Abstract
To detect the serum concentrations of secreted protein acidic and rich in cysteine (SPARC) in patients with diabetic nephropathy and SPARC mRNA and protein expressions in renal tissue of db/db mice (C57BL/KsJ, diabetic nephropathy mice), thus preliminary exploration on the role of secreted protein acidic riches in cysteine in the development of diabetic nephropathy were carried out. Serum SPARC levels in normal subjects, patients with type 2 diabetes mellitus (without diabetic nephropathy), chronic renal failure (without diabetes mellitus), and diabetic nephropathy were determined with enzyme-linked immunosorbent assay. 12-week-old db/db mice (db/db group) and its littermate wild-type control mice (NC group) were selected with 6 from each group, and the kidney tissue were taken. RT-PCR, Western blot, and immunofluorescence were used to detect the mRNA, targeted protein expressions of SPARC and the staining of renal tissue. The serum level of SPARC in diabetic nephropathy group was significantly higher than those in normal group, type 2 diabetes mellitus, and chronic renal failure group (P < 0.05 or P < 0.01). The SPARC level in the type 2 diabetes mellitus group was higher than that in normal group (P < 0.05), but there was no difference between normal group and chronic renal failure. SPARC mRNA and protein levels in renal tissue of db/db mice were higher compared with the normal control group (P < 0.05). The long term hyperglycemic state in patients with diabetic nephropathy causes pathological change of renal tissue. Simultaneously, increased secretion of SPARC from renal tissue results in elevation of serum SPARC level. SPARC correlates with the occurrence and progression of diabetes, and it may play a role in pathological change of diabetic nephropathy.
Keywords: Secreted protein acidic and rich in cysteine, diabetic nephropathy, insulin-like growth factor, transforming growth factor β1
Introduction
Secreted protein acidic and rich in cysteine (SPARC) SPARC is previously known as BM-40 and osteonectin. It is found that SPARC expression has been found not only in a variety of normal tissues, but also in some hypertrophic scar tissue and tumor tissue with high expression and playing a relevant role. In normal tissue, particularly in adipose tissue, expression of SPARC can be significantly enhanced. In high-fat diet successful induced insulin resistance mice, the expression of SPARC increased significantly in insulin resistant adipose tissue. SPARC may be involved in the occurrence of insulin resistance and associated with the occurrence of diabetes [1,2]; in gastric cancer SPARC can inhibit angiogenesis by down-regulated expression of VEGF, and MPP-7 [3]. Studies have shown that it correlates with tracheal development, tissue remodeling, cell renewal and tissue repairing and so on. In addition, SPARC has an important role in regulating the relationship among extracellular matrix, inhibiting cell proliferation, regulating cell adhesion, migration, and controlling the expression of cytokines and matrix proteins. In the study of mouse models of it can inhibit the proliferation of glomerular mesangial cells by inhibiting IGF signaling pathway [4], while it can significantly increase the expression of mesangial cells TGF-β1 and type I collagen and participate in fibrosis of organizations [4]. SPARC is closely related to the development of diabetes. Imbalanced expression of SPARC are widely associated with obesity-related metabolic disorders, including type 2 diabetes and its related complications, kidney disease, cardiovascular disease and obesity associated cancer. In 2013 Kerstin Amann and Kerstin Benz made further studies on obesity and diabetes-induced renal organizational structure changes [5]. Diabetic nephropathy is the most serious complications of diabetes, but there is few studies on the correlation between SPARC and diabetic nephropathy. In this study, we carried out the research from two perspectives, including serum and tissue including to investigate the expression of SPARC, to make preliminary explorations on the role of secreted protein acidic riches in cysteine in the occurrence and development of diabetic nephropathy.
Materials and methods
Subjects serum samples
In this study, 160 cases of volunteers were included in this study (76 male, 84 female), including 38 cases of normal volunteers (Normal) (18 men, 20 women), 41 patients of type 2 diabetes mellitus (DM) (19 men, female 22), with an duration of 1--5 years, 15 cases of lower extremity vascular ultrasound indicating mild arterial intima changes without microangiopathy; 39 cases of chronic renal failure patients (CRF) (19 men, 20 women) with chronic glomerulonephritis and without diabetes, immunosuppressive agents, dialysis or kidney transplantation in the treatment; 42 cases of diabetic nephropathy (DN) (20 male, 22 female) with an average duration of 10 years, 32 cases were at Phase II-IV and 10 cases at stage I with diabetic retinopathy, the diagnostic criteria for diabetes patients was that urinary albumin excretion rate was (UAER) 30 mg/24 h. When it was less than 30 mg/24 h, there was non-diabetic nephropathy, when it was more than diabetic nephropathy 30 mg/24 h, there was diabetic nephropathy; all the above mentioned subjects showed no long-term cardiovascular diseases, infectious diseases and other organ dysfunction. Above the subjects were from March 2012 to March 2013 who were normal subjects and patients hospitalized in the Second Affiliated Clinical College of Harbin Medical University, and all subjects were informed of consent.
