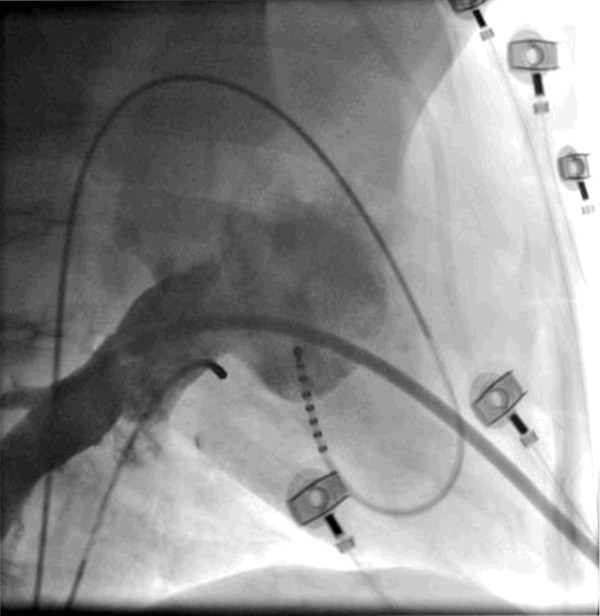
LA and PV angiogram using a right lateral projection (comparable to human RAO 30°) during rapid ventricular pacing. Please note the sheath in the lower PV exhibiting a favorable angle when introduced retrograde from the LV.
Electroanatomical mapping and ablation
Electroanatomical mapping with the Rhythmia™ mapping system was conducted as previously described [1]. In brief, rapid and automatic mapping of the LA and PV was performed by maneuvering the 8 Fr bidirectional, irrigated, deflectable mini-basket catheter with 8 splines containing 8 small (0.4 mm2), low impedance electrodes (total 64 electrodes, IntellaMap Orion™ Mapping Catheter) during sinus rhythm. At the start of each map, the undeployed mini-basket catheter was maneuvered into the LA and PV under fluoroscopic guidance. Further mapping was conducted using the initial geometric shell and mapping system for anatomical guidance. The maps were created using electrograms within 5 mm of the surface reconstruction.
Catheter ablation was conducted with an irrigated 8-mm-tip catheter equipped with radially placed micro electrodes (IntellaTip MiFi, Boston Scientific) with presets of 30 W and 40°C.
Voltage LA and PV maps before and after ablation were compared and amplitudes over 0.5 mV were defined as normal and potentials under 0.2 mV as scar.
The animals were euthanized at the end of the procedure. The experimental protocol was approved and conducted according to current standards for animal use and care.
Results
Case 1-proof of concept
In all animals, transapical puncture and LA access were achieved without complications. In case 1, the entire LA and PVs were mapped within approximately 17 minutes and contained electroanatomical information of 11.952 mapping points spread over a volume of 42.77 cc (Figure 2). The antrum of two pulmonary veins and their distal bifurcation into main lobe veins were visualized. The posterior wall of the LA and enlarged characteristic LA appendage could also be easily accessed and mapped.
Figure 2.
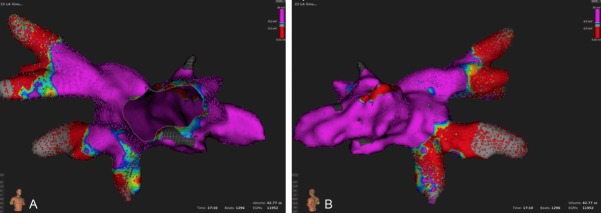
Electroanatomical voltage map of the LA and PV from anterior (A) and posterior (B). Please note two prominent PV ostia and LA appendage which is a typical finding in pigs.
The mitral annulus was identified and annotated in the review mode to create the final LA/PV model (Video S3).
Case 2-mapping reproducibility and ablation
In case 2, two maps of a predefined region of the LA including the right superior PV with an upper and lower branch were obtained by two different operators and the reproducibility was tested. The maps were acquired within approximately 10 and 12 minutes and contained 12.716 and 19.186 points within 19.89 cc. The distribution of low- and high-voltage areas was very consistent as presented in Figure 3.
Figure 3.
