Abstract
This study aims to investigate the anxiolytic effects of essential oil from S. miltiorrhiza in rats. The elevated plus maze test and the social interaction test were performed to evaluate the anxiolytic effects of essential oil. The levels of noradrenaline (NE), dopamine (DA) and serotonin (5-HT) in cerebral cortex of rats as well as the plasma corticosterone (CORT) level were examined in the rats with the treatment of essential oil. The rota-rod test was carried out to exclude any false positive results in experimental procedures related to anxiety disorders. The catalepsy test was carried out to investigate whether essential oil induces the catalepsy. Our results showed that oral administration of essential oil increased the percentage of time spent in the open arms and increased the number of entries to the open arms in the elevated plus maze test. Oral administration of essential oil also increased the time for social interaction in rats. No apparent extrapyramidal symptom (EPS) was observed in the animals with essential oil treatment. The effect of essential oil in the intracellular chloride (Cl-) concentration in the cultured human neuroblastoma cells was assessed. Treatment with essential oil (50-100 mg/kg) increased intracellular Cl- concentration in the cell culture in a dose-dependent manner, suggesting the involvement of GABAA receptor-Cl- ion channel. Together, our data indicate an anxiolytic effect induced by the essential oil from S. miltiorrhiza.
Keywords: Salvia. Miltiorrhiza, essential oil, anxiolytic effect, monoamines, GABAA receptor
Introduction
Anxiety disorders are among the most common mental disorders with approximately one-eighth of the world population affected at some point in their life [1]. Anxiolytics or anti-anxiety agents are drugs used to relieve anxiety and manage its related psychological and physical symptoms [2]. The anxiolytic drugs usually act as depressors of the central nervous system. In the search for new therapeutic products for the treatment of neurological disorders, medicinal plants have shown the pharmacological effectiveness of in a variety of animal models [3-5]. Currently, studies on herbal medicine as complementary and alternative medicine (CAM) for mild to moderate anxiety disorders are becoming popular and accepted world-widely [6]. The Salvia genus contains over 900 species, three of which, S. officinalis, S. lavandulaefolia and S. miltiorrhiza are particularly notable for their reputed beneficial effects on behavioral function, including treatment for depression, memory disorders and age-related memory decline [3,7]. Several Salvia species are capable of in vitro inhibition of both butyrylcholinesterase (BuChE) and acetylcholinesterase (AChE) [8,9]. Similar effects are also observed in vivo, at least for AChE, suggesting that relevant components of Salvia can cross the blood-brain barrier and increase cholinergic transmission via cholinesterase inhibition [7]. A series of studies have shown that both S. lavandulaefolia and S. officinalis possess properties potentially relevant to the attenuation of the cognitive decline associated with the downregulation of the cholinergic system seen in natural aging and dementia [10-13]. However, so far, the use of S. miltiorrhiza for the treatment of disorders in the central nervous system (CNS) had not been carefully studied.
S. miltiorrhiza is officially listed in the Chinese Pharmacopoeia and is used widely and successfully in clinics in China [14-16]. Over the last 50 years, the chemical constituents and biological activities of S. miltiorrhiza have been well studied [17,18]. According to their structural characteristics and physical/chemical properties, the constituents of S. miltiorrhiza have been divided into two groups. The first group contains phenolic acids such as salvianolic acid, which are water-soluble. The second group contains abietane type-diterpene quinone pigments such as tanshinone I, tanshinone IIA and tanshinone IIB, which are more lipophilic [19]. However, the essential oil constituents derived from S. miltiorrhiza and its therapeutic basis are poorly understood. An increasing number of studies have shown that essential oils can decrease anxiety-related behavior in humans and animals [20,21]. The aromatic plant-derived essential oils exhibit a variety of biological properties, such as mood enhancement, pain relief, and improved cognitive function [22,23]. Therefore, essential oil has been used in complementary and traditional medicine units as well as primary care settings [24]. The present work investigated the anxiolytic effect produced by the essential oil from S. miltiorrhiza with in vivo and in vitro approaches.