Experimental animal specimens
Choose 12-week-old db/db male mice (db/db group) and 6 cases of its littermate wild-type control mice (NC group) (Nanjing University Institute of animal models available). After the mouse were deeply anesthetized, renal tissues were rapidly thrown into liquid nitrogen, and they were finally stored in -80°C freezer for seized. All the experimental procedures were approved by Animal Experimental Center of Harbin Medical University.
Anthropometric measurements and serum samples
Anthropometric measurements were performed in the morning; subjects were fasting and wear a few clothes and no shoes to measure height and weight; height was accurate to 0.5 cm and weight was accurate to 0.5 kg; BMI was calculated (boundary of normal weight and obese was 28 kg/m2, simultaneous measurement of waist and hip circumference was performed to calculate waist-hip ratio (WHR); 24 h urine of patients with type 2 diabetes was collected in the same day and urinary albumin excretion rate (UAER) was measured to screen patients with diabetic nephropathy. Blood specimen was collected from upper limb vein after 10 h of fasting; fresh blood was allowed to stand for 30 min and then centrifuged at 4°C, 2500 r/min for 25 min; the supernatant was collected, repackaged and mixed with protease inhibitor, -80°C frozen for inspection; if precipitation appeared during storage, centrifuge again.
Detection of related indicators
Height, weight, BMI and waist-hip ratio (WHR) were detected according to the method described above; blood pressure of admission was collected; in addition, fasting blood glucose, postprandial 2 h plasma glucose, serum creatinine, blood urea nitrogen, glycated hemoglobin, fasting insulin levels, postprandial 2 h insulin levels, triglycerides, total cholesterol, low-density lipoprotein cholesterol, high density lipoprotein cholesterol and urinary protein were detected in chemical and biological laboratories and endocrine laboratory.
Reagents
ELISA kits, Trizol reagent, platinum Taq DNA polymerase, SPARC and β-actin primers (Invitrogen, USA); ThermoScript RT-PCR reverse transcription kit (Promega, USA); rabbit anti-actin antibodies, mouse anti-SPARC antibody, secondary antibody for immunofluorescence (Santacruz, USA); protein lysates, BCA assay kit (China Beyotime Biotech company); ECL immunofluorescence kit (British Amersham).
Quantitative detection of serum SPARC concentration
Double antibody sandwich ELISA was performed in accordance with the ELISA assay kit instructions. In accordance with ELISA kit: the concentration of standard product was 5.4 μg/L; the concentration of the test sample was diluted 5-fold by the dilution of the sample. Kit Performance: correlation coefficient R value of linear regression with the expected concentration was larger than 0.95. Within the batch and between batches, it should be less than 9% and 11%, respectively. Detection range: 0.2 μg/L-4 μg/L. Storage and validity: under 2-8°C, valid for 6 months (The experiment was performed in the valid period); the above information was from the SPARC-ELISA kit manufacturers. According to linear regression, linear regression equation was eventually obtained: SPARC concentration (μg/L) = 5 × (1.761 × OD value + 0.165).
Total RNA extraction and RT-PCR to detect SPARC mRNA expression levels
50 mg kidney tissue of db/db group and NC group was collected respectively; after grinding, it was placed in Trizol reagent to extract total RNA. UV spectrophotometer was used to detect RNA concentration; RT-PCR was used for the amplification of SPARC gene. Upstream primer for SPARC gene: 5-GAAGGTATGCAGCAATGACAACAA-3, downstream primer: 5-TTCGGTCAGCTCGGAATCCA-3; the product was 160 bp. Upstream primer for internal control β-actin: 5-CCCAGCACAATGAAGATCAAGATCAT-3, downstream primer: 5-ATCTGCTGGAAGGTGGACAGCGA-3; the product was 101 bp. RT reaction system was 20 μL: incubated at 42°C for 15 min, heated at 99°C for 5 min, terminate the reaction at 5°C for 5 min. PCR used 20 μL reaction system. PCR program: pre-denaturation at 95°C for 2 min, denaturation at 94°C for 15 s, annealing at 55°C for 15 s, extension at 72°C for 15 s, a total of 32 cycles, extension at 72°C for 5 min. The products were treated by 2% agarose gel electrophoresis, and scanimage gradation processing software was used to detect the electrophoresis gray value, using β-actin as an internal reference to calculate the relative level of SPARC mRNA expression. The experiment was repeated three times, and the results were stable and reliable.