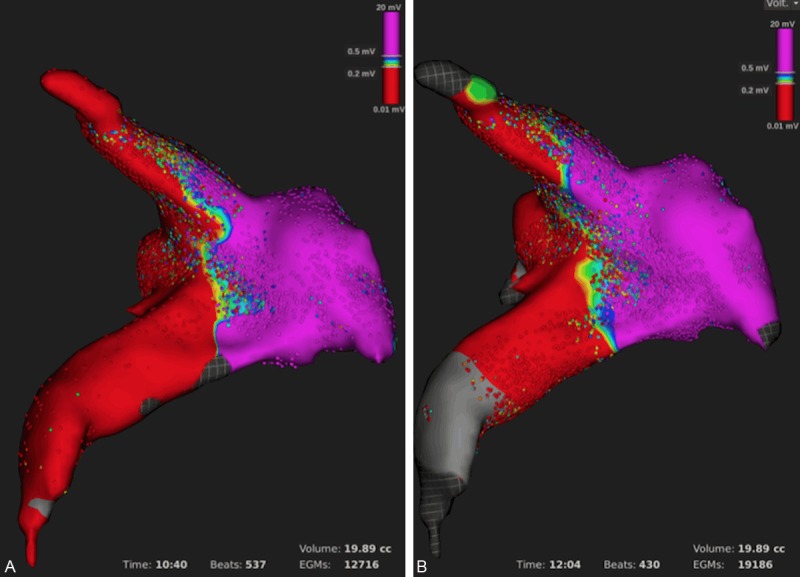
Reproducibility of the first LA and PV voltage map (A) and a second re-map (B) performed by two different operators.
The first ablation attempt resulted in incomplete PV isolation which was evidenced by atrial capture when pacing from the equatorial leads of the mini-basket catheter from within the PV. A remap that was performed within 4 minutes by collecting 19.464 points identified the gap anterior-superior. In the review mode, a high-voltage potential within the gap region was confirmed (Figure 4A). Additional map-guided ablation resulted in complete antral PV isolation that was verified by exit and entrance block. A re-map performed in 7 minutes with 32.116 points showed a large antral low-voltage area (Figure 4B).
Figure 4.
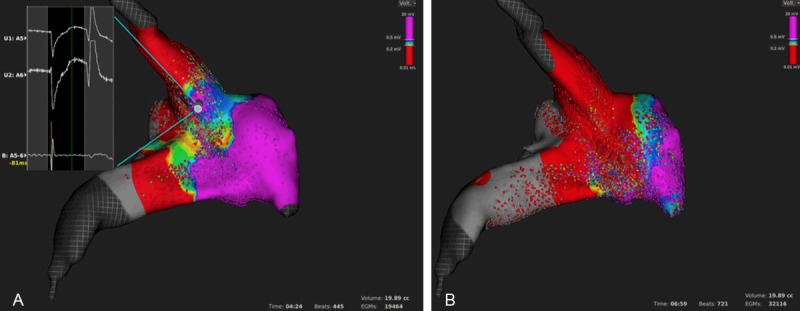
Antral PV ablation. Identification of a gap (A) by voltage mapping and corresponding uni- and bipolar electrograms (inlet). LA and PV voltage map after complete antral PV isolation (B). Please note the large antral low-voltage area as compared with the baseline map in Figure 3.
Case 3-ablation reproducibility and quality control in review mode
In case 3, antral ablation of the right upper PV branch was performed and the review mode was used for verification of successful signal modification in proximity to the ablation lesions. Figure 5 depicts the different signals obtained from the scar zone, ablation line and intact myocardium. The circumferential inspection of the antral region enabled a fast and effective verification of the continuity of the ablation line which was then confirmed by pacing maneuvers.
Figure 5.
