Abstract
Background: our research aim to study the role of AQP1 in the cardioprotective effect of remifentanil post-conditioning for myocardial ischemia/reperfusion injury. Methods: Ninety Sprague-Dawley (SD) rats were divided into 6 groups: sham operation group (Sham group), myocardial ischemia and reperfusion group (I/R group), postconditioning of remifentanil group (R-post), postconditioning of remifentanil plus AQP1 inhibitor acetazolamide group (R-post +Ace), postconditioning of remifentanil plus opioid-receptor antagonist compounds (R-post +AC), postconditioning of remifentanil plus AQP1 enhancer arginine vasopressin (R-post +AV). All groups except the sham operation group were given 30 min ischemia in left anterior descending (LAD) coronary arteries. All groups were then given 120 min reperfusion to the LAD. Before reperfusion, the R-post, R-post +Ace, R-post +AC, R-post +AV groups were given 10 min remifentanil post-conditioning. Hemodynamic data were measured every 30 min after initiation of ischemia. The rats’ hearts were exercised for detecting infarct size and water content in the left ventricle, and AQP1 expression were also detected. Results: The R-post group showed a significant reduction of the infarct size compared to the I/R group. The effect of R-post for reducing infarct size was slightly enhanced by adding acetazolamide to R-post, so significant differences could still be found when compared R-post+Ace group to the I/R group. The effect of infarct size reduction brought by R-post was blocked by the opioid-receptor antagonist compounds. This effect was also blocked by the AQP1 enhancer. Similar outcomes were found considering the water content of the left ventricle and the AQP1 expression. Conclusion: Cardioprotective effect of remifentanil post-conditioning may initiate through inhibiting the function of AQP1.
Keywords: AQP1, cardioprotective effect, remifentanil post-conditioning
Introduction
Myocardial reperfusion injury is a common clinical condition, which is usually caused by ischemia/reperfusion (I/R) treatment for coronary heart diseases [1]. Ischemia preconditioning and postconditioning are reported to be effective for myocardial IR reperfusion injury. Histological features of myocardial I/R injury are mainly myocytes swelling, contracture of myofibrils, and disruption of the sarcolemma. Myocardial edema is the first step of myocytes apoptosis, which leads to myocardial I/R injury [2,3]. Pre-conditioning refers to a number of I/R circles before myocardial ischemia. The pre-conditioning generates resistance against a subsequent prolonged period of myocardial ischemia. Post-conditioning starts I/R circles during early reperfusion and yields a similar cardioprotective effect as the pre-conditioning. Postconditioning is more practical than preconditioning, because ischemia attack is not predictable. Post-conditioning effectively reduce myocardial infarct size and edema level [4].
During the last 10 years, pharmacological postconditioning for managing myocardial I/R injury was extensively studied, several agents were used for postconditioning, including erythropoietin, adenosine, bradykinin, statins and opioids [5]. Recently, opioids post-conditioning attracts much attention for its effectiveness for myocardial I/R injury. Several studies showed promising cardioprotective effect of the opioids for decreasing myocardial I/R injury [6-9]. The selective μ opioid-receptor agonist is recently found to be clinically effective and with less side effects [5]. Remifentanil is one of the selective μ opioid-receptor agonist with a relatively short half-life. Postconditioning of Remifentanil is effective for reducing infarct size and myocardial edema of I/R injury [8,9].
Studies showed that the effect of reducing infarct size by sufentanil, a kind of selective μ opioid-receptor agonist, is related to PI3K/Akt-GSK-3b pathway, which explains how the down-stream molecular event causing myocardial apoptosis is controlled by sufentanil [10]. However, the mechanism is still unknown for post-conditioning of remifentanil, the same selective μ opioid-receptor agonist with a shorter reactive time, decreasing myocardial edema that initiates myocardial apoptosis and thus leads to I/R injury.
Aquaporins (AQP) are a family of proteins that can act as water channels, which are drug targets for reducing Somato-edema [11]. The AQP1 is related to myocardial edema [12]. A higher expression of AQP1 was observed in goats with myocardial edema [13]. Considering the close cause-effect relationship between myocardial edema and I/R injury [3], AQP1 might involve in myocytes swelling in the I/R injury process. The relation between remifentanil postconditioning and AQP1 regulation in controlling myocardial edema is not clearly demonstrated. Solving this problem would lead to a more clear understanding of the remifentanil postconditioning and a possible target for developing new drug treatment.
Methods
Animals and model preparation
Characteristics of the animals
We used 90 male Sprague-Dawley (SD) rats weighting 264.1±15.8 g in a room with artificially ventilated air. We used experimental protocols that are in accordance with the National Institutes of Health Guide for the care and use of laboratory animals [14]. We get a permission for this study from the Ethics Review Committee in Fujian Medical University.