Western blot to detect protein expression
The frozen kidney specimens were milled to a powder, cleaved by protein lysate at 4°C for 30 min, and centrifuged at 12000 rpm for 15 min; supernatant was collected. BCA was used for protein concentration determination; the determining wavelength was A540; protein concentration was calculated according to the standard curve. SDS-PAGE gel containing 10% separation gel and 5% stacking gel was perfused. Draw 60 μg total protein, constant 80 V, 25 min. When BPB frontier enter into the separating gel, the voltage was increased to 100 V until the bromophenol blue reached the bottom of the separating gel. With semi dry electric rotary constant for 80 min, the protein was transferred from the SDS-PAGE gel onto PVDF membrane. Then it was sealed by 5% skim milk for 90 min at 37°C and incubated with Mouse anti-SPARC monoclonal antibodies and Rabbit anti-actin antibody (1:1000) at 4°C overnight, and incubated with secondary antibody Goat anti-Mouse Antibody and Goat anti-Rabbit antibody (1:5000) for 1 h; the specific reaction bands were treated with ECL chemiluminescent coloring and exposed to X-ray. After being scanned into the computer, Scanimage gradation processing software was used to measure the gray values of electro-phoretic bands. Results were expressed as the ratio of gradation value SPARC gray value to β-actin gray value.
Immunofluorescence assay
The kidney tissue was fixed in paraformaldehyde for 2 h. Sections were rinsed with PBS for 30 min, treated with 0.1 mmol/L Trition-X100 for 10 min, sealed by goat serum for 4 h at 37°C, and then incubated with SPARC (1:400, primary-antibody) at 37°C overnight, rinsed with PBS for 3 times, incubated with secondary antibody (FITC-labeled goat anti-mouse antibody) for 90 min at 37°C, rinsed with PBS for 3 times; finally they were mounted by 500 ml/L glycerol -10 mmol/L PBS; immunofluorescence microscopy was used for filming.
Statistical analysis
Data were expressed as mean ± standard; data were analyzed using SPASS 13.0 Software (SPASS, Inc, Chicago, IL, USA); biochemical indicators, diabetes-related indicators and serum SPARC levels between groups and SPARC levels between men and women were compared by t-test; Logistic regression analysis was used for correlation analysis; Bidirectional detection was performed; P < 0.05 was considered statistically significant.
Results
Clinical characteristics of subjects in each group and the SPARC test results are shown in Table 1. Table 1 shows that: there were no statistical difference in age, body mass index and waist-hip ratio among groups. Blood pressure in chronic renal insufficiency group was significantly higher than that in the other three groups; blood pressure in diabetic nephropathy group was significantly higher than that in type 2 diabetic group, and the difference was statistically significant, P < 0.05 or P < 0.01. Blood glucose level in type 2 diabetic group and diabetic nephropathy group were significantly higher than that in normal and chronic renal insufficiency groups, and the difference was significant, P < 0.05; there was no statistically significant difference in blood glucose level between type 2 diabetes group and diabetic nephropathy group, but there were statistically significant difference in glycated hemoglobin level. Serum insulin level in normal group and chronic renal insufficiency group was significantly higher than that in type 2 diabetes group and diabetic nephropathy group, and the difference was significant, P < 0.05. There were statistically significant differences in renal function group between chronic renal insufficiency group and the other three groups, P < 0.05; there were no statistically significant difference in lipid levels among 2 diabetes group, chronic renal insufficiency group and diabetic nephropathy group, P > 0.05. There were statistically significant difference in serum SPARC levels between diabetic nephropathy group and the other three groups, P < 0.05 or P < 0.01; between 2 diabetic group and the normal group, the difference was also statistically significant, P < 0.05; but there was no statistically significant difference compared with renal insufficiency group. There were significant differences in SPARC, SBP, DBP, 2h-INS and HbA1C DN between DN group and DM group, P < 0.05; with DM group as the control, whether DN as the dependent variable (0 = no; 1 = Yes), and SPARC, SBP, DBP, 2h-INS, HbA1C as independent variables, Logistic regression analysis was performed (Table 2). The results showed that the concentration of serum SPARC, SBP and HbA1C was a factor of DN, P < 0.05; regression coefficient and OR value were greater than 1, indicating a positive correlation with the development of diabetic nephropathy (Figure 1).
Table 1.