Material and methods
Animals
Adult male Sprague Dawley (SD) rats (225 ± 25 g) were used in the study. Rats were housed in a temperature (22 ± 2°C) controlled animal room on a 12/12 h of light/dark illumination cycle with free access to food and water. The rats were divided into 5 groups (10 animals per group): (1) vehicle group (received 0.9% NaCl); (2) diazepam group (diazepam, 1.5 mg/kg); (3) EO-50 group (essential oil, 50 mg/kg); (4) EO-100 group (essential oil, 100 mg/kg); (5) EO-200 group (essential oil, 200 mg/kg); The animals were treated orally 60 min before the test.
The experimental procedures were in accordance with the guidelines of animal bioethics from The Act on Animal Experimentation and Animal Health and Welfare from Romania and all procedures were in compliance with Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. This study was approved by the local ethics committee and also, efforts were made to minimize animal suffering.
Isolation of essential oil (EO)
The aerial parts of S. miltiorrhiza were air-dried to a course powder. The oils were isolated by hydrodistillation using a Clevenger-type apparatus for 3 h according to the method recommended in the Chinese Pharmacopeia [25]. The essential oil was dried with anhydrous sodium sulfate and stored at 4°C until analysis.
Identification of components of essential oil
Identification of the components of the essential oil was based on GC retention indices relative to n-alkanes and computer matching with the Wiley 275 L mass spectra library [26]. In addition, the analysis included comparisons of the fragmentation patterns of the mass spectra to those reported in the literature [27].
Animal experiments
An appropriate amount of essential oil was diluted with distilled water (containing 2 drops of Tween-80 per 10 ml) to produce the final working concentration for pharmacological tests. Diazepam ampoules (10 mg/2 ml; Northeast Pharmaceutical Group Co., Ltd, China) were used as known anxiolytic drugs and were diluted to an appropriate concentration using normal saline containing 0.5% Tween-80. The animals were treated orally with control (0.9% NaCl), diazepam (1.5 mg/kg) or essential oil (50, 100 and 200 mg/kg respectively) 60 min before the tests.
Plus maze test
Animals were placed in the center of the maze. The time spent on open arms (each 49 cm long, 10 cm wide; elevated 50 cm from the ground) was used as an index of anxiety [28]. Anxiolytic activity was evaluated by the time spent in open arms or the chances staying in open arm entries.
Social interaction test
Social interaction test was conducted as previously described [29]. Social interaction of an experimental rat was evaluated in a 10-min task by crawling over or under, sniffing, following with contact, genital investigation, tumbling, boxing, or grooming within an open field apparatus. Data were expressed as cumulative time of the social interaction behavior.
Rota-rod test
Immediately after the social interaction test, each rat was placed on the apparatus to register the total time staying on the rotating bar in three successive trials with a total time of 1 min. To avoid to take into account the inability to stay on the rotating rod, rats were pre-selected 1 h before the test on the rotating rod (8 cm in diameter, 6 r.p.m.). Those staying on the rotating road for 2 min (approximately 95% of animals) were placed again on the same rotating rod to perform rota-rod procedure.
Catalepsy test
Catalepsy test was carried out as described previously [30]. Briefly, the rat forelimbs were placed on a horizontal metal bar, and the time during which both forelimbs remained on the bar was determined. If the animal maintained the imposed posture for at least 20 seconds, it was considered to be cataleptic and given one point. One extra point was given for every additional period of 20 seconds that the cataleptic posture was maintained. The animals were tested twice with 30 min intervals and only the greater duration of posture maintenance was considered.