Preparation of the ischemia and reperfusion injury model
To prepare the model, the rats were anesthetized with anchloral hydrate (3.5 ml/kg) through intraperitoneal injection. Subcutaneous stainless-steel electrodes were used to monitor the heart rate (HR) after anesthesia, and the rectal temperature was kept at 37±1°C (mean ± standard deviation) through a temperature sense probe. Animals were intubated through puncturing cricothyroid membrane and connecting to a small animal respirator (TKR-200C, manufactured by JiangXi Teli Anaesthesia & Respiration Equipment CO., LTD, China), according to Li’s method [15]. After intubation, the rats were mechanically ventilated at a rate of 60 breaths per minute with a tidal volume of 3 ml/100 g. Anesthesia was maintained with anchloral hydrate (3.5 ml/kg), additional doses were given during surgery as needed. After sterilization, we performed a left thoracotomy through the fourth left intercostal space to expose the heart. A 6-0 polypropylene suture was passed under the left anterior descending (LAD) coronary artery with a small, curved, 6.0-gauge needle. We threaded the suture ends to make a snare, with a vinyl tube with a diameter of 5mm and a length of 0.5 cm. Regional myocardial ischemia was produced by pulling the snare for 30 minutes. The snare was then released to cause reperfusion for 120 minutes, to produce myocardial reperfusion injury.
Groups and interventions
The 90 SD rats were randomly assigned to 6 groups, 15 rats in a group. (1) Sham opea was produced for 30 min, and reperfusion was given for 120 min. (3) Postconditioning of remifentanil group (R-post): After producing myocardial regional ischemia for 30 min, we gave the remifentanil post-condition to the rats, alongside with initiation of reperfusion, the remifentanil was used at a dose of 10 μg/kg. (4) Postconditioning of remifentanil plus acetazolamide group (R-post +Ace): The rats were given AQP1 inhibitor acetazolamide intramuscularly for a dose of 1 mg/100 g, just before the initiation of the remifentanil post-condition. The rest procedures were the same as the R-post group. (5) Postconditioning of remifentanil plus antagonist compounds (R-post +AC): The antagonist compounds were composed of a selective δ opioid-receptor antagonist (NTD), a selective κ receptor antagonist (nor-BNI, nor-binaltorphimine), a selective μ receptor antagonist (CTOP, D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2), which were reported to be effectively blocking remifentanil post-condition [9]. These compounds were dissolved in 0.9% saline and administrated as a bolus at the following doses: NTD (5 mg/kg); nor-BNI (5 mg/kg) and CTOP (1 mg/kg). The rest procedures were the same as the R-post group. (6) Postconditioning of remifentanil plus arginine vasopressin (R-post +AV): Arginine vasopressin is reported to significantly increase the expression of AQP1 and water permeability in first trimester-derived extravillous cytotrophoblasts [16]. The intervention procedures of each group were summarized in Figure 1.
Figure 1.
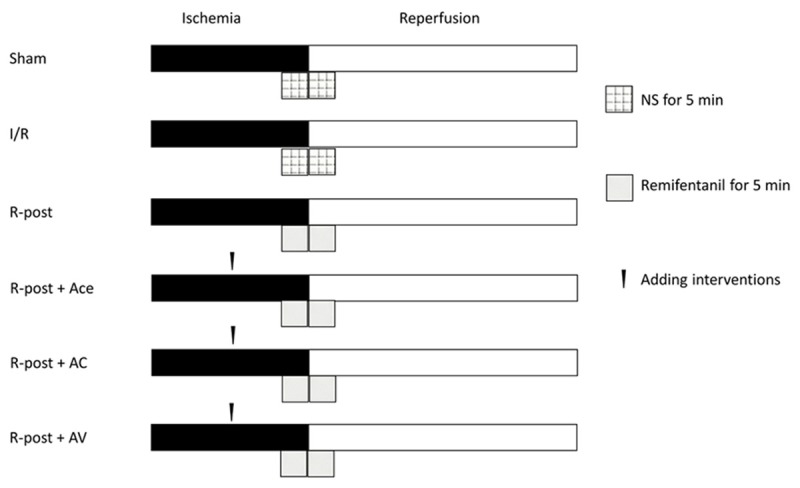
Protocols of remifentanil administration. Sham, sham operation group; I/R group, myocardial ischemia/reperfusion group; R-post, postconditioning of remifentanil group; R-post +Ace, postconditioning of remifentanil plus acetazolamide group; R-post +AC, postconditioning of remifentanil plus antagonist compounds; R-post +AV, postconditioning of remifentanil plus arginine vasopressin.
Hemodynamic measurements
The heart rate and mean arterial pressure (MAP) were measured at different time points: after operation procedure and before ischemia (the baseline period), after producing myocardial ischemia for 30 min, and after initiating reperfusion for 30, 60, 90, and 120 min.
Measurement of the enzyme related to heart function
After reperfusion for 120 min, the abdomens of the rats were opened under anesthesia; 3-5 ml blood was collected from abdominal aorta. The blood sample was kept in centrifugal tube and put into centrifuge to get the serum for measurement of the heart function, with a rotation speed of 3000 r/min for 15 min in the centrifuge. We used the Enzyme-linked immuno sorbent assay (ELISA) to measure the cardiac troponin (cTnI), actate dehydrogenase (LDH) and creatine kinase-MB (CK-MB) in the serum.