Clinical characteristics of study subjects and SPARC levels in four groups. Date are mean ± S.E.M
| Group | Normal | DM | CRF | DN |
|---|---|---|---|---|
| N (male/female) | 38 (18/20) | 41 (19/22) | 39 (19/20) | 42 (20/22) |
| Age | 48±12 | 50±16 | 51±14 | 52±13 |
| BMI (Kg/m2) | 23.66±1.58 | 24.46±2.78 | 22.36±2.25 | 23.79±2.65 |
| WHR | 0.83±0.06 | 0.88±0.08 | 0.81±0.05 | 0.86±0.06 |
| SPARC (mg/L) | 3.58±0.41 | 4.51±1.08c | 3.81±1.16 | 5.78±1.41a,b,e |
| SBP (mmHg) | 114.35±16.46 | 126.46±15.18 | 143,56±17.15a,b | 133.96±16.21c,d,e |
| DBP (mmHg) | 72,38±10.12 | 74.79±12.27 | 86.92±12.13 | 81.41±10.08c,d |
| FBG (mmol/L) | 4.97±0.96 | 8.78±1.46c,e | 4.89±0.85 | 8.69±1.65c,e |
| 2h-PBG (mmol/L) | 6.27±0.87 | 10.13±2.14c,e | 6.35±0.74 | 11.23±2.35c,e |
| f-INS (mUl/L) | 9.6±3.2 | 3.6±1.2c,e | 10.1±2.9 | 3.1±1.8c,e |
| 2h-INS (mUl/L) | 31.3±5.6 | 21.2±4.8c,e | 28.9±4.7 | 11.2±3.9c,d,e |
| Crea (mmol/L) | 73±29 | 76±28 | 436±82c,d | 79±31e |
| Bun (mmol/L ) | 4.8±1.8 | 5.1±1.7 | 13.8±2.8c,d | 5.2±1.7e |
| eGFR (ml/min) | 108.6±12.7 | 104.3±14.1 | 42.5±11.8c,d | 102.5±15.7e |
| HbA1c (%) | 5.1±0.6 | 8.3±1.7c,e | 5.4±0.7 | 9.2±1.5d |
| TG (mmol/L) | 3.56±1.62 | 6.12±1.58c | 5.96±1.42 | 5.89±1.46c |
| TC (mmol/L) | 1.88±0.78 | 4.15±1.77c | 3.99±0.98 | 4.33±1.36c |
| HDL-C (mmol/L) | 1.31±0.42 | 1.29±0.37 | 1.10±0.31 | 1.23±0.30 |
| LDL-C (mmol/L) | 1.17±0.72 | 2.71±0.81c | 1.84±0.63 | 2.12±0.71 |
| Urine A/C (mg/mmol) | 1.8±0.6 | 3.2±0.8 | 22.5±3.6c,d | 15.8±2.8c,d |
| Urine protein | - | - | ++ - +++ | ± - + + |
P < 0.01, VS Normal;
P < 0.01, VS DM;
P < 0.05, VS Normal;
P < 0.05, VS DM;
P < 0.05, VS CRF;
Normal: normal control group; DM: simple diabetes group; CRF: Chronic renal insufficiency group; DN: Diabetic nephropathy; BMI: Body mass index; WHR: Waist hip ratio; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; eGFR: Glomerular filtration rate; FBG: Fasting blood glucose; 2h-PBG: 2h postprandial blood glucose; f-INS: Fasting serum insulin; 2h-INS: 2h postprandial serum insulin; Crea: Serum creatinine; BUN: Blood urea nitrogen; HbA1c: Glycated hemoglobin; TG: Triglycerides; TC: Total cholesterol; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; Urine A/C: The ratio of albumin and creatinine in urine; SPARC: Secreted protein acidic and rich in cysteine.
Table 2.
Results of multivariable Logistic analysis for risk factors of incidence of DN
| Variable | B | SE | Wald | df | P value | OR value | 95% | CI |
|---|---|---|---|---|---|---|---|---|
| SPARC (μg/L) | 0.642 | 0.181 | 12.661 | 1 | 0.003 | 1.161 | 1.021 | 1.343 |
| SBP (mmHg) | 0.027 | 0.008 | 10.723 | 1 | 0.002 | 1.229 | 1.010 | 1.345 |
| DBP (mmHg) | 0.021 | 0.016 | 1.312 | 1 | 0.934 | 1.002 | 0.971 | 1.030 |
| 2h-INS (muI/L) | 0.074 | 0.169 | 0.192 | 1 | 0.663 | 1.078 | 0.771 | 1.502 |
| HbA1C (%) | 0.099 | 0.023 | 17,012 | 1 | 0.012 | 1.136 | 0.812 | 1.43 |
Figure 1.
