Abstract
Background: Placenta growth factor (PLGF) is a member of the vascular endothelial growth factor (VEGF) family which is associated with the progression and metastasis of cancer. However, whether it can be used to predict prognosis in multiple cancer is still inconsistent. Methods: A meta-analysis was performed by searching electronic databases updated to December 2014. Eligible studies which evaluated the relationship between PLGF expression level and survival of patients with multiple cancers were conducted. Overall survival (OS), progression-free survival (PFS), hazard ratio (HR), and 95% confidence intervals (CI) were calculated. Results: Nineteen studies with a variety of cancers were included for the meta-analysis. Combined HR suggested that high expression of PLGF significantly associated with a poor OS (HR=1.69, 95% CI, 1.32-2.16), and PFS (HR=1.8, 95% CI, 1.33-2.44) in patients with different cancers. Moreover, a subgroup analysis based on cancer type demonstrated that high expression level of PLGF predict poor OS in both digestive system carcinoma (HR=1.63, 95% CI, 1.21-2.19; I2=80.7%, P<0.001) and respiratory system tumor (HR=1.75, 95% CI, 1.28-2.41; I2=0.0%, P=0.394). For PFS, the similar result was found in respiratory system tumor (HR=1.64, 95% CI, 1.23-2.19; I2=0.0%, P=0.807), but not in digestive system carcinoma (HR=1.81, 95% CI, 0.93-3.52; I2=80.2%, P<0.001). Conclusion: Our meta-analysis demonstrates that PLGF might be regarded as a poor prognostic fact for multiple cancers. More large-scale and well-designed studies are still needed to strengthen our findings.
Keywords: Multiple cancers, placenta growth factor, prognosis, meta-analysis
Introduction
Angiogenesis is essential to multiple tumor’s growth, progression and metastasis [1] and is tightly regulated by numerous angiogenic factors [2]. Of the known angiogenic factors, Placenta growth factor (PLGF), as a number of the vascular endothelial growth factor (VEGF) family, has been established as a very potent inducer of tumor angiogenesis which can stimulate endothelial cell growth, migration, and amplification, and vascular permeability [3-6]. It is worth to note that the expression of PLGF is undetectable in majority of human tissues under healthy conditions, but in pathological conditions, it is highly expressed in several cell types, such as vascular endothelial cells, smooth muscle cells, keratin cells, hematopoietic stem cells, retinal pigment epithelial cells and various tumor cells [7]. Besides, PLGF selectively binds to Flt-1 which can regulate the contribution of monocyte and macrophages to lymphangiogenesis and angiogenesis [8,9].
Numerous of evidence suggests that PLGF levels in tumor tissue and/or serum correlated with tumor stage, metastasis, vascularity, recurrence, and survival in various human tumors [10-14]. Lots of clinical studies demonstrate that the expression of PLGF upregulated in various cancer, such as colorectal, hepatocellular, renal and others, and the high PLGF levels are significantly associated with poor prognosis [11,12,15,16]. Nevertheless, some research demonstrated that the PLGF mRNA down regulated or undetectable in several tumors. Moreover, anti-PLGF antibodies as anti-angiogenic therapies in mouse models, and constituted a functionally relevant mechanism of inhibited the growth and metastasis of tumor in some pre-clinical model. Thus, it is necessary to establish whether PLGF expression is a prognostic biomarker in human cancer. Here, we performed a meta-analysis of published studies to evaluate whether PLGF overexpression may be a prognostic biomarker for survival in multiple cancers.
Material and methods
Search strategy
We searched the relevant studies form the electronic literature database of PubMed to include in our meta-analysis. Our search identified relevant articles up to December 2014, and limited to studies conducted on human theme. Search terms included: (“placental growth factor”, or “PLGF”), and (“cancer”, OR “carcinoma”, OR “sarcoma”), and (“prognostic”, OR “survival”, “mortality”). The reference of the included studies was manually examined to complete the search.
Inclusion and exclusion criteria
Studies included in this meta-analysis had to meeting the following criteria: 1) they were clinical trials study on human who suffer from cancer. 2) They had to investigating the association between the expression levels of PLGF and survival provided by information of OS, or PFS. 3) Hazard ratio (HR) and their corresponding 95% CI estimated by the sufficient data. The criteria used to exclude of studies were: 1) reviews, letters and repeated literature; 2) the clinical trials about the new drug of anti-angiogenesis.
Data extraction and quality assessment
The following information was carefully extracted from studies using a purpose-designed from: first author, publication year, study population, source of control, different ethnicities were categorized as Asian and non-Asian, Due to the more types of cancer and a relatively small sample size, we classified four types according to the human body system, like digestive system carcinoma, respiratory system tumor and others (hematological cancer, nervous system tumors, urinary genital system tumor), study population, methods of detecting PLGF, specimens, outcomes, and HR estimates with corresponding 95% CI for PLGF which extracted from the most completely adjusted one. If the HRs and their 95% weren’t given explicitly, we calculated it from survival information using methods proposed by Parmar et al. [17]. In order to ensure the quality of our meta-analysis, we excluded the studies which without these points. Systematically evaluated was conducted by us according to the guidelines of the Meta-analysis of Observational Studies in Epidemiology [18,19].