Determination of the infarct size
After the 120 min of reperfusion and blood collection, the snare to the LAD was retightened. We then injected 1% Evans blue dye into the left internal carotid vein, to stain the normally perfused region of the heart. Evans blue negative area was the area at risk (AAR) owing to occlusion of the LAD. The AAR was clearly red-stained tissue, while the infarct size zone (IS) was pale and unstained necrotic tissue. After weighing, we frozen the hearts, cut them into 2 mm slices, incubated at 37°C for 15 min in 1% 2, 3, 5-triphenyltetrazolium in phosphate buffer (0.1 mol/L) at pH 7.4, and then fixed them for 20 min in 10% formalin to differentiate the AAR from the IS. The areas of AAR and IS in each slice were determined using a digital imaging software (Image-Pro Plus Version 6.0; Media Cybernetics, Bethesda). The volumes of the left ventricles, ARR and IS were calculated by multiplying area with the thickness of the slices and expressing them as a fraction of the left ventricle. We expressed the IS as a percentage of the AAR (IS/AAR×100%).
Percentage of water content in left ventricle
After determining the infarct size, we removed the atria and great vessels, and separated the left ventricle (including septum) and right ventricle. We calculated water content of the left ventricular as percentage difference between initial wet weight and dry weight after 24 h desiccation at 80°C, according to a previous report [17]. Moreover, myocardial tissue was taken along vertical axis of the heart and the middle point from ligation of the LAD to the apex cordis.
Western blot analysis
We collected samples from infarct border of the left ventricular for western analysis to detect AQP1 levels. About 100 mg of each sample was obtained, pulverized and homogenized in 1 ml lysis buffer for 30 min. The lysis buffer was composed of 50 mmol/L Tris-HCl (pH 7.4), 1% Nonidet P40, 0.25% sodium-deoxycholate, 150 mmol/L NaCl, 1 mmol/L ethylenediamine tetraacetic acid, 1 mmol/L phenylmethane sulfonyl fluoride, 1 mmol/L Na3VO4, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 mmol/Lethylene glycol tetra acetate, and 1 mmol/L sodium-fluoride). The samples were then centrifuged at 12,000 g for 10 min at 4°C to isolate total protein. We used bovine serum albumin (BSA, Beijing ComWin Biotech, China) as an internal standard for determination of the protein concentrations. The protein samples were mixed with SDS gel electrophoresis. Equal (50 mg) protein samples were loaded and transferred to a polyvinylidene difluoride membrane. After blocking with Phosphate Buffer Solution (PBS) containing 0.1% Tween 20 (PBS-T) and 3% BSA for 1 h at a temperature of 25°C, the membrane was incubated with the primary antibody of AQP1 for 1 h. After washing with PBS-T, the membrane was incubated with secondary antibody followed by further washing with PBS-T. We detected the signals using the Enhanced Chemiluminescent Western blot analysis system (Santa Cruz, Texas, USA) and X-ray films.
Quantitative real-time reverse transcription-polymerase chain reaction (real-time qPCR)
After finishing 120 min reperfusion, myocardium samples were taken from the infarct border of animals in all the groups except the sham group. The myocardium of the left ventricle from the sham group was collected as mock-control. Total RNA was extracted using TRIzol Reagent (Life technologies, USA) and was prepared using the UltraspectTM-II RNA system (BIotecx Laboratories, Inc., USA). Then the cDNA was synthesized first using 5 μl total RNA, 1 μl random primer, and 5 μl Rnase-free ddH2O for 5 min in 70°C and 10 seconds in 0°C. This was then reversely transcribed by in a 20 μl reaction mix containing 2 μl deoxynucleoside triphosphates (dNTPs), 1 μl Rnase inhibitor and 1 μl Reverse Transcriptase for 1 h at 37°C and stored in -20°C. The reverse transcription of cDNA was quantified by real-time qPCR using AQP1 primers: sense 5’ CATTGGCTTGTCTGTGGC 3’, antisense 5’ TTTGAGAAGTTGCGGGTG 3’. A primer of β-actin mRNA of rats was used as internal standard (sense: 5’CACCCGCGAGTACAACCTTC 3’, antisense: 5’CCCATACCCACCATCACACC 3’).
The PCR conditions were set as: 95°C for 2 min; 95°C for 10 s; 40 cycles of 60°C for 40 s, with a final extension of 60°C for 10 min. Each example was detected through this procedure for 3 times. The amount of AQP1 in the sample was determined by calculation of average.
Statistical analysis
All data are described as mean ± standard deviation (SD) or mean ± standard error (SE). Results of western blot and real-time qPCR were depicted. Data analysis was performed using the R software (www.r-project.com, version 3.1.1). Analysis of covariance (ANCOVA) was used and adjusted for baseline values, followed by post-hoc Tukey’s analyses if significant differences between groups were found. A P<0.05 was considered as statistically significant difference.