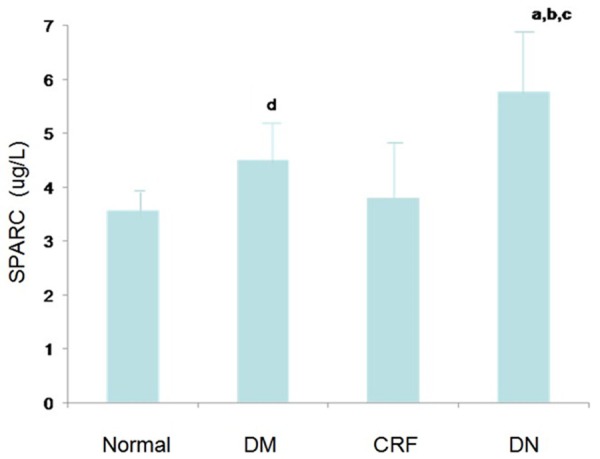
Serum SPARC levels in four groups. Date are expressed as mean (μg/L) ± S.E.M. a. P < 0.01, DN VS Normal; b. P < 0.01, DN VS DM; c. P < 0.05, DN VS CRF; d. P < 0.05, DM VS Normal.
Comparison of SPARC mRNA in kidney tissues between two groups of mice (Figure 2). SPARC mRNA expression in db/db mice (diabetic nephropathy mice) was higher than that in NC group (P < 0.05).
Figure 2.
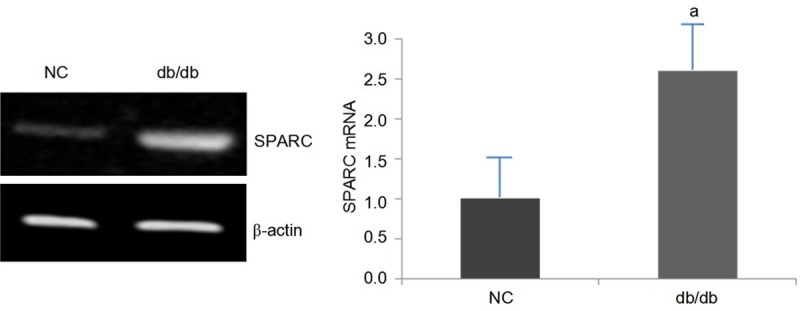
The expression of SPARC mRNA in db/db mice and control mice, aP < 0.05, db/db mice vs control mice.
Western blot to detect SPARC protein expression levels in mouse kidney (Figure 3) SPARC protein expression in db/db mice was higher than that in NC group (P < 0.05).
Figure 3.
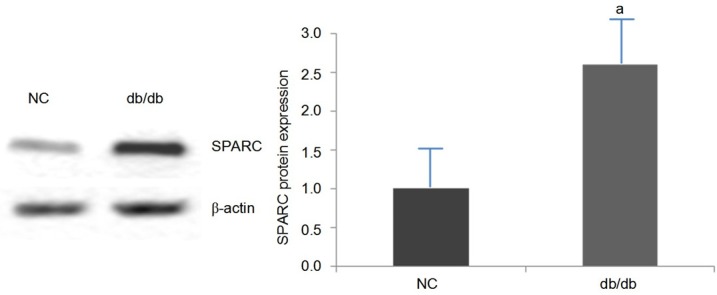
The expression of SPARC in db/db mice and control mice, aP < 0.05, db/db vs control mice.
Immunofluorescence assay
The immunofluorescence stain of kidney tissue of db/db mice and control mice (Figure 4).
Figure 4.
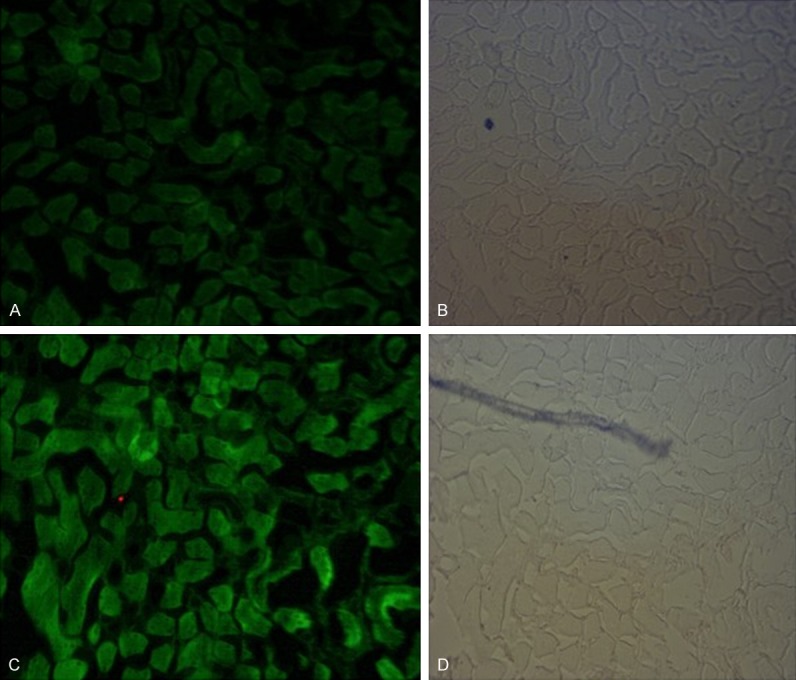
The immunofluorescence stain of kidney tissue of db/db mice and control mice (SPARC positive staining showed green fluorescence, and the more of SPARC content, the stronger of the staining; (A) was the immunofluorescence staining of kidney tissue in control group; (C) showed the immunofluorescence staining of db/db Mice kidneys; (B, D) were respectively the ordinary light microscopy of (A, C). The results showed that kidney tissue staining in db/db group was significantly stronger than that in the NC group, indicating a significant increase in SPARC protein expression in kidney tissue compared with the control group.