Statistical analysis
HR with 95% confidence intervals (CIs) was used to estimate the strength association strength between PLGF and cancer survival. Heterogeneity was tested by the Q test which was considered statistically significant when P values <0.01 and inconsistency index I2 statistic which take values between 0% and 100% (I2<25%: low heterogeneity; I2=25%-50%: medium heterogeneity; I2=50%-75%: high heterogeneity; I2=75%-100%: respectively heterogeneity). According to the heterogeneity of studies, it considered to be significant when P<0.01 or I2>50%, the random effects model (based on Der Simonian and Laird method) or fixed effects model (based on Mantel-Haenszel method) was used for meta-analysis [20,21]. By convention, poor survival for high expression level of PLGF was considered when reported HR >1. What’s more, if the 95% CI didn’t overlap 1, the impact of PLGF on survival of multiple cancers was considered with statistical significance. Robustness of the results of meta-analysis was tested through the sensitivity analyses. Subgroup analyses were performed based on cancer types, ethnicities and detection methods to investigate the value of PLGF for multiple cancers.
The publication bias was evaluated by using the methods of Begg’s and of Egger’s [22,23]. A P<0.05 was considered as statistically significant publication bias. All of the calculations were performed by Review Manager 5.2 (RevMan version 5.2; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration) and STATA version 12.0 (Stata Corporation, College Station, TX).
Results
Characteristics of studies
Nineteen eligible studies [12-15,24-39], including 2,528 cases of a variety of cancer patients, were included in our meta-analysis. The baseline data and other details were presented in Table 1. Specimens of 11 studies were collected from tumor tissue, 7 studies used serum specimens, and 1 studies used bone marrow (BM). The eligible studies were conducted in ten countries (China, Spain Germany, Belgium, Denmark, Korea, Italy, Turkey, Poland, Japan). Among them, 10 studies were performed in Asian, and the remaining 9 studies were non-Asian. 6 studies used Enzyme-linked immunosorbent assay (ELISA) to detect the expression of PLGF; 6 studies applied immunohistochemistry (IHC); 4 studies used quantitative real-time PCR (qRT-PCR) and three studies applied other methods (enzyme immunoassay, Luminex System and Roche-Elecsys).
Table 1.
Main characteristic and results of the eligible studies
| First author-year | Cancer | Country | N | Specimen source | Methods | survival analysis method | HR estimation | HR (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Chang YT-2008 | Pancreatic Cancer | China | 92 | Serum | ELISA | Kaplan-Meier analysis | Given | OS 0.98 (0.94-1.00) |
| Chen CN-2004 | gastric cancer | China | 79 | Tissue | Others | Kaplan-Meier analysis | Calculate | OS 1.46 (0.99-2.15) |
| Cheng SJ-2010 | oral squamous cell carcinoma | China | 100 | Tissue | IHC | Multivariate Cox analysis | Given | OS 4.28 (1.53-20.56) |
| Cheng SJ-2013 | oral squamous cell carcinoma | China | 63 | Tissue | PCR | Multivariate Cox analysis | Given | PFS 7.28 (3.57-18.27) |
| Sanmartín E-2013 | Non-small Cell Lung Cancer | Spain | 175 | Tissue | PCR | Kaplan-Meier analysis | Calculate | OS 1.46 (0.93-2.34) |
| PFS 1.59 (1.08-2.34) | ||||||||
| Maae E-2012 | breast cancer | Denmark | 229 | Tissue | Others | Multivariate Cox analysis | Given | PFS 1.94 (1.08-3.48) |
| Pompeo E-2009 | Malignant pleural mesothelioma | Italy | 27 | Tissue | IHC | Kaplan-Meier analysis | Calculate | OS 1.82 (1.08-3.07) |
| PFS 1.71 (1.11-2.66) | ||||||||
