Abstract
Angiotensin receptor blockers (ARBs) are the most commonly used blood pressure-lowering drugs in the world. However, the preventive value of ARBs on lung cancer is still controversial. Therefore, it was necessary for us to perform a meta-analysis to evaluate the value of ARBs on lung cancer risk. We searched the PubMed database as well as the Web of Science database. The overall effect was measured by odds ratio (OR) and corresponding 95% confidence intervals (CI). The significance of the pooled ORs was determined by the Z test with a P value less than 0.05 considering statistically significant. In this meta-analysis, we found ARBs could decrease the lung cancer risk (OR=0.81, 95% CI 0.69-0.54). The stability of the results was tested by sensitivity analysis. The result was not a significant change, suggesting that the result of our meta-analysis was stable. In conclusion, our meta-analysis demonstrated that ARBs was significantly associated with lower lung cancer.
Keywords: Angiotensin receptor blocker, lung cancer, prevent
Introduction
Angiotensin receptor blockers (ARBs) are the most commonly used blood pressure-lowering drugs in the world. ARBs are highly selective for the Ang II type 1 (AT1) receptors and block the deleterious effects of Ang II, such as vasoconstriction, aldosterone release, retention of sodium and water, sympathetic nerve activation and cell proliferation [1]. Papp et al. suggested that apoptosis was induced with purified Ang II applied to the human lung alveolar epithelial cells (AEC)-derived carcinoma cell line A549 [2]. Losartan inhibited ANG II-induced apoptosis [2]. Batra et al. confirmed that the human non-small cell lung cancer cells (A549) selectively expressed AT1 subtype receptors for Ang II that were functionally coupled to Ca2+ mobilization from both extra and intracellular sources [3]. This effect of Ang II was completely abolished by inclusion of losartan [3]. Thus, it was possible that ARBs could influence the risk of lung cancer. Recently, some studies reported these results. However, the results were still uncertain [4-11]. Thus, we did a meta-analysis to assess the association between ARBs and the risk of lung cancer.
Methods
Publication search
We searched the PubMed database from 1990 to April 2015 as well as the Web of Science database, with an index ranging from 1985 to April 2015. We ran searches based on the following terms: “Angiotensin receptor blocker”, “ARB”, “lung cancer” and “NSCLC”, including all possible combinations therein. We also conducted manual searches following up on all of the studies’ references. Lastly, we inspected several related articles from reviews and other pertinent sources such as research bibliographies.
Inclusion and exclusion criteria
A study included for meta-analysis needed to have: evaluated the correlation between ARBs and the risk of lung cancer; the research should provide sufficient information to estimate the odds ratio (OR) and corresponding 95% confidence intervals (CI). We excluded the following materials: (1) reviews, letters, conference abstracts, and case reports; (2) articles that did not offer enough data to estimate the OR and 95% CI; (3) overlapping articles. Accordingly, these articles were not applied into the scope of our meta-analysis.
Data extraction and quality assessment
We extracted the following data from each of the eligible articles: the name of the first author, publication year, nationality, study design, number of subjects. The quality of each study was evaluated according to the Newcastle-Ottawa quality assessment scale.
Statistical analysis
The overall effect was measured by OR with its 95% CI. The significance of the pooled ORs was determined by the Z test with a P value less than 0.05 considering statistically significant. Between-studies heterogeneity was assessed by the I2 test and the Q-statistic test. The random-effect model was used. The publication bias was estimated by visual funnel plot inspection. To assess whether our results were substantially influenced by the presence of any individual study, we proceed a sensitivity analysis by removing each study and recalculating the significance of the result. Statistical analyses were conducted in STATA version 11.0 (Stata Corporation, College station, TX, USA). All the tests were two-sided.
Results
Study characteristics
The process followed to select the papers used in this report is shown in Figure 1. After a thorough survey of these identified studies, we found and selected eight eligible studies for closer analysis. The total sample size of our meta-analysis was 4331054. Characteristics in this meta-analysis are summarized in Table 1. Table 2 showed the quality scores of these studies. All of the studies are high quality.
Figure 1.