Discussion
According to a budget survey conducted by the International Diabetes Federation, the worldwide prevalence of diabetes would increase three-times until 2025 [6], and in the future diabetic nephropathy will be the most common cause of end-stage renal disease. Currently the relationship between SPARC and the specific pathogenesis of diabetic nephropathy has not been reported, so this study has a positive significance. The long-term diabetes-induced hyperglycemia leads to metabolism and hemodynamic changes, resulting in pathological changes in kidney tissue, which are mainly as follows: mesangial proliferation, basement membrane thickening, glomerular sclerosis [7], and even diabetic nephropathy. It has been demonstrated that serum SPARC in patients with diabetes increased, and SPARC was related with the occurrence and development of diabetes; diabetic nephropathy as the most serious complication of diabetes, is inevitable in the long term progression of diabetes; So the relationship between the development of diabetic nephropathy and SPARC as well as the effect of SPARC on renal histopathology of diabetic nephropathy worth exploring. SPARC, secreted protein acidic and rich in cysteine, is small molecule glycoprotein manly presenting in extracellular matrix, which is highly conserved among species. Its structure is sequentially divided into I, II and III area from the N terminus of the polypeptide; I area mainly binds to immune-related antigens; II area is a cysteine-rich region, which can combine with activin, inhibin, heparin and protein poly enzyme; III region is a calcium binding site, capable of interacting with endothelial cells and binding to collagen fibers [8-10]. These structural characteristics make it possible to play a role in the regulation of cell proliferation, organizational renewal, angiogenesis and fibrosis.
In this study, the serum test results showed that blood pressure and glycated hemoglobin levels in diabetic nephropathy group were significantly higher than those in the normal group and type 2 diabetes group; although there was no significant difference in blood lipid levels, but compared with the normal group, the difference was statistically significant, suggesting that blood pressure, blood glucose and blood lipids had significant significance in the development of diabetes, and controlling blood pressure, blood glucose and blood lipids can help delay the occurrence of diabetic nephropathy. SPARC expression in Type 2 diabetes group was higher than that in the normal group, which was consistent with previous findings, confirming the conclusion that SPARC may be associated with diabetes occurrence. In diabetic nephropathy group, serum SPARC expression was significantly higher than that in type 2 diabetes group; To consider that serum SPARC expression would increase when diabetic patients had kidney disease, and the kidney immunofluorescence of diabetic nephropathy mice confirmed that SPARC expression in kidney tissues significantly enhanced compared with control, it can be considered that the increased serum SPARC in diabetic nephropathy may be derived from diseased kidney tissue. There was no significant difference in serum SPARC between chronic renal insufficiency group and normal control group, chronic renal insufficiency group and type 2 diabetes group; while there were statistically significant differences between type 2 diabetes group and normal control group; So we speculated that elevated blood glucose was an important variable leading to an increase in serum SPARC expression; the effect of simple renal pathological changes without abnormal serum glucose levels on SPARC expression was not obvious, but when long-term glucose disorder was complicated with changes in kidney tissue pathology, serum SPARC expression will appear a peak, so serum SPARC expression in diabetic nephropathy group was significantly higher than that in type 2 diabetes group. Only based on the serum test results, the following conclusions could be obtained: SPARC may be related to the occurrence of type 2 diabetes; elevated blood glucose was an important factor leading to changes in serum SPARC expression; simple renal tissue pathological changes had no significant effect on expression of serum SPARC; when long-term elevated blood glucose was complicated with pathological changes in diabetic kidney tissue, serum SPARC expression will change significantly; meanwhile Logistic multivariate regression analysis confirmed that SPARC was a factor of diabetic nephropathy, so it can be considered that SPARC maybe related with the occurrence and development of diabetic nephropathy.