| Hilfenhaus G-2013 | neuroendocrine tumors (NETs) | Germany | 87 | Serum | Others | Kaplan-Meier analysis | Given | OS 2.35 (1.08-5.10) |
| PFS 4.87 (1.18-20.13) | ||||||||
| Ho MC-2007 | hepatocellular carcinoma | China | 71 | Tissue | PCR | Kaplan-Meier analysis | Calculate | PFS 2.06 (0.15-27.5) |
| Coenegrachts L-2013 | endometrial carcinomas | Belgium | 128 | Tissue | PCR | Kaplan-Meier analysis | Calculate | OS 1.24 (0.51-3.03) |
| PFS 0.95 (0.44-2.02) | ||||||||
| Rahbari NN-2011 | colorectal liver metastases | Germany | 107 | Serum | ELISA | Multivariate Cox analysis | Given | PFS 0.26 (0.08-0.81) |
| Rahbari NN-2011 | pancreatic cancer | Germany | 67 | Serum | ELISA | Multivariate Cox analysis | Given | OS 0.87 (0.35-2.12) |
| Kemik Ö-2012 | colorectal cancer | Turkey | 158 | Serum | ELISA | Multivariate Cox analysis | Given | OS 3.00 (1.53-6.21) |
| Sujka-Kordowska P-2012 | ALL | Poland | 264 | BM | IHC | Kaplan-Meier analysis | Calculate | PFS 2.12 (1.08-4.16) |
| Nagaoka S-2010 | hepatocellular carcinoma | Japan | 78 | Serum | ELISA | Kaplan-Meier analysis | Given | OS 1.36 (0.91-2.03) |
| Sung CY-2012 | colorectal cancer | Korea | 83 | Tissue | IHC | Kaplan-Meier analysis | Calculate | OS 2.17 (1.01-4.65) |
| Wei SC-2009 | colorectal cancer | China | 86 | Serum | ELISA | Multivariate Cox analysis | Given | OS 3.20 (1.01-10.10) |
| 71 | PFS 2.46 (1.27-4.78) | |||||||
| Xu HX-2010 | hepatocellular carcinoma | China | 394 | Tissue | IHC | Kaplan-Meier analysis | Calculate | OS 1.58 (1.22-2.04) |
| PFS 1.43 (1.10-1.87) | ||||||||
| 102 | Multivariate Cox analysis | Given | OS 2.05 (1.09-3.85) | |||||
| PFS 1.95 (1.03-3.69) | ||||||||
| Zhang LJ-2005 | non-Small cell Lung cancer | China | 91 | Tissue | IHC | Multivariate Cox analysis | Given | OS 2.74 (1.27-6.10) |
Note: N, number of patients; IHC, immunohistochemistry; ELISA, Enzyme-linked immunosorbent assay; RT-PCR, reverse transcription-polymerase chain reaction; HR, hazard ratio; CI, confidence interval; OS, overall survival; PFS, progression free survival; BM, bone marrow.
The prognostic value of PLGF status for survival in patients with several cancers (Non-small Cell Lung Cancer, hepatocellular carcinoma (HCC), pancreatic cancer, endometrial carcinomas, colorectal cancer, hepatocellular carcinoma, neuroendocrine tumors (NETs), breast cancer, oral squamous cell carcinoma (OSCC), gastric cancer, Pancreatic Cancer, Malignant pleural mesothelioma, acute lymphoblastic leukaemia (ALL) were reported among the studies. Due to the more types of cancer and a relatively small sample size, we classified four types according to the human body system, like digestive system carcinoma, respiratory system tumor and others (hematological cancer, nervous system tumors, urinary genital system tumor). The HR estimation of 12 studies was directly reported, while others calculated using the information given by authors. Of the 19 studies, 14 studies offered OS, and 11 studies offered PFS.
Meta-analysis
The combined HR of 15 included studies including 1747 cancer patients showed that high PLGF level was associated with poor overall survival (HR=1.69, 95% CI, 1.32-2.16). Furthermore, there was significant heterogeneity among the studies (I2=78.6%, P=0.000), as shown in Figure 1. As for PFS, the pooled HR of 12 eligible studies including 1678 cancer patients was 1.8 (95% CI, 1.33-2.44), which suggests that high PLGF level represent an indicator of poor prognosis for multiple cancer, and significant heterogeneity was found between studies (I2=63.8%, P=0.001), as shown in Figure 2.
Figure 1.
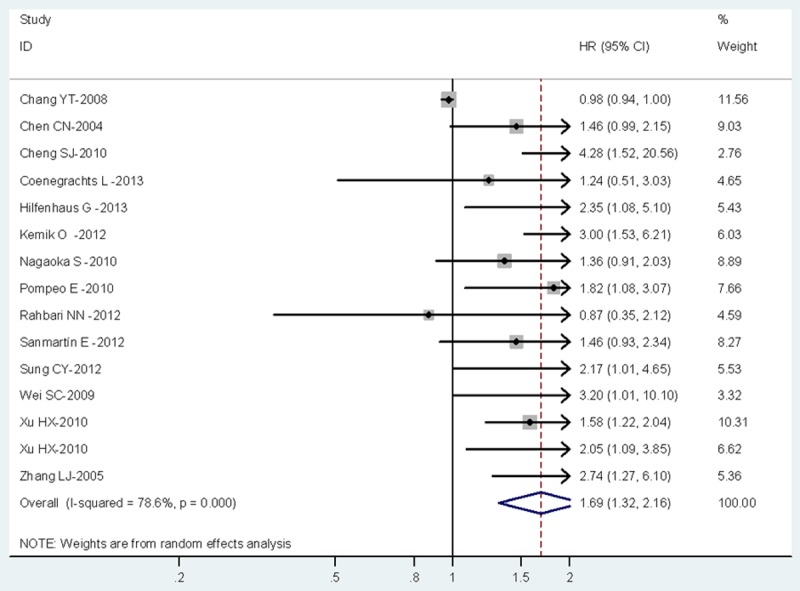
Meta-analysis (forest plot) of 15 included studies evaluation PLGF in Overall survival.
Figure 2.