Estimation of cortex monoamines and plasma corticosterone
Immediately after catalepsy testing, rats were decapitated and trunk blood was collected. The blood samples were centrifuged at 3,000× g for 10 min, and plasma was collected and stored at -20°C and for later use. Whole brain tissues were stored at -80°C. The concentrations of noradrenaline (NE), dopamine (DA) and serotonin (5-HT) were detected from brain tissues with high-performance liquid chromatography (HPLC) by using method described [31]. Plasma corticosterone levels were examined by HPLC-internal standard method [32].
Intracellular Cl- measurement
Human neuroblastoma SH-SY5Y cells (Shanghai Yanyu Co., Ltd, China) were cultured in minimum essential medium (MEM) supplemented with 10% fetal bovine serum (FBS) in a humidified incubator of 95% air and 5% CO2 at 37°C. The cells were washed twice and suspended at a concentration of 4 × 105 cells/ml in Hank’s solution. Cells were incubated overnight with the N-(6-Methoxyquinolyl) acetomethylester (MQAE) and/or essential oil in a final concentration of 5 mM at room temperature. Fluorescence (365 nm excitation and 450 nm emission) of well-stirred samples was monitored in a cuvette. Data were presented as relative fluorescence F/F0, where F0 is minimize fluorescence without chloride (Cl-) ions and F is the fluorescence as a function of time [33].
Statistical analyses
Data were analyzed by a one-way analysis of variance (ANOVA). The differences between means were analyzed by Duncan’s multiple-range test. The data were presented as the mean ± SEM. The P value (<0.05) was used as the standard for statistical significance.
Results
Chemical composition
The identified components of the essential oil and their percentages were shown in Table 1. The components were listed in order of their elution from the RTx-1MS column. The essential oil of S. miltiorrhiza consisted of at least 50 components, which accounted for 99.3% of the total mass of essential oils (Table 1). The main components of the essential oil were ferruginol (35.26%), 1-naphthalenepropanol, α-ethenyldecahydro-α, 5, 5, 8a-tetramethyl-2-methylene-, [1S-[1α(R), 4αβ, 8aα]] (14.12%) and humulane-1 (17.37%).
Table 1.
Percentage composition of the essential oil from S. miltiorrhiza analyzed by GC-MS
| No. | tR/min | Compound | % |
|---|---|---|---|
| 1 | 8.75 | Phenyl-acetaldehyde | 2.74 |
| 2 | 10.14 | 4, 6-Dimethyldodecane | 0.26 |
| 3 | 12.39 | Nonylcyclopropane | 0.07 |
| 4 | 13.73 | Myrtenol | 0.10 |
| 5 | 15.60 | Tetradecane | 0.04 |
| 6 | 16.16 | 1, 7, 7-Trimethyl-bicyclo[2.2.1]hept-2-ylester | 0.10 |
| 7 | 16.26 | Bornyl acetic ether | 0.14 |
| 8 | 16.66 | 8-Methylheptadecane | 1.30 |
| 9 | 16.89 | Cis-3-Methylcyclohexanol | 0.08 |
| 10 | 19.26 | 3, 4-Dihydro-. alpha. -ionone | 0.05 |