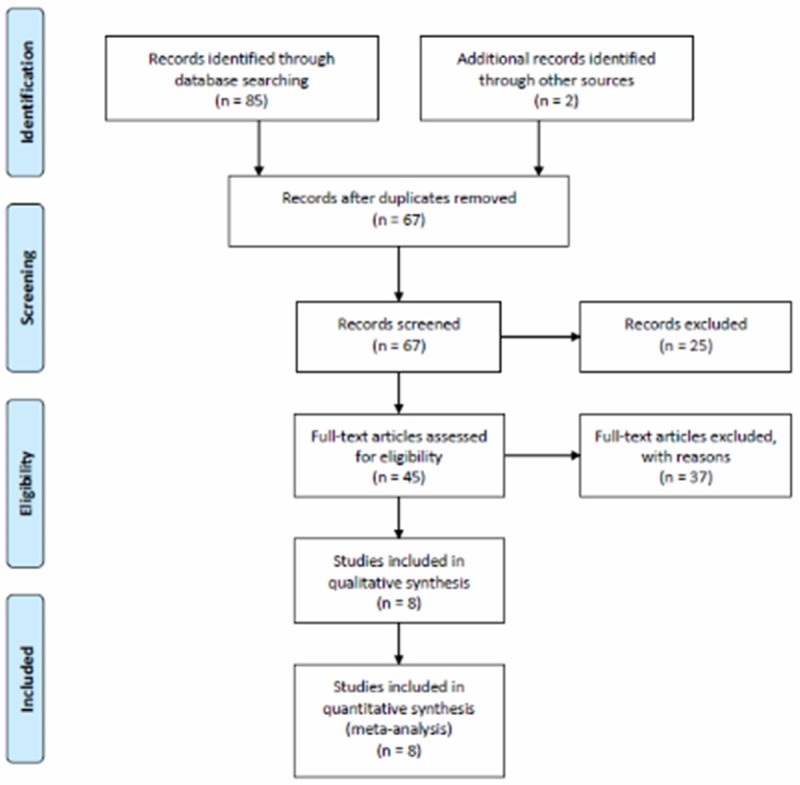
Flow chart of the literature search according to PRISMA statement.
Table 1.
Characteristics of the studies
| First author | Location/Year | Study design | No. of participants | Adjusted |
|---|---|---|---|---|
| Rao GA | USA/2013 | Retrospective cohort | 1228960 | Yes |
| Wang KL | Taiwan/2013 | Retrospective cohort | 85842 | Yes |
| Pasternak B | Denmark/2011 | Retrospective cohort | 317158 | Yes |
| Huang CC | Taiwan/2011 | Retrospective cohort | 109002 | Yes |
| Bhaskaran K | UK/2012 | Retrospective cohort | 1542323 | Yes |
| Azoulay L | UK/2012 | Case-control | 410167 | Yes |
| Hallas J | Denmark/2012 | Case-control | 597668 | Yes |
| Chang CH | Taiwan/2011 | Case-control | 39934 | Yes |
Table 2.
Quality scores of studies using Newcastle-Ottawa Scale (maximum score of 9)
| Study | Selection | Comparability | Outcome | Overall quality |
|---|---|---|---|---|
| Rao GA | 4 | 2 | 3 | 9 |
| Wang KL | 4 | 2 | 2 | 8 |
| Pasternak B | 4 | 2 | 3 | 9 |
| Huang CC | 4 | 2 | 2 | 8 |
| Bhaskaran K | 4 | 2 | 3 | 9 |
| Azoulay L | 4 | 2 | 3 | 9 |
| Hallas J | 4 | 2 | 3 | 9 |
| Chang CH | 4 | 2 | 3 | 9 |
Results of meta-analysis
The meta-analysis gave the result that the pooled OR was 0.81 (95% CI 0.69-0.54) for the ARBs users compared to the non-ARBs user (Figure 2). The stability of the results was tested by sensitivity analysis. The result was not a significant change, suggesting that the result of our meta-analysis was stable (Figure 3). As shown in Figure 4, five studies were identified as the outliners. When the five studies were excluded, the heterogeneity was decreased from 90% to 0%.
Figure 2.
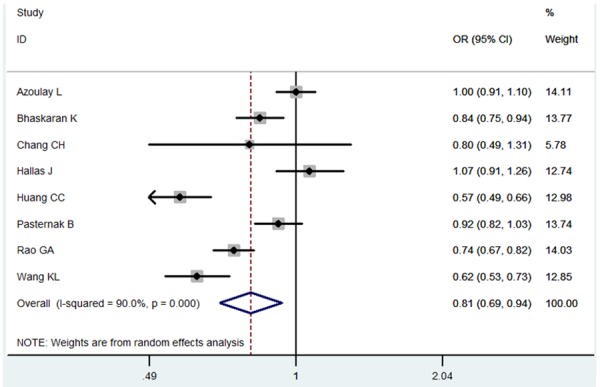
Meta-analysis for the association between ARBs and lung cancer.
Figure 3.