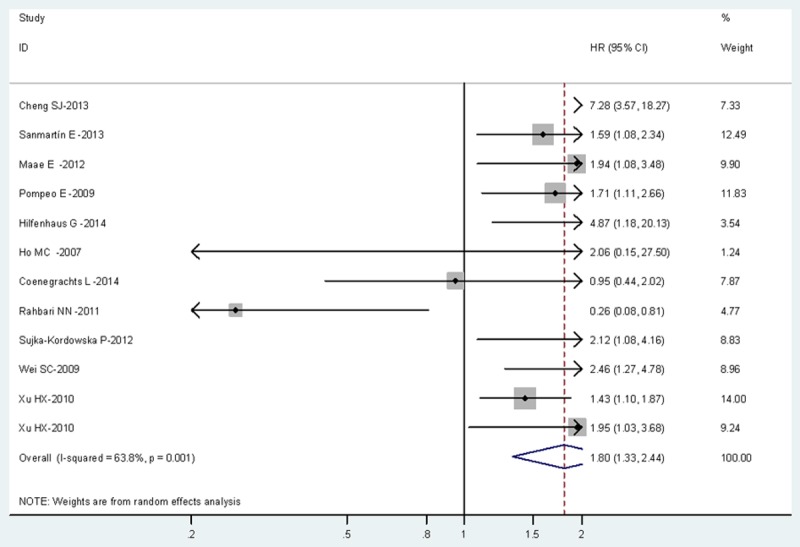
Meta-analysis (forest plot) of 12 included studies evaluation PLGF in Progression-Free Survival.
Tumor type analysis
Analysis by tumor type was performed in Table 2. It showed high expression of PLGF was associated with poor OS in digestive system carcinoma (HR=1.63, 95% CI, 1.21-2.19), and significant heterogeneity was observed in studies (I2=80.7%, P<0.001). But not obvious associated with poor PFS in those cancer patients (HR=1.81, 95% CI, 0.93-3.52; I2=80.2%, P<0.001). In respiratory system tumor, the similar result found high expression of PLGF predicted poor OS (HR=1.75, 95% CI, 1.28-2.41) without heterogeneity (I2=0.0%, P=0.394) and PFS (HR=1.64, 95% CI, 1.23-2.19) without heterogeneity (I2=0.0%, P=0.807) either. In other system cancers, it also suggest a poor PFS (HR=1.79, 95% CI, 1.24-2.59; I2=38.6%, P=0.181).
Table 2.
Meta-analysis: HRs value of OS and PFS in overall and subgroups of multiple cancer according to study design
| Subgroup | No. of cases | No. of studies | Pooled HR (95% CI) | P-value | Heterogeneity | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| I2 (%) | P-value | ||||||
| OS | Overall | 1747 | 15 | 1.69 (1.32-2.16) | <0.001 | 78.6 | <0.001 |
| Tumor type | |||||||
| Digestive system carcinoma | 1239 | 10 | 1.63 (1.21-2.19) | 0.001 | 80.7 | <0.001 | |
| Respiratory system tumor | 293 | 3 | 1.75 (1.28-2.41) | 0.001 | 0.0 | 0.394 | |
| Others | 215 | 2 | 1.78 (0.99-3.20) | 0.053 | 11.1 | 0.289 | |
| Ethnicity | |||||||
| Asian patients | 1105 | 9 | 1.68 (1.23-2.28) | 0.001 | 81.6 | <0.001 | |
| non-Asian patients | 642 | 6 | 1.70 (1.31-2.21) | <0.001 | 20.4 | 0.279 | |
| Detection methodI | |||||||
| IHC | 797 | 6 | 1.79 (1.47-2.19) | <0.001 | 0.0 | 0.525 | |
| ELSIA | 481 | 5 | 1.45 (0.93-2.25) | 0.104 | 75.6 | 0.003 | |
| qRT-PCR | 303 | 2 | 1.41 (0.94-2.12) | 0.100 | 0.0 | 0.750 | |
| Others | 166 | 2 | 1.61 (1.13-2.27) | 0.007 | 13.5 | 0.282 | |
| Survival analysis method | |||||||
| Kaplan-Meier analysis | 1143 | 9 | 1.46 (1.14-1.89) | 0.003 | 77.8 | <0.001 | |
| Multivariate Cox analysis | 604 | 6 | 2.29 (1.63-3.21) | <0.001 | 23.5 | 0.257 | |
| Specimen source | |||||||
| Serum | 568 | 6 | 1.56 (1.02-2.40) | 0.042 | 76.4 | 0.001 | |
| Tissue | 1179 | 9 | 1.67 (1.42-1.96) | <0.001 | 0.0 | 0.661 | |
| PFS | Overall | 1678 | 12 | 1.80 (1.33-2.44) | <0.001 | 63.8 | <0.001 |
| Tumor type | |||||||
| Tumor type | 808 | 6 | 1.81 (0.93-3.52) | 0.079 | 80.2 | <0.001 | |
| Respiratory system tumor | 202 | 2 | 1.64 (1.23-2.19) | 0.001 | 0.0 | 0.807 | |
| Others | 668 | 4 | 1.79 (1.24-2.59) | 0.002 | 38.6 | 0.181 | |
| Ethnicity | |||||||
