Abstract
Purpose: To compare the pharmacodynamic effects of an anodyne spray (XQAS) containing extracts of two herbs, Cynanchum paniculatum (CP) and Illicium henryi (IH), with those of spray containing the vehicle alone, CP alone (CPS) or IH alone (IHS), when applied topically acute soft tissue injury (STI) in an animal model. Methods: Acute closed STI was modeled by hammer blow in the hind leg muscle of rat. In the acute test, XQAS, vehicle and normal saline (NS) were applied topically with instantly and repeatedly every 2 h for 8 h after modeling. In the chronic test, XQAS, vehicle, NS, CPS and IHS were applied topically respectively with instantly and repeatedly every 8 h for 96 h after modeling. Results: XQAS (150 μl/time) rapidly suppressed STI-caused muscle swelling, high contents of inflammatory mediators such as prostaglandin-E2, interleukin-lβ, nitric oxide and so on. XQAS (100 and 250 μl/time) also showed chronic effects with dose-dependent suppressions of muscle swelling, up-regulated mRNA expressions of nuclear factor-κB p65 (NF-κB p65), cyclooxygenase-2 and interleukin-lβ, high contents of inflammatory mediators, and muscle cells impairment and necrosis induced by STI, while XQAS was more effective than CPS or HIS on treating STI. Conclusion: XQAS can suppress STI-caused increased gene expressions of NF-κB p65 and its downstream genes which mediate biosyntheses of inflammatory mediators, resulting in suppressed swelling, inflammatory reaction and cell impairment in the injured muscle. There is a synergistic effect between CPS and IHS on curing STI.
Keywords: Xiangqing anodyne spray (XQAS), soft-tissue injury (STI), inflammation, synergistic effect, rapid effect
Introduction
Closed or open soft-tissue injury (STI) is a nearly invariable consequence of musculoskeletal trauma and the severity of STI is one of the most decisive prognostic determinants of the outcome of complex injury to the extremities. Owing to the high frequency of high-velocity or high-energy trauma and sports-related injuries, the prevalence of severe soft-tissue trauma continues to increase [1]. The occurrence of STI is presumed to have a very complicated mechanism. Inflammation can be conceptualized as a response of the immune system to the injury. This phase is intimately related with the subsequent phases of regeneration and fibrosis [2]. It is believed that, since there is a consistent and lasting response to inflammation, inflammation is the most important reaction when STI occurs. The inflammatory response is dependent on two factors, namely the extent of actual physical damage and the degree of muscle visualization at the time of injury [3,4]. STI care can be traced back to early civilizations, and many of these treatments were based on the use of herbal remedies. Approximately one-third of all traditional medicines in use are for the treatment of wounds and soft tissue disorders, compared to only 1%-3% of modern drugs [5]. Many traditional remedies are based on systematic observations and methodologies have been time-tested. However, many of them are still lacking scientific evidence. There are only a few prospective randomized, controlled trials that have proved the clinical efficacy of these traditional healing agents for STI [6,7].
Xiangqing anodyne spray (XQAS), a spray formulation applied topically, is composed of the ethanol extracts of Cynanchum paniculatum (CP) and Illicium henryi (IH) with additional penetration enhancers. In traditional Chinese medicine, the ethanol or water-soluble extract of CP has been used for relieving pain such as rheumatic arthralgia, lumbago, pain due to traumatic injuries, abdominal pain, toothache etc, as well as skin diseases such as eczema, rubella and neurodermatitis [8]. Paeonol (2’-hydroxy-4’-methoxyacetophenone), a main active compound in the radix of Cynanchum paniculatum, has been reported as an analgesic and anti-inflammatory drug with a beneficial effect in prevention and treatment of thromboembolic diseases [9]. The water-soluble extract of root bark of IH has also been applied to clinical as an analgesic agent by intramuscular injection in China [10]. The flavonoids such as quercetin, quercitrin and other quercetin glycosides are main active compounds in the IH and exhibited pronounced antinociceptive properties [11,12].
XQAS is expected to have a comprehensive property of both CP and IH in treating STI. Using the mass-drop injury model introduced by Stratton SA, Heckmann R, Francis RS [13], we tried to look at whether XQAS can rapidly relieve swelling and inflammation; Further, the long-term effects of XQAS and the advantage compared with the extract of CP and IH were investigated with a special focus on the formation of certain important inflammatory mediators such as cytokines interleukin-lβ (IL-1β), tumor necrosis factor α (TNF-α), nitric oxide (NO) and prostaglandin E-2 (PGE-2).
Material and methods
Xiangqing anodyne spray (XQAS) preparation
The XQAS was prepared as following: Firstly, weigh 50 g dried roots of the plant Cynanchum paniculatum (Family-Asclepiadaceae) and Illicium henryi (Family-Magnoliaceae) respectively and crush through 20 mesh sieve, add eight-fold 75% ethanol as solvent and dip for 48 h, then filtrate extract by percolation and collect percolate to be concentrated; Secondly, the penetration enhancers were added in XQAS, which contain 1% Azone, 2% peppermint oil, 1% and 2% PEG 400 borneol; Finally, XQAS contains 0.50 g/ml Cynanchum paniculatum (crude drug) and 0.50 g/ml Illicium henryi (crude drug), containing 0.858 mg/ml paeonol and 7.33 mg/ml quercetin analyzed by high performance liquid chromatography. With the same method, the Cynanchum paniculatum spray (CPS) and Illicium henryi spray (IHS) were also prepared respectively, containing 1.0 g/ml crude drug respectively with equal penetration enhancers with XQAS.