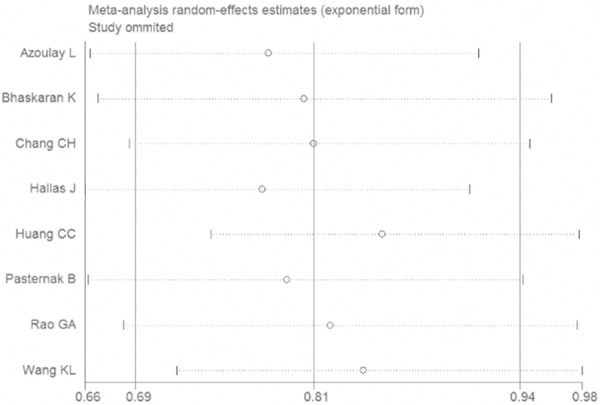
Sensitivity analysis for the association between ARBs and lung cancer.
Figure 4.
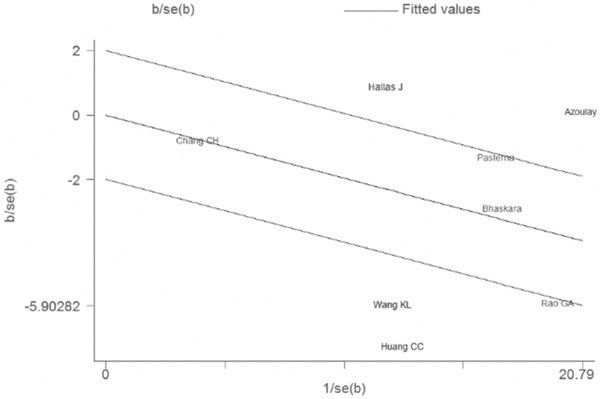
Galbraith plot of for the association between ARBs and lung cancer.
We did a subgroup analysis according to the study design. In the case-control studies, no significant result was found (OR=1.01, 95% CI 0.93-1.10). However, in the cohort studies, the result was still positive (OR=0.73, 95% CI 0.62-0.86).
Publication bias assessment
The data of the eight studies were included in a funnel plot, which was used to analyze the publication bias of the literature included in this meta-analysis (Figure 5). The result did not suggest any evidence of publication bias (P=0.568).
Figure 5.
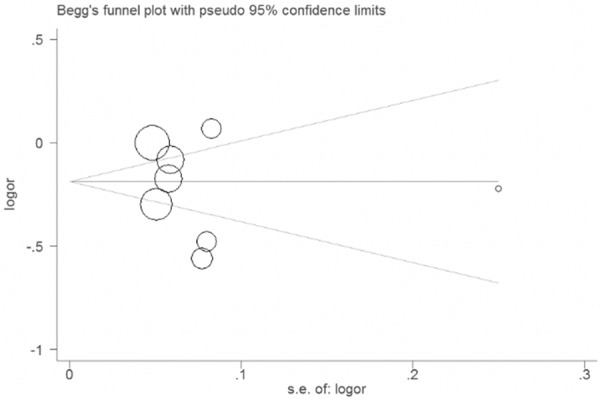
Funnel plot of for the association between ARBs and lung cancer.
Discussion
Lung cancer is the leading cause of cancer death worldwide. In 2013 alone, an estimated 228,190 US cases will be diagnosed and 159,480 US deaths are estimated to occur [12]. The 5-year survival for all lung cancer patients is a dismal 15% [12]. Clearly there is need for novel preventive approaches in lung cancer.
In this meta-analysis, we found ARBs could decrease the lung cancer risk. An increased incidence of some neoplastic diseases during the use of ARBs was first shown by the CHARM study, which compared candesartan with placebo in patient with chronic heart failure [13]. In the LIFE (Losartan Intervention For Endpoint reduction in Hypertension) study, the risk of neoplastic diseases was increased by 12% compared with the control group, but this difference was not significant [14]. In the PRoFESS (Prevention Regimen For Effectively avoiding Second Strokes) study, lung cancer showed a non-significant increase of 24% in the ARB (telmisartan) group compared to placebo [15]. Thus, the result of our study should be validated in the future studies.
Some strengths of our study deserve particular mention. First, this is the first meta-analysis of the association between ARBs and lung cancer. Second, the overall outcome was based on individual adjusted ORs. Third, the number of included subjects is very large. Thus, the statistic power is good enough. We should also be aware of some limitations in this meta-analysis. First, because of the lack of a relevant prospective study, all of the included studies were retrospective. Therefore, more prospective studies are needed to confirm our result. Second, as in all meta-analysis, the possibility of publication bias is of concern. However, a formal statistical test did not provide evidence for such bias. Finally, most of the studies met our inclusion criteria conducted in European and Asian, which might influence the generalizability of the findings to other types of geographic areas.