| Asian patients | 701 | 5 | 2.44 (1.35-4.39) | 0.003 | 73.4 | 0.005 | |
| non-Asian patients | 977 | 7 | 1.50 (1.02-2.22) | 0.040 | 59.5 | 0.022 | |
| Detection method | |||||||
| IHC | 787 | 4 | 1.59 (1.30-1.95) | <0.001 | 0.0 | 0.615 | |
| ELSIA | 178 | 2 | 0.84 (0.09-7.60) | 0.879 | 90.8 | 0.001 | |
| qRT-PCR | 437 | 4 | 2.15 (0.87-5.29) | 0.096 | 79.1 | 0.002 | |
| Others | 276 | 2 | 2.22 (1.29-3.81) | 0.004 | 27.7 | 0.240 | |
| Survival analysis method | |||||||
| Kaplan-Meier analysis | 1059 | 6 | 1.52 (1.27-1.82) | <0.001 | 0.0 | 0.703 | |
| Multivariate Cox analysis | 619 | 6 | 2.14 (1.06-4.33) | 0.033 | 78.2 | <0.001 | |
| Specimen source | |||||||
| Serum | 225 | 3 | 1.45 (0.30-7.02) | 0.646 | 85.1 | 0.001 | |
| Tissue | 1189 | 8 | 1.81 (1.34-2.44) | <0.001 | 57.5 | 0.021 | |
| BM | 264 | 1 | 2.12 (1.08-4.16) | 0.029 | - | - | |
Other subgroup analysis
We performed other subgroup analysis on ethnicity, high expression of PLGF associate with poor OS either in Asian patients (HR=1.68, 95% CI, 1.23-2.28, P=0.001; I2=81.6%, P<0.001) or non-Asian patients (HR=1.70, 95% CI, 1.31-2.21, P<0.001; I2=20.4%, P=0.279). Moreover, the same founding for the PFS in Asian patients (HR=2.44, 95% CI, 1.35-4.39, P=0.003; I2=73.4%, P=0.005) and non-Asian patients (HR=1.50, 95% CI, 1.02-2.22, P=0.040; I2=59.5%, P=0.022). Studies detected by IHC found high expression of PLGF indicated worse OS (HR=1.79, 95% CI, 1.47-2.19, P=0.000; I2=0.0%, P=0.525) and PFS (HR=1.59, 95% CI, 1.30-1.95, P=0.000; I2=0.0%, P=0.615). However, in studied which detected by ELISA or PCR, the combined HR didn’t show obvious association between high expression of PLGF and survival. Then, other subgroup analysis including sources of specimen and survival analysis method were performed, all of them suggested that high PLGF level was an indicator of poor prognosis for multiple cancer. As shown in Table 2.
Publication bias
At last, the Publication bias of our meta-analysis was performed by Begg’s funnel plot and Egger’s test. 12 studies evaluating PFS of patients with multiple cancer yield a Begg’s and Egger’s test which score of P=0.304 and P=0.519 respectively. Meanwhile according to the funnel plot (Figure 3), there were no publication biases. However, for evaluating high PLGF level on OS of patients in 16 studies, publication biases was found (Begg’s test P=1.0 and Egger’s test P<0.001) (Figure 4).
Figure 3.
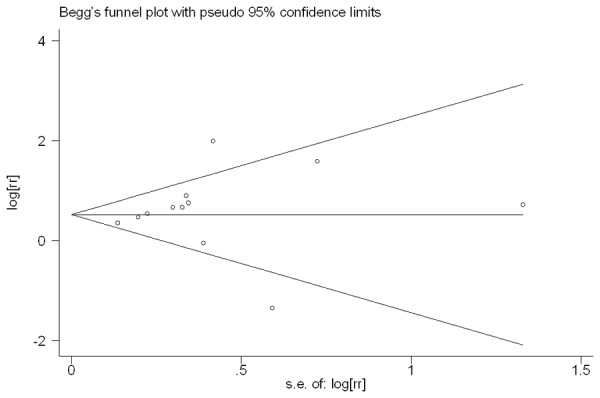
Funnel plot of the 12 evaluable studies assessing PLGF in multiple cancer for progression-free survival.
Figure 4.
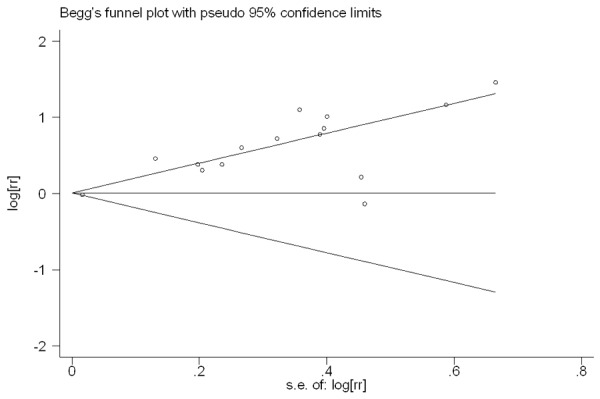
Funnel plot of the 15 evaluable studies assessing PLGF in multiple cancer for overall survival.