Animals
All animal experiments were performed in accordance with China state regulations on animal experimentation and approved by Animal Experimental Ethical Center of Southeast University (protocol No. 20120023). Male Sprague-Dawley rats (250 to 300 g) supplied by Suzhou Industrial Park, Matt Ireland Ltd, were randomized by bodyweight and housed in polypropylene cages. The animals were maintained on a standard 12 h light-12 h dark cycle, in a temperature-controlled environment (24 ± 2°C), with free access to water and chow. They were acclimatized for at least 1 week before starting the experiments.
Closed soft-tissue injury (STI) modeled by hammer blow
As previously reported [13], the rats were anesthetized by ethyl ether, the inside of hind leg was epilated with depilatory Na2S solvent in advance and fixed in lateral, the thigh muscle were hit with a cylindrical hammer (200 g in weight, 1.0 cm in top diameter and 1.5 cm in bottom diameter) by free falling vertically from inside of a hard smooth plastic tube (75 cm in length and 1.5 cm in inner diameter), only to result in closed STI but not to make femoral fracture.
Acute treatment with XQAS on the STI
The rats were randomly divided into four groups (n = 8 per group): control (Ctrl), model (Mod), vehicle treatment (Veh) and XQAS treatment (XQAS). Except for the control group, rats were made by two-time blows to hind leg muscle. After modeling, each rat was instantly applied topically with smearing corresponding fluid drug on the surface of hit hind leg with normal saline (NS, 150 μl/time) in the Ctrl and Mod groups, penetration enhancers (150 μl/time) in the Veh group, and XQAS (150 μl/time) in the XQAS group, respectively, and during the 8 h after modeling, each group was administrated repeatedly every 2 h. The circumference locating in the damaged leg muscle was measured at 0 h (Before making the model), 1 h, 2 h, 3 h, 4 h, 6 h and 8 h, muscle swelling rate was then calculated (MSR (%) = (D/C-1) × 100%, where D represents the circumference at 0 h, while C represents the circumference at the time after hitting) [14]. At the end of the experiment, the rat were sacrificed after anesthetization with urethane (1.0 g/kg), then the leg muscle was dissected and stored at -80°C until further examination.
Repeated treatment with XQAS on the STI
The rats were randomly divided into seven groups (n = 6 per group): control (Ctrl), model (Mod), vehicle treatment (Veh), high-dose XQAS treatment (HXQAS), low-dose XQAS treatment (LXQAS), CPS treatment (CPS) and IHS treatment (IHS). Except for the control group, rats were subject to three-time blows to hind leg muscle. After modeling, the rats in each group were instantly applied topically with NS (100 μl/time) in the Ctrl and Mod groups, penetration enhancers (100 μl/time) in the Veh group, XQAS in the HXQAS (250 μl/time) and LXQAS (100 μl/time) groups, CPS (100 μl/time) in the CPS group, or IHS (100 μl/time) in the IHS group, respectively. Topical application was repeated every 8 h for 96 h after modeling, and the circumference locating in the damaged leg muscle was measured at 0 h (Before making the model), 4 h, 24 h, 48 h, 72 h and 96 h to calculate MSR. At the end of experiment, rats were processed like acute trial, then the leg muscle each rat was divided into two parts: one was stored at -80°C to be use to measure biochemical parameters and detect relational mRNA expressions; the other was fixed in neutral buffered formalin to be used to observe the morphological change.
Biochemical analysis of the muscle tissue
The muscle was homogenized (Jinda Biochemistry Company, Shanghai, China) in 0.02 M phosphate buffered solution (PBS pH 7.4). The parameter was measured according to the protocols of respective kits. The levels of interleukin-1 beta (IL-1β), tumor necrosis factor-alpha (TNF-α) and prostaglandin-E2 (PGE-2) in the muscle tissue were measured by ELISA kits provided by Abcam (HK) Ltd. The content of melondialdehyde (MDA), nitric oxide (NO) and lactate dehydrogenase (LDH) were measured by spectrophotometer kits respectively, provided by Nanjing Jiancheng Bioengineering Institute.
Determinations of mRNA expressions in the muscle tissue
The muscle tissues of six rats of each group were used for polymerase chain reaction (PCR) examination via performing reverse transcription-PCR (RT-PCR) assay. Total RNA was isolated from tissues using RNAiso plus Isolation Reagent (TAKARA, Otsu, Shiga, Japan). Total RNA solution was first reserve transcribes and then immediately amplified in a GeneAmp PCR system (Eppendorf). RT-PCR for Nuclear factor-κB p65 (NF-κB p65), Cyclooxygenase -2 (COX-2) and IL-1β were carried out in the same system (Eppendorf). Primers used in the RT-PCR are shown in Table 1. The gel was photographed by GeneGenius automatic gel imaging and analysis system (Syngene, Cambridge, UK) and the bands on the film were scanned by densitometry for quantitation. To exclude variations due to RNA quantity and quality, the data for all genes were adjusted to GAPDH.