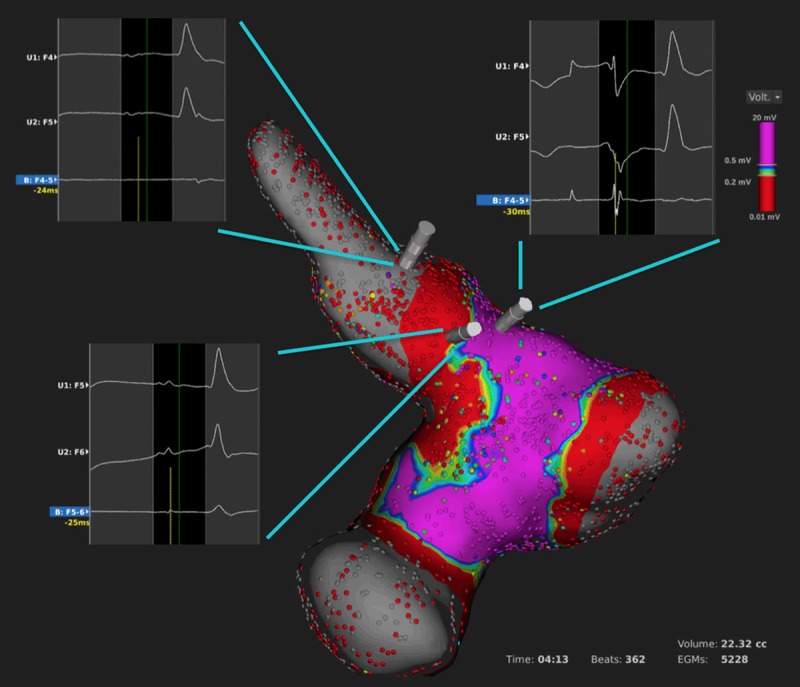
LA and PV voltage map after complete isolation of the upper PV and verification of the ablation lesions in the review mode. Please note the different unipolar and bipolar electrograms within the PV (left upper inlet), along the ablation line (left lower inlet) and outside the ablation line (right upper inlet).
Discussion
To the best of our knowledge, this is the first report of LA mapping and PV ablation using a new rapid high-density mapping system and novel ablation catheter equipped with microelectrodes. Furthermore, we successfully elaborated an alternative experimental approach to the LA and PV overcoming the anatomical limitations of a porcine model.
As presented in our case series, a rapid, very high-density acquisition of electroanatomical signals of the LA and PV can be performed. Voltage mapping of ablation lines can provide additional information about lesion extension and possible gap location. This approach may therefore be useful for improving AF ablation outcomes, simplify the procedure and reduce procedure times. Nevertheless, our preliminary results regarding LA and PV mapping and ablation are limited to an experimental setting and validation of the system in human cases is mandatory.
The suggested percutaneous transapical access was easy, safe and offered superior catheter maneuverability in the small pig LA and PV. Aside from LA and PV-based experimental procedures, it might be also beneficial for different pre-clinical studies including mapping and ablation of ventricular tachycardias, testing of wireless intracardiac stimulation devices or new minimal-invasive congenital heart failure treatment techniques. However the role of the transapical approach in non-acute experimental setting must be established, especially since closure of the percutaneous puncture site was not attempted.
Although the experience with the transapical approach is limited to our experiment and a small case series describing among other procedures one ventricular tachycardia ablation, those initial results are promising as this may offer an alternative approach in highly-selected patients [4].
Acknowledgements
The authors would like to thank Sebastian Weickert, Susanne Ossmann, Sarah Klein, Heart Center Leipzig, and Roberto Bavila and Steve Mure, Boston Scientific for their support of this project.
Disclosure of conflict of interest
None.
Video S1
Video S2
Video S3
References
- 1.Nakagawa H, Ikeda A, Sharma T, Lazzara R, Jackman WM. Rapid high resolution electroanatomical mapping: evaluation of a new system in a canine atrial linear lesion model. Circ Arrhythm Electrophysiol. 2012;5:417–24. doi: 10.1161/CIRCEP.111.968602. [DOI] [PubMed] [Google Scholar]
- 2.Vandecasteele T, Vandevelde K, Doom M, Van Mulken E, Simoens P, Cornillie P. The pulmonary veins of the pig as an anatomical model for the development of a new treatment for atrial fibrillation. Anat Histol Embryol. 2015;44:1–12. doi: 10.1111/ahe.12099. [DOI] [PubMed] [Google Scholar]
- 3.Dukkipati SR, Neuzil P, Skoda J, Petru J, d’Avila A, Doshi SK, Reddy VY. Visual balloon-guided point-by-point ablation: reliable, reproducible, and persistent pulmonary vein isolation. Circ Arrhythm Electrophysiol. 2010;3:266–73. doi: 10.1161/CIRCEP.109.933283. [DOI] [PubMed] [Google Scholar]
- 4.Brown SC, Boshoff DE, Rega F, Eyskens B, Budts W, Heidbüchel H, Meyns B, Gewillig M. Transapical left ventricular access for difficult to reach interventional targets in the left heart. Catheter Cardiovasc Interv. 2009;74:137–42. doi: 10.1002/ccd.21939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