Pathological changes of diabetic nephropathy in kidney tissues mainly manifest as mesangial hyperplasia initially; proliferation of mesangial cells requires IGF-mediated signal transduction pathway [11,12]. IGF protein family, as a potent mitogen for various cells, plays an important role in cell differentiation, organizational renewal and wound healing [13]; kidneys are vital organs for IGF protein family synthesis [14], including IGF1, IGF2 and IGF1 receptor; the front two take effect by binding to IGF-1 receptor. Long-term hyperglycemia in diabetic nephropathy can stimulate renal tissue to express IGFs. In 2009, Movva SIREESHA et al reported that renal biopsy of patients with type 2 diabetes and diabetic nephropathy showed a significantly higher expression of IGF2 compared with normal control group [15]. In fact, these high expressed IGF proteins could be suppressed by SPARC; study showed that in SPARC-knockout mice, the expression of IGF1, IGF2 and IGF1 receptor was significantly higher; IGFs expression was significantly inhibited in renal tissue after being treated with recombinant human SPARC; When the wild-type group (with normal SPARC expression) was treated with recombinant human SPARC, the expression of IGF-1 receptor and the IGF-1-mediated mesangial cell proliferation were inhibited [4], indicating that SPARC can inhibit IGF signaling pathway by inhibiting the expression of IGFs to inhibit the proliferation of mesangial cells. Obviously this function of SPARC had a protective effect on the initial renal pathological changes.
The results of this experiment showed that SPARC in serum and kidney tissues of diabetic nephropathy was highly expressed; but the protective effect of the SPARC cannot fully suppress the pathological changes of the kidney, and glomerular sclerosis and interstitial fibrosis were still inevitable in diabetic nephropathy. Studies have shown that TGF-β1 may be involved in the formation of glomerular sclerosis and interstitial fibrosis. However, TGF-β1 generation can be regulated by SPARC; the study of SPARC-knockout mice showed that TGF-β1 mRNA level in mesangial cells was normal, but TGF-β1 protein was reduced compared with the normal control group, suggesting that SPARC can induce the synthesis of TGF-β1 in mesangial cells [16]. Under vitro hyperglycemia state, TGF-β1 protein was involved in regulating the generation of type I collagen, type IV collagen and glomerular basement membrane components [14]. For diabetic nephropathy, SPARC expression in kidney tissue increased, and thus inducing the increased synthesis of TGF-β1; under the stimulation of high TGF-β1 expression and high glucose, the generation of glomerular basement membrane components, laminin, and type IV collagen increased correspondingly [17], resulting in glomerular sclerosis and interstitial fibrosis. The comparative study between chronic STZ-induced SPARC-knockout mice model of diabetic nephropathy and mice model of diabetic nephropathy reserving SPARC gene showed that: in the same state of diabetic nephropathy, the degree of final worsening kidney function and pathological kidney damage in SPARC knockout mice was lighter than mice with SPARC gene [17], indicating that SPARC was closely related with the pathological kidney damage of diabetic nephropathy.
In summary, it can be assumed that the SPARC was correlated with the occurrence of type 2 diabetes and the progression of diabetic nephropathy; under the condition of long-term hyperglycemia and diabetic nephropathy, SPARC expression in kidney tissue would increase; the increased SPARC would enter into the blood circulation and cause further increase in serum SPARC. Although SPARC can inhibit mesangial proliferation by inhibiting IGFs signal transduction pathway to play a protective role in the progression of diabetic nephropathy, in the further development of renal pathological changes, the increased expression of SPARC in kidney tissues would up-regulate TGF-β1 expression to induce the production of extracellular matrix and abnormal deposition of collagen, resulting in basement membrane thickening, glomerular sclerosis and interstitial fibrosis to increase kidney lesions. Therefore, SPARC can be considered to play a protective role in the development of diabetic nephropathy, but then it will further aggravate the renal pathological damage by inducing the expression of TGF-β1, and the role of SPARC in aggravating kidney pathological damage was more pronounced. SPARC was closely related to the development of diabetic nephropathy, and the specific mechanism needs further exploration; from the perspective of SPARC studying diabetic nephropathy has new significance.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81370903 to H.S.).
Disclosure of conflict of interest
None.