Table 1.
Primer used for RT-PCR
| Gene | Forward primer (5’-3’) | Reverse primer (5’-3’) |
|---|---|---|
| NF-κB P65 | GGGACTATGACTTGAATGCG | CAGGCTAGGGTCAGCGTAT |
| COX-2 | CAATGAGTACCGCAAACGC | CTCCGAAGGTGCTAGGTTT |
| IL-1β | GATGACGACCTGCTAGTGT | CTTCTTCTTTGGGTATTGTT |
| GAPDH | ATGTATCCGTTGTGGATCTG | GATGGTATTCGAGAGAAGGG |
Histological observation of muscle tissue
After fixation in neutral buffered formalin, the muscle was sectioned and processed routinely for hematoxylin-eosin (HE) staining for histopathological examination. All histopathological examinations were performed by a trained pathologist. The muscle injury, including mainly muscle fibers degeneration and necrosis, interstitial ecchymosis and inflammatory cell infiltrates, in each sample was assessed using the method reported by Jonathan R. Bunn, John Canning, George Burke, et al [15].
Statistical analysis
Results were expressed as mean ± standard deviation (SD). For statistical significance, except MRS differences between groups were tested using analysis of covariance (the baseline value in the control as a covariate), the other data were tested using one-way analysis of variance (ANOVA) and both followed by LSD’s multiple comparison test. P<0.05 was considered significant.
Results
Acute treatment with XQAS markedly depressed hammers blow-induced STI
As shown in Figure 1A, significantly MSR in the injured hind leg of model rats increased during 8 h after hitting (all P<0.01 vs. Ctrl); while XQAS markedly reduced MSR beginning from 1 h after hitting (P<0.05 or P<0.01 vs. Veh), suggesting a rapid and markedly inhibition of muscle swelling by XQAS.
Figure 1.
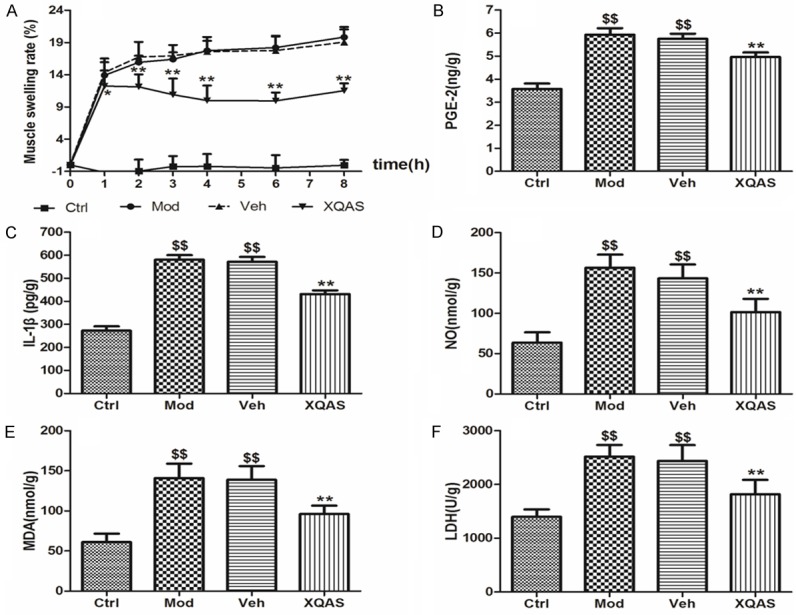
Data of MSR (A), PGE-2 (B), IL-1β (C), NO (D), MDA (E) and LDH (F) in the acute injured hind leg muscle of rats with the control (Ctrl), model (Mod), vehicle treatment (Veh) and Xiangqing anodyne spray treatment (XQAS). The data were shown as mean ± SD (n = 8 per group). **P<0.01 vs. Veh; $$P<0.01 vs. Ctrl.
Levels of PGE-2, IL-1β and NO (Figure 1B-D), biomarkers of inflammation in the injured muscle of model rat were notably elevated at 8 h after the hitting (P<0.01 vs. Ctrl, respectively) while they were markedly reduced in the XQAS-treated rats (P<0.01 vs. Veh, respectively). Levels of MDA and LDH, biomarkers of cellular impairment in the injured muscle (Figure 1E, 1F) were also elevated significantly in the model (P<0.01 vs. Ctrl, respectively); while XQAS treatment significantly lowered them (P<0.01 vs. Veh, respectively). The data showed that the marked inflammation and cellular impairment in the muscle induced by hammer blow could be suppressed rapidly by XQAS treatment, suggesting a rapid protective effect of XQAS on injured muscle cells.
Repeated treatment with XQAS showed a marked protection on muscle against hitting injury with a dose-dependent manner and advantage of combination of CPS and IHS
Repeated treatment with XQAS could constantly reduce MSR in the injured muscle induced by hammer blow. Elevated MSR in the injured hind leg of model rats was significant during 96 h after hitting (all P<0.01 vs. Ctrl), while significantly lower MRS exhibited in the HXQAS group beginning from 4 h after hitting (all P<0.01 vs. Veh) and the LXQAS group beginning from 48 h after hitting (all P<0.05 vs. Veh). Moreover, the depressing effect of high-dose XQAS treatment on MRS was more significant than low-dose XQAS (P<0.05 or P<0.01, LXQAS vs. HXQAS), suggesting a dose-dependent manner. CPS or IHS alone didn’t show marked depressing effect on MRS (all P>0.05 vs. Veh, respectively), indicating a synergetic effect between CPS and IHS in the XQAS. The results are shown in Figure 2A.