Results
Hemodynamic data
Compared to baseline, heart rate of the rats were slightly reduced (P>0.05). Between group differences were not found considering heart rate, at baseline, ischemia for 30 min, and reperfusion for 30, 60, 90 and 120 min. The changes of MAP from baseline to reperfusion for 120 min were similar to the heart rate. However, between-group comparison showed that MAP of the rats in the R-post and R-post +Ace group were significantly lower than those in the sham group at 90 and 120 min after reperfusion (P<0.05). Table 1 showed more details.
Table 1.
Hemodynamic parameters
| Baseline | Ischemia | Reperfusion | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 0 min | 30 min | 30 min | 60 min | 90 min | 120 min | |
| Hear rate (beats/min) | ||||||
| Sham | 398.4rat.6 | 393.5±12.5 | 396.4±15.3 | 399.4±17.5 | 395.5±16.3 | 396.3±17.2 |
| I/R | 393.5±13.4 | 388.2±15.6 | 390.2±10.5 | 391.2±12.6 | 389.6±17.4 | 386.4±14.1 |
| R-post | 402.6±23.6 | 400.6±18.4 | 393.6±16.6 | 388.4±13.6 | 386.7±13.7 | 382.6±12.5 |
| R-post +Ace | 390.5±13.8. | 392.7±12.3 | 385.7±20.7 | 378.7±12.7 | 376.3±12.4 | 382.5±15.6 |
| R-post +AC | 405.6±22.9 | 399.3±15.7 | 393.7±12.8 | 395.9±16.7 | 390.5±18.8 | 388.3±16.8 |
| R-post +AV | 391.7±19.4 | 393.7±13.8 | 388.3±14.8 | 376.3±15.8 | 380.7±14.8 | 384.8±15.9 |
| MAP (mmHg) | ||||||
| Sham | 120.6±11.6 | 118.2±12.5 | 116.6±8.5 | 118.2±10.8 | 116.5±11.7 | 114.6±9.3 |
| I/R | 118.5±12.7 | 112.5±9.6 | 108.5±10.5 | 109.7±12.3 | 110.3±9.7 | 112.7±11.5 |
| R-post | 117.6±9.7 | 111.6±6.2 | 107.5±8.6 | 102.3±6.0 | 99.5±8.7* | 100.4±6.8* |
| R-post +Ace | 121.3±11.6 | 114.7±10.5 | 107.7±10.5 | 105.0±7.3 | 104.3±6.8* | 103.7±8.9* |
| R-post +AC | 119.3±10.6 | 112.8±8.6 | 109.9±7.5 | 107.1±5.6 | 106.4±7.8 | 107.3±8.5 |
| R-post +AV | 118.4±9.8 | 115.3±9.7 | 108.6±6.7 | 105.5±6.6 | 107.9±8.6 | 106.8±10.2 |
Compared to the sham group, P<0.05.
Sham, the sham operation group; I/R, the ischemia and reperfusion group; R-post, the postconditioning of remifentanil group; R-post +Ace, postconditioning of remifentanil plus acetazolamide group; R-post +AC, postconditioning of remifentanil plus antagonist compounds group; R-post +AV, postconditioning of remifentanil plus arginine vasopressin group. MAP, mean arterial pressure.
Myocardial infarct size
At the end of the reperfusion, the R-post group showed a significant reduction of the infarct size compared to the I/R group (25.4±8.5 vs. 51.6±6.5, P<0.05). The effect of R-post reducing infarct size was slightly enhanced by adding AQP1 inhibitor acetazolamide to R-post, so significant differences still could be found when compared the R-post +Ace group to the I/R group (22.5±7.6 vs. 51.6±6.5, P<0.05). The effect of infarct size reduction induced by R-post was blocked by the opioid-receptor antagonist compounds (R-post vs. R-post +AC, 25.4±8.5 vs. 50.9±6.9, P<0.05). This effect was also blocked by the AQP1 enhancer arginine vasopressin (R-post vs. R-post +AV, 25.4±8.5 vs. 46.3±8.2, P<0.05). Table 2 and Figure 2 showed more details.
Table 2.
Infarct size in the left ventricle
| Group | Body weight (g) | LVW (g) | AAR/LVW (%) | IS/AAR (%) |
|---|---|---|---|---|
| Sham | 264.3±7.7 | 0.50±0.05 | - | - |
| I/R | 264.1±10.6 | 0.49±0.03 | 54.8±8.3 | 51.6±6.5 |
| R-post | 263.6±14.2 | 0.48±0.02 | 56.8±7.5 | 25.4±8.5* |
| R-post +Ace | 264.3±14.5 | 0.51±0.06 | 57.6±9.2 | 22.5±7.6† |
| R-post +AC | 261.6±10.5 | 0.48±0.03 | 55.1±7.1 | 50.9±6.9‡ |
| R-post +AV | 261.3±7.2 | 0.47±0.04 | 54.5±8.2 | 46.3±8.2‡ |
LVW, left ventricle weight; AAR, area at risk; IS, ischemia size.