In conclusion, our meta-analysis demonstrated that ARBs was significantly associated with lower lung cancer. However, further large prospective studies should be carried out to confirm whether ARBs has a preventive value in lung cancer.
Acknowledgements
Supported by Science and Technology Planning Project of Guangdong Province, China (2009B030801011) and Guangdong medical science research fund (B2012194, B2011100).
Disclosure of conflict of interest
None.
References
- 1.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–72. [PubMed] [Google Scholar]
- 2.Papp M, Li X, Zhuang J, Wang R, Uhal BD. Angiotensin receptor subtype AT(1) mediates alveolar epithelial cell apoptosis in response to ANG II. Am J Physiol Lung Cell Mol Physiol. 2002;282:L713–8. doi: 10.1152/ajplung.00103.2001. [DOI] [PubMed] [Google Scholar]
- 3.Batra VK, Gopalakrishnan V, McNeill JR, Hickie RA. Angiotensin II elevates cytosolic free calcium in human lung adenocarcinoma cells via activation of AT1 receptors. Cancer Lett. 1994;76:19–24. doi: 10.1016/0304-3835(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 4.Chang CH, Lin JW, Wu LC, Lai MS. Angiotensin receptor blockade and risk of cancer in type 2 diabetes mellitus: a nationwide case-control study. J. Clin. Oncol. 2011;29:3001–7. doi: 10.1200/JCO.2011.35.1908. [DOI] [PubMed] [Google Scholar]
- 5.Huang CC, Chan WL, Chen YC, Chen TJ, Lin SJ, Chen JW, Leu HB. Angiotensin II receptor blockers and risk of cancer in patients with systemic hypertension. Am J Cardiol. 2011;107:1028–33. doi: 10.1016/j.amjcard.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 6.Pasternak B, Svanström H, Callréus T, Melbye M, Hviid A. Use of angiotensin receptor blockers and the risk of cancer. Circulation. 2011;123:1729–36. doi: 10.1161/CIRCULATIONAHA.110.007336. [DOI] [PubMed] [Google Scholar]
- 7.Azoulay L, Assimes TL, Yin H, Bartels DB, Schiffrin EL, Suissa S. Long-term use of angiotensin receptor blockers and the risk of cancer. PLoS One. 2012;7:e50893. doi: 10.1371/journal.pone.0050893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhaskaran K, Douglas I, Evans S, van Staa T, Smeeth L. Angiotensin receptor blockers and risk of cancer: cohort study among people receiving antihypertensive drugs in UK General Practice Research Database. BMJ. 2012;344:e2697. doi: 10.1136/bmj.e2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hallas J, Christensen R, Andersen M, Friis S, Bjerrum L. Long term use of drugs affecting the renin-angiotensin system and the risk of cancer: a population-based case-control study. Br J Clin Pharmacol. 2012;74:180–8. doi: 10.1111/j.1365-2125.2012.04170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao GA, Mann JR, Shoaibi A, Pai SG, Bottai M, Sutton SS, Haddock KS, Bennett CL, Hebert JR. Angiotensin receptor blockers: are they related to lung cancer? J Hypertens. 2013;31:1669–75. doi: 10.1097/HJH.0b013e3283621ea3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang KL, Liu CJ, Chao TF, Huang CM, Wu CH, Chen TJ, Chiang CE. Long-term use of angiotensin II receptor blockers and risk of cancer: a population-based cohort analysis. Int J Cardiol. 2013;167:2162–6. doi: 10.1016/j.ijcard.2012.05.096. [DOI] [PubMed] [Google Scholar]
- 12.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 13.Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759–66. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- 14.Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC. Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. Lancet Oncol. 2010;11:627–36. doi: 10.1016/S1470-2045(10)70106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yusuf S, Diener HC, Sacco RL, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, Bornstein N, Chan BP, Chen ST, Cunha L, Dahlöf B, De Keyser J, Donnan GA, Estol C, Gorelick P, Gu V, Hermansson K, Hilbrich L, Kaste M, Lu C, Machnig T, Pais P, Roberts R, Skvortsova V, Teal P, Toni D, VanderMaelen C, Voigt T, Weber M, Yoon BW PRoFESS Study Group. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008;359:1225–37. doi: 10.1056/NEJMoa0804593. [DOI] [PMC free article] [PubMed] [Google Scholar]