References
- 1.Guan YY, Song HY, Zhang LP, Li K, Dong CL, Li Q, Sun YQ. Study on the mechanism of SPARC in the development of insulin resistance. Chinese Journal of Endocrinology and Metabolism. 2011;27:161–164. [Google Scholar]
- 2.Wu D, Li L, Yang M, Liu H, Yang G. Elevated plasma levels of SPARC in patients with newly diagnosed type 2 diabetes mellitus. Eur J Endocrinol. 2011;165:597–601. doi: 10.1530/EJE-11-0131. [DOI] [PubMed] [Google Scholar]
- 3.Zhang JL, Chen GW, Liu YC, Wang PY, Wang X, Wan YL, Zhu J, Gao HQ, Yin J, Wang W, Tian ML. Secreted Protein Acidic and Rich in Cysteine (SPARC) Supresses Angiogenesis by Down-Regulating the Expression of VEGF and MMP-7 in Gastric Cancer. PLoS One. 2012;7:e44618. doi: 10.1371/journal.pone.0044618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francki A, Motamed K, McClure TD, Kaya M, Murri C, Blake DJ, Carbon JG, Sage EH. SPARC regulates cell cycle progression in mesangial cells via its inhibition of IGF-dependent signaling. J Cell Biochem. 2003;88:802–811. doi: 10.1002/jcb.10424. [DOI] [PubMed] [Google Scholar]
- 5.Amann K, Benz K. Structural renal changes in obesity and diabetes. Semin Nephrol. 2013;1:23–33. doi: 10.1016/j.semnephrol.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Pichler RH, Bassuk JA, Hugo C, Reed MJ, Eng E, Gordon KL, Pippin J, Alpers CE, Couser WG, Sage EH, Johnson RJ. SPARC is expressed by mesangial cells in experimental mesangial proliferative nephritis and inhibits platelet-derived-growth-factor-mediated mesangial cell proliferation in vitro. Am J Pathol. 1996;148:1153–1167. [PMC free article] [PubMed] [Google Scholar]
- 7.Momeni A, Behradmanesh MS, Kheiri S, Karami Horestani M. Evaluation of spironolactone plus hydrochlorothiazide in reducing proteinuria in type 2 diabetic nephropathy. J Renin Angiotensin Aldosterone Syst. 2015;16:113–118. doi: 10.1177/1470320313481485. [DOI] [PubMed] [Google Scholar]
- 8.Yan Q, Sage EH. SPARC, a matricellular glycoprotein with important biological functions. J Histochem Cytochem. 1999;47:1495–1506. doi: 10.1177/002215549904701201. [DOI] [PubMed] [Google Scholar]
- 9.Stenner DD, Romberg RW, Tracy RP, Katzmann JA, Riggs BL, Mann KG. Monoclonal antibodies to native noncollagenous bone-specific proteins. Proc Natl Acad Sci U S A. 1984;81:2868–2872. doi: 10.1073/pnas.81.9.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sage EH, Vernon RB. Regulation of angiogenesis by extracellular matrix: the growth and the glue. J Hypertens Suppl. 1994;12:S145–152. [PubMed] [Google Scholar]
- 11.Paik S, Rosen N, Jung W, You JM, Lippman ME, Perdue JF, Yee D. Expression of insulin-like growth factor-II mRNA in fetal kidney and Wilms tumor. Lab Invest. 1989;61:522–526. [PubMed] [Google Scholar]
- 12.Bach LA. IGF-I and IGF binding proteins in diabetic-related kidney growth. Growth Regul. 1992;2:30–39. [PubMed] [Google Scholar]
- 13.Mathews LS, Hammer RE, Behringer RR, D’Ercole AJ, Bell GI, Brinster RL, Palmiter RD. Growth enhancement of transgenic mice expressing human insulin-like growth factor I. Endocrinology. 1988;123:2827–2833. doi: 10.1210/endo-123-6-2827. [DOI] [PubMed] [Google Scholar]
- 14.Alpers CE, Hudkins KL, Segerer S, Sage EH, Pichler R, Couser WG, Johnson RJ, Bassuk JA. Localization of SPARC in developing, mature, and chronically ingured human allograft kidneys. Kidney Int. 2002;62:2073–2086. doi: 10.1046/j.1523-1755.2002.00680.x. [DOI] [PubMed] [Google Scholar]
- 15.Sireesha M, Sambasivan V, Kumar VK, Radha S, Raj AY, Qurratulain H. Relevance of insulin-like growth factor 2 in the etiopathophysiology of diabetic nephropathy: Possible roles of phosphatase and tensin homolog on chromosome 10 and secreted protein acidic and rich in cysteine as regulators of repair. J Diabetes. 2009;1:118–124. doi: 10.1111/j.1753-0407.2009.00025.x. [DOI] [PubMed] [Google Scholar]
- 16.Francki A, Bradshaw AD, Bassuk JA, Howe CC, Couser WG, Sage EH. SPARC regulates the expression of collagen type I and transforming growth factor-betal in mesangial cells. J Biol Chem. 1999;274:32145–32152. doi: 10.1074/jbc.274.45.32145. [DOI] [PubMed] [Google Scholar]
- 17.Taneda S, Pippin JW, Sage EH, Hudkins KL, Takeuchi Y, Couser WG, Alpers CE. Amelioration of diabetic nephropathy inSPARC-Null mice. J Am Soc Nephrol. 2003;14:968–980. doi: 10.1097/01.asn.0000054498.83125.90. [DOI] [PubMed] [Google Scholar]