Discussion
Although various pre-clinical models and clinical studies have been conducted, the role of PLGF in tumor growth and tumor angiogenesis is still controversial [40]. Moreover, increasing clinical settings have been demonstrated the relationship between expression of PLGF and prognosis for multiple cancers. Here, we performed the first meta-analysis by collected complete articles and pooled the prognostic value to explore the association between PLGF and cancer prognosis.
In present meta-analysis, included 19 studies with 2528 cases, were identified and analyzed. The results shown that PLGF over-expression is a poor prognostic factor in multiple cancers with statistical significance for OS (HR=1.69, 95% CI, 1.32-2.16), and PFS (HR=1.8, 95% CI, 1.33-2.44). According to the different of tumor type, ethnicity, detection and survival analysis method, and specimen source, we conducted the subgroup analysis. For tumor type, the analysis indicated a statistically significant detrimental effect of PLGF on OS in digestive and respiratory systems carcinomas. Furthermore, high PLGF expression also significantly related with worse PFS in respiratory system carcinoma and others, but not on PFS in digestive respiratory system carcinoma. Several studies have confirmed that PLGF was high expressed in digestive and respiratory systems cancers [28,35]. Further studies showed that inhibiting the production of PLGF in NSCLC can reduce the activity of tumor cells [41]. Thus, PLGF may be used as a prognostic marker and therapeutic target for those two systems carcinomas.
Furthermore, there are several limitations for our analysis. (1) Non-English literature was included in our meta-analysis which leads to lose some potential important survival data; meanwhile there might be selection bias. (2) Different detection methods (IHC, ELISA, RT-PCR or others) were used to detect the expression of PLGF in multiple cancers. (3) The sources of specimen were different from tissue, serum or bone marrow in different studies. (4) No consistent standard for cut-off values in our eligible studies. All of above might cause clinical and statistical heterogeneity. Moreover, the HRs itself may source of heterogeneity owing to the methodology for calculating from unreported articles. In order to decrease the influence of this heterogeneity, we used a random effects model and performed subgroup analysis. However, due to the number of studies of each tumor type especially in the other system type and the patient cases of subgroup were limited, we still need more researches to analyze the value of PLGF expression in multiple cancers. Although our results demonstrated that high PLGF expression is poor prognostic factor for OS and PFS in cancer patients, we could not identify it as an independent prognostic fact of all the tumors. On the contrary, in the Rahbari NN et al. 2011 study, we found the low levels of circulating PLGF predicted a poor recurrence-free survival [34].
Publication bias is a major concern for all forms of meta-analysis [42]. The present analysis only included the published studies; we did not search for unpublished. Nevertheless, most of those published articles with a positive results; the negative results are often rejected or not even given in the articles. And part of the results was based on unadjusted HRs which may cause serious confounding bias. Under those possibility of publication bias, our meta-analysis could not completely exclude bias although no publication bias for OS was indicated. Much less, a publication bias for PFS. All of those affect the prognosis. As a result, it is necessary to flexibly regard these results.
To sum up, due to the heterogeneity, biases, and other limitations our paper is imperfect, but it is worth noting that PLGF is a candidate prognostic biomarker. The present meta-analysis demonstrated that PLGF over-expression is associated with poor outcome in multiple cancers. To strengthen our findings, more large-scale and well-designed studies need to further investigate the associations of PLGF with survival of multiple cancers.
In conclusion, we demonstrated that PLGF over-expression is significantly associated with poor overall survival and progression-free survival, especially in respiratory and digestive system carcinoma. High expression of PLGF may predict poor prognosis in different cancer. At the same time, the target of PLGF could become an effective target for anticancer therapy.
Disclosure of conflict of interest
None.