| 11 | 20.33 | 2, 4a, 5, 6, 7, 8, 9, 9a-Octahydro-3, 5, 5-trimethyl-9-methylene-,(4aS-cis) -1H-benzocycloheptene | 0.31 |
| 12 | 20.35 | Caryophyllene | 0.74 |
| 13 | 20.48 | Crocetane | 0.04 |
| 14 | 20.59 | n-Pentadecane | 0.10 |
| 15 | 21.23 | α-Caryophyllene | 0.20 |
| 16 | 21.63 | Heptadecane | 0.12 |
| 17 | 21.71 | 9-Methylnonadecane | 0.04 |
| 18 | 21.94 | -D Germacrene D | 0.15 |
| 19 | 22.02 | β-Sesquiphellandrene | 0.09 |
| 20 | 22.50 | 2, 4-Di-tert-butylphenol | 1.27 |
| 21 | 23.85 | (-) -Isoledene | 0.17 |
| 22 | 23.93 | Trans-Nerolidol | 0.14 |
| 23 | 24.07 | Tetradecylaldehyde | 0.06 |
| 24 | 27.48 | cis,. alpha. -Santalol | 0.22 |
| 25 | 35.06 | Naphthalene | 0.63 |
| 26 | 35.31 | 3-Methyl-2-pent-2-enyl-cyclopent-2-enone | 1.31 |
| 27 | 35.92 | 8-Hexylpentadecane | 0.10 |
| 28 | 36.23 | Cupressene | 0.26 |
| 29 | 36.28 | γ-Gurjunenepoxide | 0.17 |
| 30 | 36.63 | 9, 9’-Biphenanthrene, octacosahydro- | 0.34 |
| 31 | 36.82 | β-Humulene | 0.33 |
| 32 | 37.51 | 2-Furoicacid, 2-methyloct-5-yn-4-yl ester | 0.45 |
| 33 | 38.07 | 2-(7-Heptadecynyloxy) tetrahydro-2H-pyran | 0.57 |
| 34 | 38.81 | 7-Isopropyl-1, 1,4a-trimethyl-1, 2, 3, 4, 4a, 9, 10, 10a-octahydrophenanthrene | 12.32 |
| 35 | 38.85 | Kaurene | 0.32 |
| 36 | 39.17 | 1-Naphthalenepropanol, alpha. -ethenyldecahydr-. alpha. , 5, 5, 8a-tetramethyl-2-Methylene-, [1S-[1. alpha. (R), 4a. beta, 8a, alpha.] | 17.37 |
| 37 | 39.52 | Acetate, (2, 4a, 5, 8a2tetramethyl-1, 2, 3, 4, 4a, 7, 8, 8a-Octahydro-1-naphthalenyl) ester | 1.21 |
| 38 | 40.35 | Humulane-1, 6-dien-3-ol | 14.12 |
| 39 | 40.53 | 4, 4-Dimethy-5-androstene | 0.20 |
| 40 | 41.15 | n-Heneicosane | 0.50 |
| 41 | 42.45 | α-Retinene | 0.40 |
| 42 | 42.58 | 4a, 7, 7, 10a-Tetramethyldodecahydrobenzo[f]chromen-3-ol | 0.15 |
| 43 | 42.71 | 10, 12, 14-Nonacosatriynoicacid | 0.87 |
| 44 | 43.12 | Andrographolide | 0.39 |
| 45 | 44.10 | n-Docosane | 0.67 |
| 46 | 44.69 | 3-Methyl-5-(1,4,4-trimethylcyclohex-2-enyl) pentan-1-ol | 0.11 |
| 47 | 45.83 | 4, 4-Dimethyl-androsta-5, 7-diene | 0.26 |
| 48 | 45.96 | 13, 15-Octacosadiyne | 0.17 |
| 49 | 46.90 | Ferruginol | 35.26 |
| 50 | 49.72 | n-Hexatriacontane | 0.97 |
Anxiolytic effects of EO in plus maze test
The plus maze test was conducted to evaluate anxiolytic effect of EO. It was found that the percentage of time spent in the open arms increased significantly in EO100 and EO200 groups as compared with control group (Figure 1A). Also the number of entries to the open arms increased in EO100 and EO200 groups as compared with controls (Figure 1B). There was no change of the percentage of time spent in the poem arms (P>0.05), and no change of number of entries into the open arms in DO50 group compared to control group (P>0.05).
Figure 1.