Compared to I/R group, P<0.05.
Compared to I/R group, P<0.05.
Compared to I/R group, P<P<0.05.
Figure 2.

Myocardial infarct size. The images of myocardial infarct size showed that the R-post+Ace group presented the smallest ischemia size while the R-post+AV group presented with the largest size. The exact data were given in Table 2.
Percentage of water content in the left ventricle
The myocardial water content was calculated by comparing wet to dry weights of non-ischemic myocardium in the left ventricular after 120 min reperfusion. Table 3 showed that the water content increased after I/R (Sham vs. I/R group, 77.82±0.51 vs. 81.65±0.66, P<0.05), R-post reversed this effect of increasing water content (I/R group vs. R-post group, 81.65±0.66 vs. 78.66±0.82, P<0.05). Both opioid-receptor antagonist compounds and arginine vasopressin blocked the effect of R-post (R-post +AC and R-post +AV vs. R-post group, 81.03±0.74 and 81.12±0.58 vs. 78.66±0.82, P<0.05). The effect of R-post was slightly enhanced by adding acetazolamide (R-post+Ace vs. R-post, 77.15±0.69 vs. 78.66±0.82, P>0.05). Table 3 showed the details.
Table 3.
Water content in the left ventricle
| Group | Water content (%) |
|---|---|
| Sham | 77.82±0.51 |
| I/R | 81.65±0.66* |
| R-post | 78.66±0.82† |
| R-post +Ace | 77.75±0.69 |
| R-post +AC | 81.03±0.74‡ |
| R-post +AV | 81.12±0.58‡ |
Compared to sham group, P<0.05.
Compared to I/R group, P<0.05.
Compared to R-post group, P<0.05.
Measurement of CTnI, LDH and CK-MB
CTnI, LDH and CK-MB are indicators for myocardial injury. As shown in Table 4, CTnI, LDH and CK-MB increased significantly after 120 min reperfusion (I/R vs. sham group, P<0.05), and R-post decreased the level of these indicators (R-post vs. I/R group, P<0.05). Adding acetazolamide to R-post enhanced the reduction of CTnI, LDH and CK-MB (R-post +Ace vs. R-post, P>0.05). However, reduction of these enzymes could be blocked by opioid-receptor antagonist compounds or arginine vasopressin (R-post +AC and R-post_AV vs. R-post, P<0.05).
Table 4.
Measurement of CTnI, LDH and CK-MB
| Group | CTnI (ng/ml) | LDH (U/L) | CK-MB (U/L) |
|---|---|---|---|
| Sham | 0.86±0.17 | 225.23±32.94 | 270.35±42.78 |
| I/R | 4.59±1.11* | 443.17±50.61* | 584.65±53.36* |
| R-post | 2.28±0.67† | 276.52±45.91† | 437.69±75.16† |
| R-post +Ace | 1.78±0.46 | 266.33±36.58 | 405.27±58.36 |
| R-post +AC | 4.33±1.32‡ | 439.60±54.42‡ | 589.02±63.38‡ |
| R-post +AV | 3.89±0.86‡ | 408.11±41.33‡ | 558.32±53.43‡ |
CTnI, cardiac troponin I; LDH, lactate dehydrogenase; CK-MB, creatine kinase MB.
Compared to sham group, P<0.05.
Compared to I/R group, P<0.05.
Compared to R-post group, P<0.05.
Western blot analysis
Expression of AQP1 was measured by densitometry of western blot analysis. The expression of AQP1 was compared to β-actin and presented as AQP1/β-actin ratio. A higher ratio indicated a higher expression of AQP1, and therefor informed a higher myocardial edema level. Figure 3 showed that the AQP1/β-actin ratio was significantly increased at the end of reperfusion (I/R vs. sham group, P<0.05). R-posed significantly decreased the AQP1/β-actin ratio that was raised by I/R injury (R-post vs. I/R group, P<0.05), while adding acetazolamide to R-post showed the same cardioprotective effect (R-post +Ace vs. I/R group, P<0.05). However, this effect was reversed after opioid-receptor antagonist compounds or arginine vasopressin was added to R-post (R-post +AC and R-post +AV, P<0.05).
Figure 3.