Figure 2.
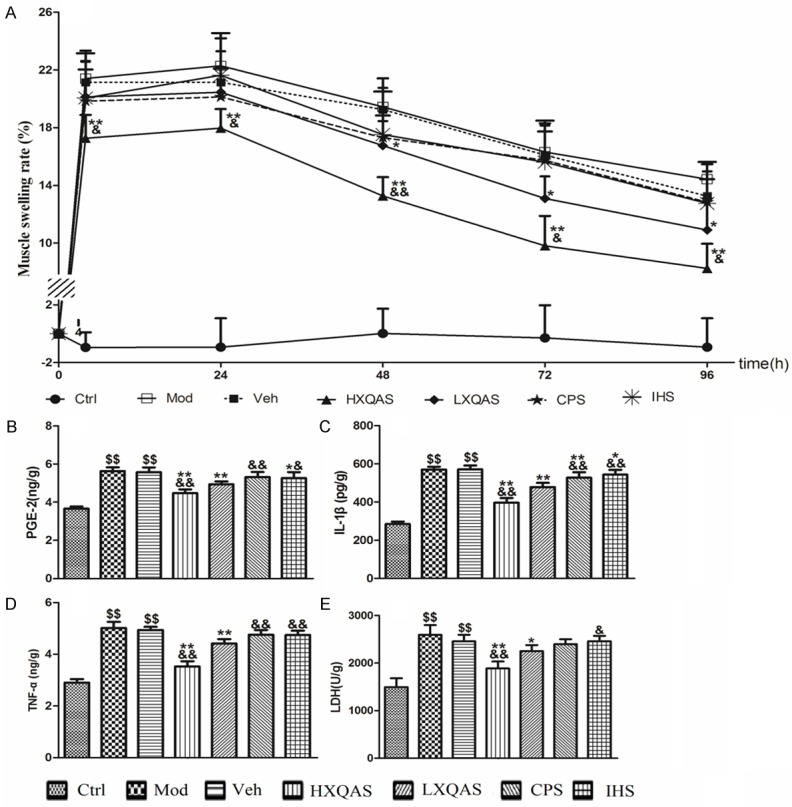
Data of MSR (A), PGE-2 (B), IL-1β (C), TNF-α (D) and LDH (E) in the injured hind leg muscle of rats with the control (Ctrl), model (Mod), vehicle treatment (Veh), high-dose Xiangqing anodyne spray (XQAS) treatment (HXQAS), low-dose XQAS treatment (LXQAS), Cynanchum paniculatum spray treatment (CPS) and Illicium henryi spray treatment (IHS). The data were shown as mean ± SD (n = 6 per group). $$P<0.01 vs. Ctrl; *P<0.05, **P<0.01 vs. Veh; &P<0.05, &&P<0.01 vs. LXQAS.
Repeated treatment with XQAS inhibited productions of inflammatory cytokines with protecting muscle cells from hammer blow-induced impairment
Levels of PGE-2, IL-1β, TNF-α and LDH in the injured hind leg muscle of model rats were significantly raised on day 4 after hitting (P<0.01 vs. Ctrl, respectively); vehicle treatment did not change this status (P>0.05 vs. Mod, respectively), while XQAS treatment in both the HXQAS and LXQAS groups markedly decreased the levels of aforementioned biomarkers (P<0.05 or P<0.01 vs. Veh, respectively) and showed more marked decrease in the HXQAS group than LXQAS group (P<0.01, respectively), in a dose-dependant manner. CPS or IHS treatment decreased level of IL-1β in the CPS group (P<0.01 vs. Veh) and decreased levels of PGE-2 and IL-1β in the IHS group (P<0.05 vs. Veh, respectively) but without decreased levels of LDH and TNF-α in both groups (P>0.05 vs. Vet, respectively). The results are shown in Figure 2B-E.
Repeated treatment with XQAS suppressed hammer blow-induced elevations of mRNA expressions of NF-κB p65, COX-2 and IL-1β in the hind leg muscle of rats
The NF-κB p65, COX-2 and IL-1β mRNA expressions were significantly up-regulated in the injured muscle of model rats detected on day 4 post hitting (P<0.01 vs. Ctrl, respectively), while high- or low-dose XQAS treatment both markedly down-regulated mRNA expressions of them (P<0.05 or P<0.01 vs. Veh, respectively), and HXQAS group showed stronger down-regulation on the COX-2 mRNA expression (P<0.05) and stronger down-regulated tendency on the mRNA expressions of NF-κB p65 and IL-1β (P>0.05, respectively) than LXQAS. CPS treatment only displayed a slight depressing effects, with decreased mRNA expression of IL-1β (P<0.05 vs. Veh) and decreased tendency on the mRNA expressions of NF-κB p65 and COX-2 (P>0.05 vs. Veh, respecitively), while IHS treatment merely showed decreased tendency on the mRNA expressions of NF-κB p65, COX-2 and IL-1β (P>0.05 vs. Veh, respectively). The depressant effects of low-dose XQAS treatment on the mRNA expressions of NF-κB p65 and COX-2 were more significant than CPS or IHS treatment alone (both P<0.05 or P<0.01, respectively). The results are shown in Figure 3.