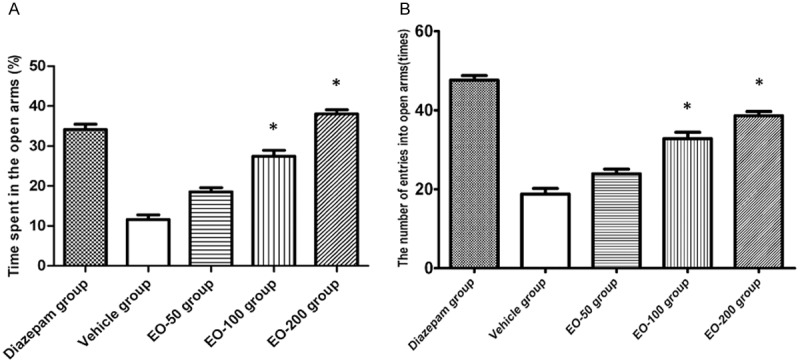
A. The percentage of the time spent in the open arms in diazepam group, vehicle group and EO-50, EO-100 and EO-200 groups. B. The number of entries into open arms in diazepam group, vehicle group and EO-50, EO-100 and EO-200 groups. Values are shown as means ± SEM. *Denotes the P<0.05 as compared to vehicle group.
EO increases social interaction time
The social interaction test is a valuable behavioural model for testing anxiolytic and neuroleptic drugs. The results for time spent in social interaction were illustrated (Figure 2). The interaction time was significantly increased in EO-100 (P<0.05) and EO-200 group (P<0.01) as compared to the vehicle group.
Figure 2.
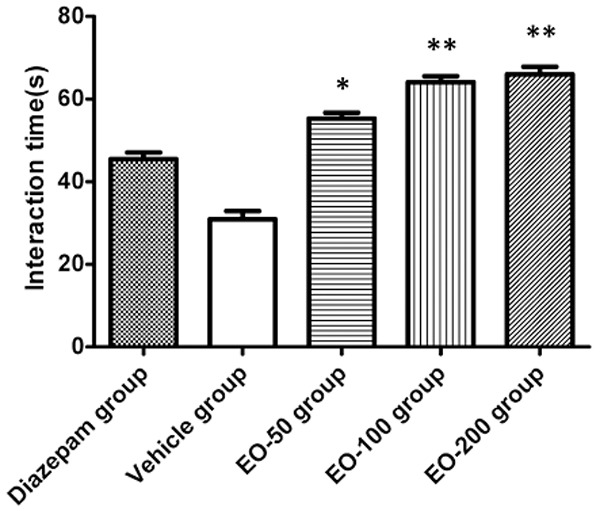
The social interaction time in diazepam group, vehicle group and EO-50, EO-100 and EO-200 groups. Values are shown as means ± SEM. *Denotes the P<0.05 as compared to vehicle group, **denotes the P<0.01 as compared to vehicle group.
Anxiolytic effects of EO in rota-rod test
The rota-rod test was carried out to measure locomotor impairment in rats. It was found that the proportion of animals in EO50, EO100 or EO200 group did not differ statistically from the vehicle group (Table 2), indicating an absence of treatment-induced motor impairment.
Table 2.
Effect of EO on motor coordination evaluated by the rota-rod test
| Different groups | Motor impairment* |
|---|---|
| Diazepam group | 1/8 |
| Vehicle group | 2/6 |
| EO-50 group | 1/6 |
| EO-100 group | 1/7 |
| EO-200 group | 0/8 |
Results are expressed as the fraction of total animals in each experimental group that were unable to perform the test.
Anxiolytic effects of EO in catalepsy test
Most antipsychotic drugs induce frequent extrapyramidal symptoms, and they often require concomitant medication with anti-Parkinson’s agents [34-36]. The catalepsy test was then conducted. There is no reduction of cataleptic scores in EO50, EO100 or EO200 groups as compared to vehicle group. There was also no difference of cataleptic score between Diazepam group and vehicle group (Table 3).
Table 3.
Effect of EO on catalepsy test
| Different groups | Scoring (point)* |
|---|---|
| Diazepam group | 16.3±0.5 |
| Vehicle group | 14.2±0.6 |
| EO-50 group | 18.8±0.8 |
| EO-100 group | 16.0±0.9 |
| EO-200 group | 17.3±0.7 |
Values are shown as means ± SEM.