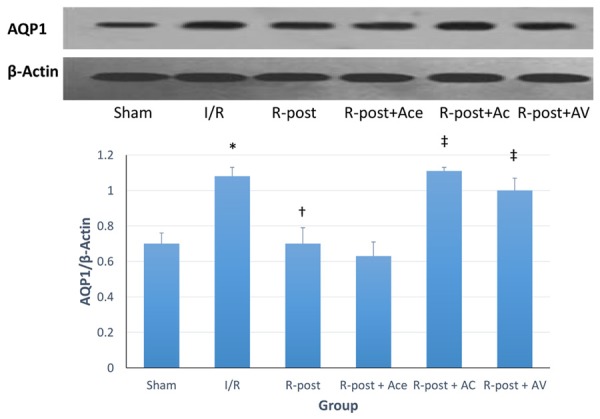
Western blot analysis. Expression of AQP1 was characterized using densitometry of western blot analysis. The expression of AQP1 was compared to β-actin and presented as AQP1/β-actin ratio. *Compared to sham group, P<0.05. †Compared to I/R group, P<0.05. ‡Compared to R-post group, P<0.05.
Real-time qPCR analysis
Real-time qPCR was performed to detect the expression of AQP1mRNA in the myocytes, higher AQP1mRNA indicated a higher myocardial edema. Figure 4 showed that the expression of AQP1mRNA was raised after 120 min reperfusion (I/R vs. sham group, P<0.05), and R-post significantly suppressed the abnormal increase of AQP1mRNA (R-post vs. I/R group, P<0.05). Adding acetazolamide to R-post slightly enhanced this suppression (R-post +Ace vs. I/R group, P<0.05), however, the suppression of expressing AQP1mRNA was eliminated by opioid-receptor antagonist compounds or arginine vasopressin (R-post +AC and R-post +AV, P<0.05).
Figure 4.
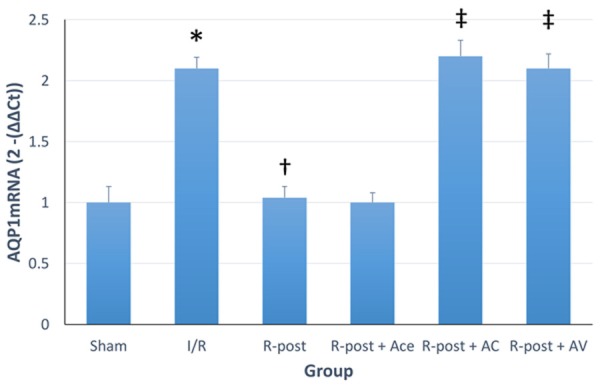
Real-time qPCR analysis. Real-time qPCR was performed to detect the expression of AQP1mRNA in the myocytes, higher AQP1mRNA indicated a higher myocardial edema. *Compared to sham group, P<0.05. †Compared to I/R group, P<0.05. ‡Compared to R-post group, P<0.05.
Discussion
In this study, we confirmed the cardioprotective effect of R-post in myocardial I/R injury in rats. R-post reduced the infarct size and water content in the swelling myocytes. This cardioprotective effect was slightly enhanced by adding AQP1 inhibitor acetazolamide to R-post, whereas this effect could be blocked by opioid-receptor antagonist compounds or AQP1 enhancer arginine vasopressin. So for the first time, we demonstrated the importance of AQP1 in the R-post effect on myocardial I/R injury.
Aquaporins (AQP) are membrane proteins with six transmembrane helices which usually oligomerize into tetramers forming pores. The pores are highly permeable to water and small solutes and therefore important for water transport and osmoregulation [3]. Studies showed that AQP1 and AQP4 are involved in myocardial edema, which is mainly caused by myocardial I/R injury [12]. I/R induced myocardial edema contribute to myocytes death, through increasing sarcolemmal fragility [18], activating phospholipases [19], causing damage to cytoskeletal scaffold [20], bringing impairment to Na+-K+-ATPase in early reperfusion [21,22]. It is widely accepted that high AQP1 expression leads to high water permeability and thus leads to myocardial edema. Our study results showed that increase of AQP1 expression was related an increase of water content in the left ventricle. However, the cause-effect relationship between myocardial edema and I/R injury is still controversial. Our study results showed that the infarct size and the edema level are consistent. And combining with the result that adding AQP1 enhancer arginine vasopressin increased the infarct size, we hypothesized that the cardioprotective effect of R-post might be mediated by blocking the AQP1 channels. Blocking AQP1 will decrease the permeability of water and thus control the myocardial edema and therefore may stop the process of myocardial apoptosis, which is an independent contributor to myocardial infarct size [23]. Stopping the process of myocardial apoptosis may prevent further myocardial damages like myocardial stunning, cardiac dysfunction and even lethal reperfusion injury [1].
A direct evidence of the cardioprotective effect of R-post mediated by blocking AQP1 channels was that adding R-post would directly decrease the level of AQP1 in the myocardium. Acetazolamide is an AQP1 channels inhibitor, which reduce the permeability of water into myocytes and thus decrease myocardial edema [24]. Our study result showed that adding acetazolamide on the basis of R-post did not significantly reduced infarct size and water content, compared to using R-post only, which also indicated that effect of R-post might initiate through blockage of AQP1 pathway. We then verified this assumption through first adding opioid-receptor antagonist compounds to R-post, to observe the changes of AQP1 expression in the myocardium. The increase of AQP1 after adding AC compounds confirmed that effect of R-post and expression of AQP1 are related, which was further testified by adding AQP1 enhancer arginine vasopressin to watch the attenuation of cardioprotective effect by R-post.