Figure 3.
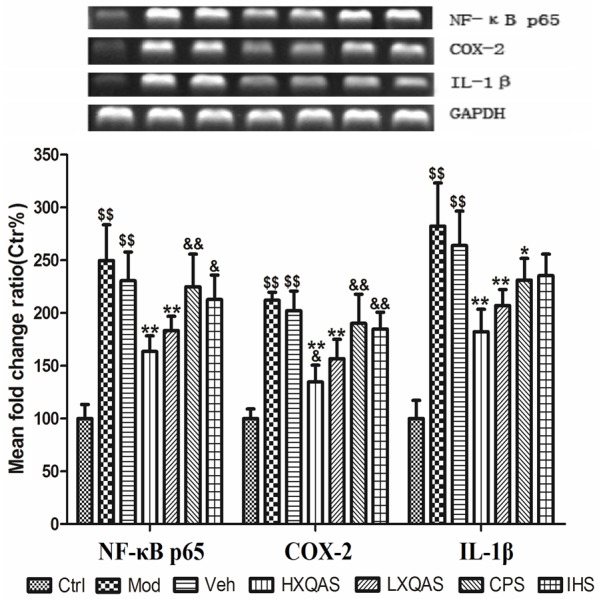
Relative expressions of NF-κB p65, COX-2 and IL-1β mRNA in the injured hind leg muscle of rats with the control (Ctrl), model (Mod), vehicle treatment (Veh), high-dose Xiangqing anodyne spray (XQAS) treatment (HXQAS), low-dose XQAS treatment (LXQAS), Cynanchum paniculatum spray treatment (CPS) and Illicium henryi spray treatment (IHS). The mRNA expressions were detected by reverse transcription-PCR assay. The data are shown as mean ± SD (n = 6 per group) and a representative photo for PCR assay was shown here. $$P<0.01, vs. Ctrl; *P<0.05, **P<0.01 vs. Veh; &P<0.05, &&P<0.01 vs. LXQAS.
Long-term treatment with XQAS lowered blow-induced muscle degeneration and necrosis detected by histopathological finding
In the control rats, no injury was found in the hind leg muscle with the normal muscle cells; in the injured muscle of model rats, severe muscle injury with severe muscle fibers degeneration and collagen fiber hyperplasia, muscle cells necrosis, massive inflammatory cell infiltrates and interstitial ecchymosed in the necrosis area were found; in the vehicle-treated rats, severe muscle injury was similar with the model rats; in the high-dose XQAS-treated rats, muscle injury was mild with greatly reduced area of muscle fibers degeneration and necrosis following little inflammatory cell infiltrates and interstitial ecchymosed; in the low XQAS-treated rats, muscle injury was also improved significantly, with reduced area of muscle fibers necrosis following a little inflammatory cell infiltrates and interstitial ecchymosed; in the CPS- or IHS-treated rats, slight improvements of muscle injury but less than that in the low-dose XQAS-treated rats were found, with a few uninjured muscle fibers shown in the necrosis area. Representative histopathological findings in each group were shown in Figure 4.
Figure 4.
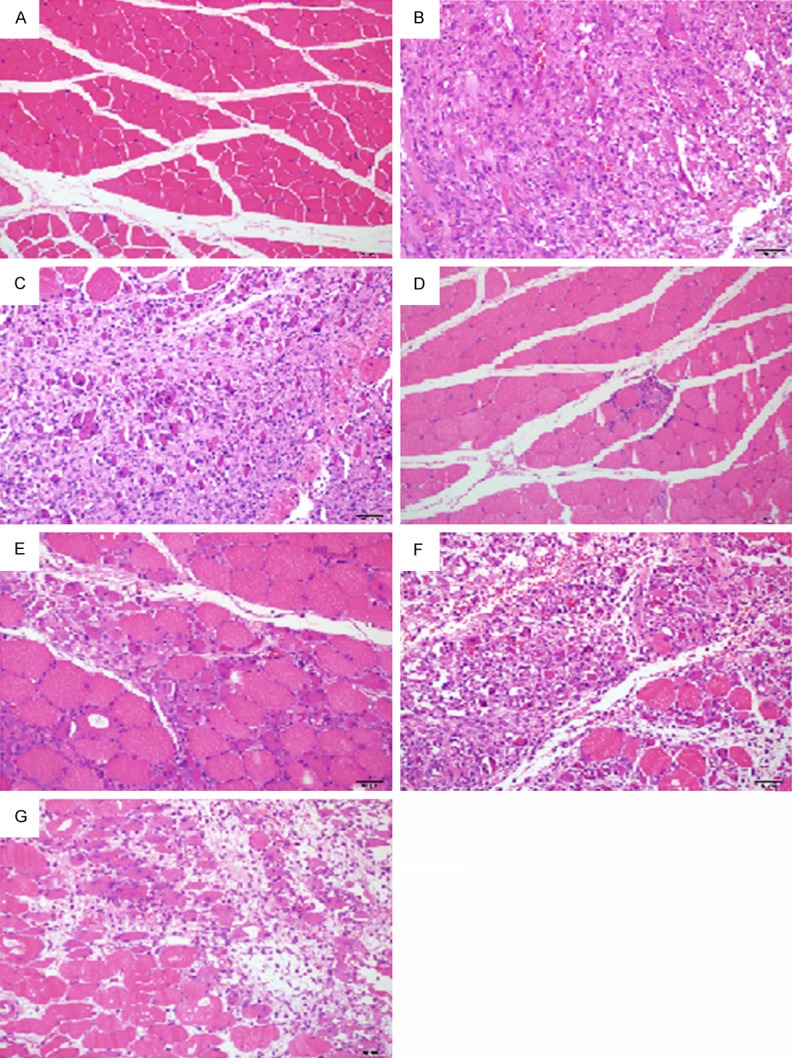
Representative histopathological micrographs (× 200, HE staining) in the injured muscle of rats with the control (A), model (B), vehicle treatment (C), high or low-dose Xiangqing anodyne spray treatment (D, E), Cynanchum paniculatum spray (F) or Illicium henryi spray (G) treatment. Normal myocyte in (A); Severe myocyte degeneration and necrosis following marked fibrosis, massive inflammatory cell infiltrates and interstitial ecchymosed in (B, C); A little area of the degeneration and necrosis in (D); Markedly reduced area of the degeneration and necrosis in (E); Markedly difference in (F, G) compared to (B) was normal myocyte shown in the necrosis area.