Anxiolytic effects of EO on the levels of NE, DA and 5-HT
The influences of EO on the levels of NE, DA and 5-HT in cerebral cortex, as well as plasma corticosterone were examined. There was significant decrease of NE, DA, and 5-HT levels in cortex in the diazepam group as compared to the vehicle rats (P<0.01). There was significant decrease in NE, DA, and 5-HT levels in EO100 or EO200 groups as compared to the vehicle group (Table 4). In addition, the levels of plasma corticosterone in EO100 and EO200 groups were lower than that in vehicle group (P<0.05 and P<0.01 respectively) (Figure 3).
Table 4.
Effect of EO on levels of NE, DA and 5-HT
| Different groups | NE (ng/g) | DA (ng/g) | 5-HT (ng/g) |
|---|---|---|---|
| Diazepam group | 311.6±25.6 | 421.0±20.0 | 1213.6±40.8 |
| Vehicle group | 278.9±22.3* | 388.8±23.3* | 1012.2±38.6* |
| EO-50 group | 301.5±22.0 | 411.2±23.6 | 1200.6±44.3 |
| EO-100 group | 270.6±23.6* | 370.7±20.7* | 900.6±32.5** |
| EO-200 group | 266.8±19.6** | 356.3±23.6** | 876.8±20.6** |
Values are shown as means ± SEM.
P<0.05 vs. Vehicle group;
P<0.01 vs. Vehicle group.
Figure 3.
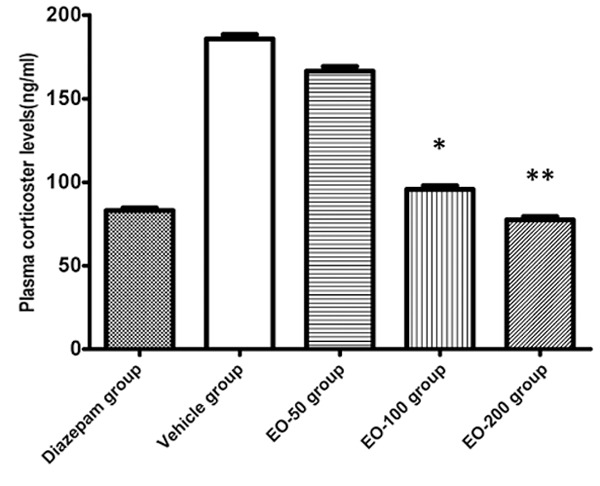
The levels of plasma corticosterone in diazepam group, vehicle group and EO-50, EO-100 and EO-200 groups. Values are shown as means ± SEM. *Denotes the P<0.05 as compared to vehicle group, **denotes the P<0.01 as compared to vehicle group.
Intracellular Cl- measurement assay
To explore the possible neuronal inhibition mediated by Cl- influx, the effect of EO on intracellular Cl- concentration in cultured human neuroblastoma cell was examined. There was an increase of F/F0 ratio at 100 s EO treatment compared to 50s treatment in the EO50, EO100 or EO200 groups (Figure 4). Prolonged EO treatment (>100 s) did not further increase the F/F0 ratio.
Figure 4.
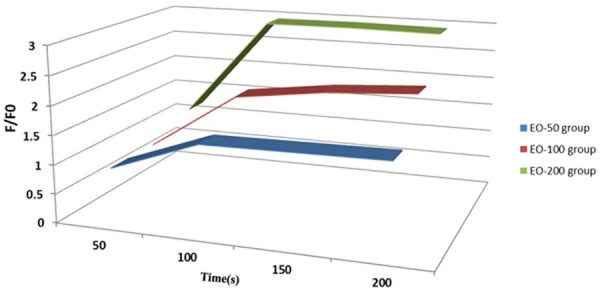
Intracellular Cl- concentration in the cultured human neuroblastoma cells with EO (50, 100 or 200) treatment.