References
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 3.Odorisio T, Schietroma C, Zaccaria ML, Cianfarani F, Tiveron C, Tatangelo L, Failla CM, Zambruno G. Mice overexpressing placenta growth factor exhibit increased vascularization and vessel permeability. J Cell Sci. 2002;115:2559–2567. doi: 10.1242/jcs.115.12.2559. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, VandenDriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 5.Pipp F, Heil M, Issbrücker K, Ziegelhoeffer T, Martin S, van den Heuvel J, Weich H, Fernandez B, Golomb G, Carmeliet P, Schaper W, Clauss M. VEGFR-1-Selective VEGF Homologue PLGF Is arteriogenic: evidence for a monocyte-mediated mechanism. Circ Res. 2003;92:378–385. doi: 10.1161/01.RES.0000057997.77714.72. [DOI] [PubMed] [Google Scholar]
- 6.Maglione D, Guerriero V, Viglietto G, Delli-Bovi P, Persico MG. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci U S A. 1991;88:9267–9271. doi: 10.1073/pnas.88.20.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci Signal. 2009;2:re1. doi: 10.1126/scisignal.259re1. [DOI] [PubMed] [Google Scholar]
- 8.Tchaikovski V, Fellbrich G, Waltenberger J. The molecular basis of VEGFR-1 signal transduction pathways in primary human monocytes. Arterioscler Thromb Vasc Biol. 2008;28:322–328. doi: 10.1161/ATVBAHA.107.158022. [DOI] [PubMed] [Google Scholar]
- 9.Murakami M, Zheng Y, Hirashima M, Suda T, Morita Y, Ooehara J, Ema H, Fong GH, Shibuya M. VEGFR-1 tyrosine kinase signaling promotes lymphangiogenesis as well as angiogenesis indirectly via macrophage recruitment. Arterioscler Thromb Vasc Biol. 2008;28:658–664. doi: 10.1161/ATVBAHA.107.150433. [DOI] [PubMed] [Google Scholar]
- 10.Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PLGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8:942–956. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- 11.Parr C, Watkins G, Boulton M, Cai J, Jiang WG. Placenta growth factor is over-expressed and has prognostic value in human breast cancer. Eur J Cancer. 2005;41:2819–2827. doi: 10.1016/j.ejca.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Wei SC, Tsao PN, Yu SC, Shun CT, Tsai-Wu JJ, Wu CH, Su YN, Hsieh FJ, Wong JM. Placenta growth factor expression is correlated with survival of patients with colorectal cancer. Gut. 2005;54:666–672. doi: 10.1136/gut.2004.050831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei SC, Liang JT, Tsao PN, Hsieh FJ, Yu SC, Wong JM. Preoperative serum placenta growth factor level is a prognostic biomarker in colorectal cancer. Dis Colon Rectum. 2009;52:1630–1636. doi: 10.1007/DCR.0b013e3181afbdaf. [DOI] [PubMed] [Google Scholar]
- 14.Maae E, Olsen DA, Steffensen KD, Jakobsen EH, Brandslund I, Sørensen FB, Jakobsen A. Prognostic impact of placenta growth factor and vascular endothelial growth factor A in patients with breast cancer. Breast Cancer Res Treat. 2012;133:257–265. doi: 10.1007/s10549-012-1957-0. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Chen J, Ke Y, Mansel RE, Jiang WG. Expression of Placenta growth factor (PLGF) in non small cell lung cancer (NSCLC) and the clinical and prognostic significance. World J Surg Oncol. 2005;3:68. doi: 10.1186/1477-7819-3-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen CN, Hsieh FJ, Cheng YM, Cheng WF, Su YN, Chang KJ, Lee PH. The significance of placenta growth factor in angiogenesis and clinical outcome of human gastric cancer. Cancer Lett. 2004;213:73–82. doi: 10.1016/j.canlet.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 17.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 18.Fu X, Han Y, Wu Y, Zhu X, Lu X, Mao F, Wang X, He X, Zhao Y, Zhao Y. Prognostic role of microRNA-21 in various carcinomas: a systematic review and meta-analysis. Eur J Clin Invest. 2011;41:1245–1253. doi: 10.1111/j.1365-2362.2011.02535.x. [DOI] [PubMed] [Google Scholar]
- 19.Xu W, Xu J, Liu S, Chen B, Wang X, Li Y, Qian Y, Zhao W, Wu J. Effects of common polymorphisms rs11614913 in miR-196a2 and rs2910164 in miR-146a2 on cancer susceptibility: a meta-analysis. PLoS One. 2011;6:e20471. doi: 10.1371/journal.pone.0020471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 24.Chang YT, Chang MC, Wei SC, Tien YW, Hsu C, Liang PC, Tsao PN, Jan IS, Wong JM. Serum vascular endothelial growth factor/soluble vascular endothelial growth factor receptor 1 ratio is an independent prognostic marker in pancreatic cancer. Pancreas. 2008;37:145–150. doi: 10.1097/MPA.0b013e318164548a. [DOI] [PubMed] [Google Scholar]
- 25.Chen CN, Hsieh FJ, Cheng YM, Cheng WF, Su YN, Chang KJ, Lee PH. The significance of placenta growth factor in angiogenesis and clinical outcome of human gastric cancer. Cancer Lett. 2004;213:73–82. doi: 10.1016/j.canlet.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 26.Cheng SJ, Lee JJ, Kok SH, Chou CH, Chang HH, Chiang ML, Chen HM, Kuo MY, Chiang CP. Expression of placenta growth factor: an independent factor for prediction of progression of oral cancer. Head Neck. 2010;32:1363–1369. doi: 10.1002/hed.21335. [DOI] [PubMed] [Google Scholar]