Discussion
Closed soft tissue injury (STI) frequently occurred in daily life, and effective treatment of STI is important to avoid sequelae. As the muscle tissue is damaged, local tissue mediators are released, including histamines, kinin, prostaglandins, and leukotrienes etc, which result in inflammation, edema and pain. Inflammation starts from migration of leukocytes and damaged soft tissue cells [16,17]. Therefore, early diagnosis and treatment can improve functional results and attenuate structural damage in the early and acute period of skeletal muscle injury [15]. As the extension of injury time, severe muscle fibers degeneration and necrosis following marked collagen fiber hyperplasia, massive inflammatory cell infiltrates and interstitial ecchymosed will emerge in the site of damaged tissue to create irreversible structural changes [18]. Hence, rapid attenuating tissue swelling and inhibiting inflammation by preventing injured cells necrosis are necessary for treatment of STI. Our results showed that XQAS had a rapid effect on treating acute closed STI by rapidly attenuating muscle swelling and myocyte impairment and markedly depressing productions of pro inflammatory factors in the injured muscle. In particular, we found that in the muscle subjected to severe acute closed STI, long-term treatment with repeated administration of XQAS for four days could effectively prevent muscle cells necrosis and fibrosis, with marked reduction in myocyte necrosis and inhibition in collagen fiber hyperplasia and inflammatory cell infiltrates in the injured muscle. Moreover, XQAS showed a dose-dependent effect on STI, which comes from its extractive components getting from Cynanchum paniculatum (CP) and Illicium henryi (IH) and exhibited synergistic effects between two extracts.
Nuclear Factor κB (NF-κB) is thought as a genetic switch to control the expressions of many target genes and directly participates in inflammation [19]. A key component of NF-κB is heterodimer transcription factor p50/p65 in the cytoplasm, an inactive state combined with inhibitory proteins (inhibitor kappa B, IκBα) [20]. When the cells receive various stimuli, such as angiogenesis, oxidative stress and various cytokines, NF-κB is released rapidly from IκB to activate the downstream gene expressions of multiple cytokines, chemotactic cytokine and matrix proteins involved in inflammation, immunological responses and/or proliferation [21], such as IL-1β and Cyclooxygenase (COX). COX, an enzyme that converts arachidonic acid to PGs, has been found to have two isoforms namely COX-1 and COX-2. COX-2 is responsible for production of large amounts of pro inflammatory PGs, especially PGE-2, at the inflammatory site [22]. PGE-2, IL-1β, NO and TNF-α are important inflammatory mediators. PGE-2 has an effect on dilating blood vessels, resulting in fever and pain. It can also work synergistically with other inflammatory mediators to exacerbate inflammatory response [23]; IL-1β is widely involved in many pathological processes, such as tissue damage and edema formation [15]; NO modulates vascular diameter, causing vasodilatation and increasing perfusion, which is essential for remodeling the damaged muscle but resulting in tissue edema [24,25]; TNF-α, a polypeptide cytokine with a wide range of biological activity, involves in inflammatory reaction of ischemic tissue injury and plays an important role in immune and inflammatory response [26]. In addition, MDA, as a product of free radical damage, is an important component of pathological inflammation injury, and its content can indirectly represent the degree of free radicals injury [27]; LDH, as a key enzyme in the cell, can directly mark to muscle injury and oxidative stress [28]. The results suggested that XQAS can suppress over-expressed NF-κB p65 mRNA in the injured muscle, as well as its downstream inflammatory mediators such as COX-2 and IL-1β mRNA, resulting in rapidly inhibition on releases of inflammatory mediates such as PGE-2, IL-1β, NO and TNF-α, and blunting inflammatory reaction, cell injury and tissue edema.