Although we found some evidence that the effect of R-post might be mediated by blocking of AQP1, several questions are not solved. First, which is the best dose of remifentanil in R-post in order to stop myocardial edema? We did not include a dose-response study, since the best dose of remifentanil is studied [9,25]. We selected a dose of 10 μg/kg, which yields the smallest infarct size. Although the infarct size and myocardial edema are highly correlated, we could not be sure if 10 μg/kg is the best dose for limiting myocardial edema. Second, dose R-post limit intracellular or extracellular myocardial edema? Reperfusion induced interstitial edema could have beneficial effects, because of increased wall thickness and stiffness that lead to collagen deposition and fibrosis and thus limit infarct expansion [3]. However, to distinguish intracellular or extracellular myocardial edema is still a technological problem in a lab study, this question might not be fully addressed now.
This study has some limitations. First, we did not perform a cell culture in vitro to determine whether R-post reduce myocardial edema intracellularly or extracellularly. Adding this in vitro study, we will be able to decide future studies, whether we aim at the reduction of interstitial edema by R-post or at the intracellularly edema. However, the experimental protocols that induce I/R myocytes edema are not well developed, which is a challenge both in bench work and at the bedside. Second, we did not measure the myocardial apoptosis using the TUNEL staining. Myocardial apoptosis is an independent contributor to infarct size, measuring the staining of the apoptosis will be useful to examine the cardioprotective effect of R-post. However, considering that the relationship between myocardial edema and apoptosis is clearly confirmed and our primary aim was to clarify the involvement of AQP1 in R-post cardioprotective effect, we did not use the TUNEL staining.
In a word, our study results indicate the protective effect of R-post may initiate through myocardial edema controlling, which work through blockage of AQP1.
Acknowledgements
This study is financially supported by a grant from the Department of education of Fujian Province (Grant number: JA10153).
Disclosure of conflict of interest
None.
References
- 1.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 2.Egan JR, Butler TL, Cole AD, Aharonyan A, Baines D, Street N, Navaratnam M, Biecker O, Zazulak C, Au CG. Myocardial ischemia is more important than the effects of cardiopulmonary bypass on myocardial water handling and postoperative dysfunction: a pediatric animal model. J Thorac Cardiovasc Surg. 2008;136:1265–1273.e1262. doi: 10.1016/j.jtcvs.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Dorado D, Andres-Villarreal M, Ruiz-Meana M, Inserte J, Barba I. Myocardial edema: A translational view. J Mol Cell Cardiol. 2012;52:931–939. doi: 10.1016/j.yjmcc.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Thuny F, Lairez O, Roubille F, Mewton N, Rioufol G, Sportouch C, Sanchez I, Bergerot C, Thibault H, Cung TT. Post-conditioning reduces infarct size and edema in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2012;59:2175–2181. doi: 10.1016/j.jacc.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 5.Kim JM, Jang YH, Kim J. Morphine and remifentanil-induced cardioprotection: its experimental and clinical outcomes. Korean J Anesthesiol. 2011;61:358–366. doi: 10.4097/kjae.2011.61.5.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zatta AJ, Kin H, Yoshishige D, Jiang R, Wang N, Reeves JG, Mykytenko J, Guyton RA, Zhao ZQ, Caffrey JL. Evidence that cardioprotection by postconditioning involves preservation of myocardial opioid content and selective opioid receptor activation. Am J Physiol Heart Circ Physiol. 2008;294:H1444–H1451. doi: 10.1152/ajpheart.01279.2006. [DOI] [PubMed] [Google Scholar]
- 7.Rentoukas I, Giannopoulos G, Kaoukis A, Kossyvakis C, Raisakis K, Driva M, Panagopoulou V, Tsarouchas K, Vavetsi S, Pyrgakis V. Cardioprotective role of remote ischemic periconditioning in primary percutaneous coronary intervention: enhancement by opioid action. JACC Cardiovasc Interv. 2010;3:49–55. doi: 10.1016/j.jcin.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Kim HS, Cho JE, Hong SW, Kim SO, Shim JK, Kwak YL. Remifentanil protects myocardium through activation of anti-apoptotic pathways of survival in ischemia-reperfused rat heart. Physiol Res. 2010;59:347–356. doi: 10.33549/physiolres.931772. [DOI] [PubMed] [Google Scholar]