- 27.Cheng SJ, Cheng SL, Lee JJ, Chen HM, Chang HH, Kok SH, Chiang ML, Kuo MY. Increased placenta growth factor mRNA level is significantly associated with progression, recurrence and poor prognosis of oral squamous cell carcinoma. J Formos Med Assoc. 2013;112:253–258. doi: 10.1016/j.jfma.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Sanmartín E, Sirera R, Usó M, Blasco A, Gallach S, Figueroa S, Martínez N, Hernando C, Honguero A, Martorell M, Guijarro R, Rosell R, Jantus-Lewintre E, Camps C. A gene signature combing the tissue expression of three angiogenic factors is a prognostic marker in early-stage non-small cell lung cancer. Ann Surg Oncol. 2014;21:612–620. doi: 10.1245/s10434-013-3330-x. [DOI] [PubMed] [Google Scholar]
- 29.Pompeo E, Albonici L, Doldo E, Orlandi A, Manzari V, Modesti A, Mineo TC. Placenta growth factor expression has prognostic value in malignant pleural mesothelioma. Ann Thorac Surg. 2009;88:426–431. doi: 10.1016/j.athoracsur.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 30.Hilfenhaus G, Göhrig A, Pape UF, Neumann T, Jann H, Zdunek D, Hess G, Stassen JM, Wiedenmann B, Detjen K, Pavel M, Fischer C. Placental growth factor supports neuroendocrine tumor growth and predicts disease prognosis in patients. Endocr Relat Cancer. 2013;20:305–319. doi: 10.1530/ERC-12-0223. [DOI] [PubMed] [Google Scholar]
- 31.Ho MC, Chen CN, Lee H, Hsieh FJ, Shun CT, Chang CL, Lai YT, Lee PH. Placenta growth factor not vascular endothelial growth factor A or C can predict the early recurrence after radical resection of hepatocellular carcinoma. Cancer Lett. 2007;250:237–249. doi: 10.1016/j.canlet.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Coenegrachts L, Schrauwen S, Van Bree R, Despierre E, Luyten C, Jonckx B, Stassen JM, Vergote I, Amant F. Increased expression of placental growth factor in high-grade endometrial carcinoma. Oncol Rep. 2013;29:413–8. doi: 10.3892/or.2012.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahbari NN, Reissfelder C, Mühlbayer M, Weidmann K, Kahlert C, Büchler MW, Weitz J, Koch M. Correlation of circulation angiogenic factors with circulating tumor cells and disease recurrence in patients undergoing curative resection for colorectal liver metastases. Ann Surg Oncol. 2011;18:2182–2191. doi: 10.1245/s10434-011-1761-9. [DOI] [PubMed] [Google Scholar]
- 34.Rahbari NN, Schmidt T, Falk CS, Hinz U, Herber M, Bork U, Büchler MW, Weitz J, Koch M. Expression and prognostic value of circulating angiogenic cytokines in pancreatic cancer. BMC Cancer. 2011;11:286. doi: 10.1186/1471-2407-11-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kemik O, Sarbay Kemik A, Sümer A, Purısa S, Tükenmez B, Tüzün S. Preoperative serum placenta growth factor level as a new marker for stage II or III colorectal cancer patients. Turk J Gastroenterol. 2012;23:104–109. doi: 10.4318/tjg.2012.0333. [DOI] [PubMed] [Google Scholar]
- 36.Sujka-Kordowska P, Malinska A, Ostalska-Nowicka D, Zabel M, Nowicki M. CD105 and placental growth factor--potent prognostic factors in childhood acute lymphoblastic leukaemia. Leuk Res. 2012;36:846–851. doi: 10.1016/j.leukres.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 37.Nagaoka S, Yoshida T, Akiyoshi J, Akiba J, Hisamoto T, Yoshida Y, Abe M, Koga H, Toirimura T, Ueno T, Sata M. The ratio of serum placenta growth factor to soluble vascular endothelial growth factor receptor-1 predicts the prognosis of hepatocelluar carcinoma. Oncol Rep. 2010;23:1647–1654. doi: 10.3892/or_00000807. [DOI] [PubMed] [Google Scholar]
- 38.Sung CY, Son MW, Ahn TS, Jung DJ, Lee MS, Baek MJ. Expression of placenta growth factor in colorectal carcinomas. J Korean Soc Coloproctol. 2012;28:315–320. doi: 10.3393/jksc.2012.28.6.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu HX, Zhu XD, Zhuang PY, Zhang JB, Zhang W, Kong LQ, Wang WQ, Liang Y, Wu WZ, Wang L, Fan J, Tang ZY, Sun HC. Expression and prognostic significance of placental growth factor in hepatocellular carcinoma and peritumoral liver tissue. Int J Cancer. 2011;128:1559–1569. doi: 10.1002/ijc.25492. [DOI] [PubMed] [Google Scholar]
- 40.Ribatti D. The controversial role of placental growth factor in tumor growth. Cancer Lett. 2011;307:1–5. doi: 10.1016/j.canlet.2011.02.043. [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Ye L, Zhang WG. Placenta growth factor, PLGF, influences the motility of lung cancer cell, the role of Rho associated kinase, Rock1. J Cell Biochem. 2008;105:313–320. doi: 10.1002/jcb.21831. [DOI] [PubMed] [Google Scholar]
- 42.Begg CB, Berlin JA. Publication bias: A problem in interpreting medical data. J R Stat Soc A. 1988;151:419–463. [Google Scholar]