Paeonol and quercetin are considered as major active compounds in the CP and IH. Paeonol exerts its anti-inflammatory and analgesic effects associated with decreased production of pro inflammatory cytokines, such as NO and PGE-2, accompanied by down-regulated protein expressions of iNOS and COX-2 which were elevated in the inflammatory tissue [29]. It was showed that quercetin can suppress generation of proinflammatory cytokine TNF-α in a dose-dependent manner via modulation to NF-κB and IκBα [30]. Therefore, the effect of XQAS on damaged tissue could be due to paeonol and quercetin. But XQAS, a combination of CPS and IHS, showed more effective than CPS or IHS treatment alone, suggesting that there could be a synergism between quercetin and paeonol on curing STI.
Taken together, XQAS treatment produced rapid effects on STI with the muscle swelling, inflammation and cell injury being suppressed quickly in the injured muscle; XQAS also showed significant effects on closed STI-induced muscle necrosis and fibrosis to prevent muscle cells impairment and degeneration. The mechanism of action of XQAS relates with suppressed expression of NF-kB p65 in the damaged tissue and with attenuated expressions of its downstream genes which mediate syntheses of inflammatory mediators. Moreover, XQAS treatment also exhibits significantly greater effectiveness than CPS or IHS treatment alone, suggestion synergistic effect of combination of the extracts of Cynanchum paniculatum and Illicium henryi on soft-tissue injury.
Acknowledgements
The authors are grateful to Professor Rong Hu (China Pharmaceutical University) for her contribution in manuscript revision, and Wenxia Bai, an associate researcher (Jiangsu center for safety evaluation of drugs, China) for her contribution to histopathological examination.
Disclosure of conflict of interest
None.
References
- 1.Gierer P, Mittlmeier T, Bordel R, Schaser KD, Gradl G, Vollmar B. Selective cyclooxygenase-2 inhibition reverses microcirculatory and inflammatory sequelae of closed soft-tissue trauma in an animal model. J Bone Joint Surg Am. 2005;87:153–160. doi: 10.2106/JBJS.C.01510. [DOI] [PubMed] [Google Scholar]
- 2.Gates C, Huard J. Management of skeletal muscle injuries in military personnel. Operative Techniques in Sports Medicine. 2005;13:247–256. [Google Scholar]
- 3.Kearns SR, Daly AE, Sheehan K, Murray P, Kelly C, Bouchier-Hayes D. Oral vitamin C reduces the injury to skeletal muscle caused by compartment syndrome. J Bone Joint Surg Br. 2004;86:906–911. doi: 10.1302/0301-620x.86b6.14177. [DOI] [PubMed] [Google Scholar]
- 4.Beiner JM, Jokl P, Cholewicki J, Panjabi MM. The effect of anabolic steroids and corticosteroids on healing of muscle contusion injury. Am J Sports Med. 1999;27:2–9. doi: 10.1177/03635465990270011101. [DOI] [PubMed] [Google Scholar]
- 5.Mantle D, Gok MA, Lennard TW. Adverse and beneficial effects of plant extracts on skin and skin disorders. Adverse Drug React Toxicol Rev. 2001;20:89–103. [PubMed] [Google Scholar]
- 6.Liu JM, Wang L, Geng YP, Wang QB, Luo LJ, Zhong Y. Genetic diversity and population structure of Lamiophlomis rotata (Lamiaceae), an endemic species of Qinghai-Tibet Plateau. Genetica. 2006;128:385–394. doi: 10.1007/s10709-006-7517-y. [DOI] [PubMed] [Google Scholar]
- 7.Li MZ, Jia ZP, Shen T, Zhang RX, Zhang HX, Li ZY. Effect of Herba Lamiophlomis Rotata extract on rats blood conglomeration parameters by oral administration. Zhong Yao Cai. 2006;29:160–163. [PubMed] [Google Scholar]
- 8.Deng C, Yao N, Wang B, Zhang X. Development of microwave-assisted extraction followed by headspace single-drop microextraction for fast determination of paeonol in traditional Chinese medicines. J Chromatogr A. 2006;1103:15–21. doi: 10.1016/j.chroma.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Hirai A, Terano T, Hamazaki T, Sajiki J, Saito H, Tahara K, Tamura Y, Kumagai A. Studies on the mechanism of antiaggregatory effect of Moutan Cortex. Thromb Res. 1983;31:29–40. doi: 10.1016/0049-3848(83)90005-1. [DOI] [PubMed] [Google Scholar]
- 10.Liu JS, Zhou QR. [The toxic principle of Illicium henryi Diels and structure of 6-deoxypseudoanisatin] . Yao Xue Xue Bao. 1988;23:221–223. [PubMed] [Google Scholar]
- 11.Filho AW, Filho VC, Olinger L, de Souza MM. Quercetin: further investigation of its antinociceptive properties and mechanisms of action. Arch Pharm Res. 2008;31:713–721. doi: 10.1007/s12272-001-1217-2. [DOI] [PubMed] [Google Scholar]