- 9.Wong G, Li R, Jiang L, Irwin M. Remifentanil post-conditioning attenuates cardiac ischemiafentanil post. Remifent κ or δ opioid receptor activation. Acta Anaesthesiol Scand. 2010;54:510–518. doi: 10.1111/j.1399-6576.2009.02145.x. [DOI] [PubMed] [Google Scholar]
- 10.Wu QL, Shen T, Ma H, Wang JK. Sufentanil postconditioning protects the myocardium from ischemia-reperfusion via PI3K/Akt-GSK-3beta pathway. J Surg Res. 2012;178:563–570. doi: 10.1016/j.jss.2012.05.081. [DOI] [PubMed] [Google Scholar]
- 11.Kortenoeven MLA, Fenton RA. Renal aquaporins and water balance disorders. Biochim Biophys Acta. 2014;1840:1533–1549. doi: 10.1016/j.bbagen.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Page E, Winterfield J, Goings G, Bastawrous A, Upshaw-Earley J, Doyle D. Water channel proteins in rat cardiac myocyte caveolae: osmolarity-dependent reversible internalization. Am J Physiol. 1998;274:H1988–2000. doi: 10.1152/ajpheart.1998.274.6.H1988. [DOI] [PubMed] [Google Scholar]
- 13.Ding FB, Yan YM, Huang JB, Mei J, Zhu JQ, Liu H. The involvement of AQP1 in heart oedema induced by global myocardial ischemia. Cell Biochem and Funct. 2013;31:60–64. doi: 10.1002/cbf.2860. [DOI] [PubMed] [Google Scholar]
- 14.National Research Council Committee for the Update of the Guide for the C and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. Washington (DC): National Academies Press (US) National Academy of Sciences; 2011. The National Academies Collection: Reports funded by National Institutes of Health. [Google Scholar]
- 15.Li-ying Z, Zhong-yuan X, Fang X, Bang-chang C. Effect of radix paeoniae rubra on expression of p38 MAPK/iNOS/HO-1 in rats with lipopolysaccharide-induced acute lung injury. Chin J Traumatol. 2007;10:269–274. [PubMed] [Google Scholar]
- 16.Belkacemi L, Beall MH, Magee TR, Pourtemour M, Ross MG. AQP1 gene expression is upregulated by arginine vasopressin and cyclic AMP agonists in trophoblast cells. Life Sci. 2008;82:1272–1280. doi: 10.1016/j.lfs.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Ran X, Wang H, Chen Y, Zeng Z, Zhou Q, Zheng R, Sun J, Wang B, Lv X, Liang Y. Aquaporin-1 expression and angiogenesis in rabbit chronic myocardial ischemia is decreased by acetazolamide. Heart Vessels. 2010;25:237–247. doi: 10.1007/s00380-009-1179-5. [DOI] [PubMed] [Google Scholar]
- 18.Ruiz-Meana M, García-Dorado D, González MA, Barrabés JA, Soler-Soler J. Effect of osmotic stress on sarcolemmal integrity of isolated cardiomyocytes following transient metabolic inhibition. Cardiovasc Res. 1995;30:64–69. [PubMed] [Google Scholar]
- 19.Nakamura H, Nemenoff RA, Gronich JH, Bonventre JV. Subcellular characteristics of phospholipase A2 activity in the rat kidney. Enhanced cytosolic, mitochondrial, and microsomal phospholipase A2 enzymatic activity after renal ischemia and reperfusion. J Clin Invest. 1991;87:1810–8. doi: 10.1172/JCI115202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steenbergen C, Hill ML, Jennings RB. Volume regulation and plasma membrane injury in aerobic, anaerobic, and ischemic myocardium in vitro. Effects of osmotic cell swelling on plasma membrane integrity. Circ Res. 1985;57:864–875. doi: 10.1161/01.res.57.6.864. [DOI] [PubMed] [Google Scholar]
- 21.Inserte J, Garcia-Dorado D, Hernando V, Soler-Soler J. Calpain-mediated impairment of Na+/K+-ATPase activity during early reperfusion contributes to cell death after myocardial ischemia. Circ Res. 2005;97:465–473. doi: 10.1161/01.RES.0000181170.87738.f3. [DOI] [PubMed] [Google Scholar]
- 22.Inserte J, Garcia-Dorado D, Hernando V, Barba I, Soler-Soler J. Ischemic preconditioning prevents calpain-mediated impairment of Na+/K+-ATPase activity during early reperfusion. Cardiovasc Res. 2006;70:364–373. doi: 10.1016/j.cardiores.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Kajstura J, Cheng W, Reiss K, Clark WA, Sonnenblick EH, Krajewski S, Reed JC, Olivetti G, Anversa P. Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest. 1996;74:86–107. [PubMed] [Google Scholar]
- 24.Gao J, Wang X, Chang Y, Zhang J, Song Q, Yu H, Li X. Acetazolamide inhibits osmotic water permeability by interaction with aquaporin-1. Anal Biochem. 2006;350:165–170. doi: 10.1016/j.ab.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Chun KJ, Park YH, Kim JS, Jang Y, Kim JH, Kim J, Lee MY. Comparison of 5 different remifentanil strategies against myocardial ischemia-reperfusion injury. J Cardiothorac Vasc Anesth. 2011;25:926–930. doi: 10.1053/j.jvca.2011.02.019. [DOI] [PubMed] [Google Scholar]