- 12.Gadotti VM, Schmeling LO, Machado C, Liz FH, Filho VC, Meyre-Silva C, Santos AR. Antinociceptive action of the extract and the flavonoid quercitrin isolated from Bauhinia microstachya leaves. J Pharm Pharmacol. 2005;57:1345–1351. doi: 10.1211/jpp.57.10.0014. [DOI] [PubMed] [Google Scholar]
- 13.Stratton SA, Heckmann R, Francis RS. Therapeutic ultrasound: its effects on the lntegrity of a nonpenetrating wound*. J Orthop Sports Phys Ther. 1984;5:278–281. doi: 10.2519/jospt.1984.5.5.278. [DOI] [PubMed] [Google Scholar]
- 14.Suleyman H, Demirezer LO, Kuruuzum A, Banoglu ZN, Gocer F, Ozbakir G, Gepdiremen A. Antiinflammatory effect of the aqueous extract from Rumex patientia L-roots. J Ethnopharmacol. 1999;65:141–148. doi: 10.1016/s0378-8741(98)00175-5. [DOI] [PubMed] [Google Scholar]
- 15.Bunn JR, Canning J, Burke G, Mushipe M, Marsh DR, Li G. Production of consistent crush lesions in murine quadriceps muscle--a biomechanical, histomorphological and immunohistochemical study. J Orthop Res. 2004;22:1336–1344. doi: 10.1016/j.orthres.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Barlow Y, Willoughby J. Pathophysiology of soft-tissue repair. Br Med Bull. 1992;48:698–711. doi: 10.1093/oxfordjournals.bmb.a072572. [DOI] [PubMed] [Google Scholar]
- 17.Noble KA. Pathophysiology corner: inflammation I. J Perianesth Nurs. 2005;20:56–58. doi: 10.1016/j.jopan.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Schaser KD, Vollmar B, Menger MD, Schewior L, Kroppenstedt SN, Raschke M, Lubbe AS, Haas NP, Mittlmeier T. In vivo analysis of microcirculation following closed soft-tissue injury. J Orthop Res. 1999;17:678–685. doi: 10.1002/jor.1100170509. [DOI] [PubMed] [Google Scholar]
- 19.Perkins ND. The Rel/NF-κB family: friend and foe. Trends Biochem Sci. 2000;25:434–440. doi: 10.1016/s0968-0004(00)01617-0. [DOI] [PubMed] [Google Scholar]
- 20.Henkel T, Machleidt T, Alkalay I, Kronke M, Ben-Neriah Y, Baeuerle PA. Rapid proteolysis of I-kappa-B-alpha is necessary for activation of transcription factor NF-kappa-B. Nature. 1993;365:182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- 21.Zhang YW, Wu CY, Cheng JT. Merit of Astragalus polysaccharide in the improvement of early diabetic nephropathy with an effect on mRNA expressions of NF-kappaB and IkappaB in renal cortex of streptozotoxin-induced diabetic rats. J Ethnopharmacol. 2007;114:387–392. doi: 10.1016/j.jep.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Lee SH, Soyoola E, Chanmugam P, Hart S, Sun W, Zhong H, Liou S, Simmons D, Hwang D. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J Biol Chem. 1992;267:25934–25938. [PubMed] [Google Scholar]
- 23.Choi JH, Jung BH, Kang OH, Choi HJ, Park PS, Cho SH, Kim YC, Sohn DH, Park H, Lee JH, Kwon DY. The anti-inflammatory and anti-nociceptive effects of ethyl acetate fraction of cynanchi paniculati radix. Biol Pharm Bull. 2006;29:971–975. doi: 10.1248/bpb.29.971. [DOI] [PubMed] [Google Scholar]
- 24.Rubinstein I, Abassi Z, Coleman R, Milman F, Winaver J, Better OS. Involvement of nitric oxide system in experimental muscle crush injury. J Clin Invest. 1998;101:1325–1333. doi: 10.1172/JCI810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moncada S, Higgs EA. Endogenous nitric oxide: physiology, pathology and clinical relevance. Eur J Clin Invest. 1991;21:361–374. doi: 10.1111/j.1365-2362.1991.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 26.Tracey KJ, Beutler B, Lowry SF, Merryweather J, Wolpe S, Milsark IW, Hariri RJ, Fahey TJ 3rd, Zentella A, Albert JD, et al. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234:470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- 27.Venditti P, DiMeo S. Antioxidants, tissue damage, and endurance in trained and untrained young male rats. Arch Biochem Biophys. 1996;331:63–68. doi: 10.1006/abbi.1996.0283. [DOI] [PubMed] [Google Scholar]
- 28.Childs A, Jacobs C, Kaminski T, Halliwell B, Leeuwenburgh C. Supplementation with vitamin C and N-acetyl-cysteine increases oxidative stress in humans after an acute muscle injury induced by eccentric exercise. Free Radic Biol Med. 2001;31:745–753. doi: 10.1016/s0891-5849(01)00640-2. [DOI] [PubMed] [Google Scholar]
- 29.Chou TC. Anti-inflammatory and analgesic effects of paeonol in carrageenan-evoked thermal hyperalgesia. Br J Pharmacol. 2003;139:1146–1152. doi: 10.1038/sj.bjp.0705360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nair MP, Mahajan S, Reynolds JL, Aalinkeel R, Nair H, Schwartz SA, Kandaswami C. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-kappa beta system. Clin Vaccine Immunol. 2006;13:319–328. doi: 10.1128/CVI.13.3.319-328.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