Discussion
We present here the evidence in support of the anxiolytic effect of essential oil isolated from S. miltriorrhiza in rats.
Studies have demonstrated that the aromatic plant-derived essential oils exhibit a variety of biological properties [22-24]. The inhaled aromas from the essential oils are believed to be useful for a vast array of symptoms and conditions and a large body of published literatures have generally focused on the human brain and emotions [22-24,37,38]. The present study shows that the extract of essential oil of S. miltiorrhiza produces anxiolytic-like effects in rats. Furthermore, the essential oil of S. miltiorrhiza induced the increased of intracellular Cl- concentration in cultured human neuroblastoma cells, suggesting that its anxiolytic effects are probably mediated through a GABAergic mechanism of action.
The elevated plus-maze test is one of the most widely accepted tests for measuring anxiety in rats [39]. The social interaction test is a valuable behavioral model for testing anxiolytic and neuroleptic drugs [40]. We applied these two classical methods to estimate anxiolytic effects of the essential oil of S. miltiorrhiza. Our results demonstrate that treatment with EO induces anxiolytic-like performance, including the increase of the percentage of the time spent in the open arms, the increase of number of entries into open arms, and the recovery of the normally short interaction time.
The activation of hypothalamic-pituitary-adrenocortical (HPA) axis is a key feature of the physiological responses to anxiety [41]. The monoamine neurotransmitters NE, DA and 5-HT in cerebral cortex are involved in generating symptoms of anxiety [42]. The interplay between NE and 5-HT systems might play the prominent role in the regulation of anxiety [43]. We showed that EO treatment clearly reduces the elevation of cortex monoamine levels. Therefore, the effects of EO on brain monoamine levels might be involved in the mechanisms of anti-anxiety. Furthermore, EO treatment markedly inhibits the elevation of plasma corticosterone, which is also an indication of activation of HPA axis during anxiety.
Activation of Cl- influx leads to hyperpolarization of neurons and inhibition of excitability. EO treatment of cultured human neuroblastoma cells increases intracellular Cl- concentration, supporting the anxiolytic-like effects observed in the animal tests. In addition, EO displays a dose-dependent manner in the increase of intracellular Cl- concentration. Our data indicate that the chloride channels, such as GABA receptors, would be a potential target of EO in mediating the anxiolytic-like effects.
Conclusion
We demonstrate here that oral administration of essential oil of S. miltiorrhiza presents the anxiolytic-like effects in rats. The oral administration of essential oil does not induce extrapyramidal symptom. Essential oil treatment increases intracellular chloride concentration, indicating GABAA receptor Cl- channel would be a potential target for induction of anxiolytic effect.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81371240), Natural Science Foundation of Shaanxi Province (No. 2011JQ4001), International Science & Technology Cooperation Program of China (2011DFA32560), and Program for Shaanxi Province Key Research Team of Science and Technology Innovation (2012KCT-14).
Disclosure of conflict of interest
None.
References
- 1.Eisenberg JM. Health services research in a market-oriented health care system. Health Aff (Millwood) 1998;17:98–108. doi: 10.1377/hlthaff.17.1.98. [DOI] [PubMed] [Google Scholar]
- 2.Skolnick P. Anxioselective anxiolytics: on a quest for the Holy Grail. Trends Pharmacol Sci. 2012;33:611–620. doi: 10.1016/j.tips.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howes MJ, Perry NS, Houghton PJ. Plants with traditional uses and activities, relevant to the management of Alzheimer’s disease and other cognitive disorders. Phytother Res. 2003;17:1–18. doi: 10.1002/ptr.1280. [DOI] [PubMed] [Google Scholar]
- 4.Sarris J, McIntyre E, Camfield DA. Plant-based medicines for anxiety disorders, Part 1: a review of preclinical studies. CNS Drugs. 2013;27:207–219. doi: 10.1007/s40263-013-0044-3. [DOI] [PubMed] [Google Scholar]
- 5.Sarris J, McIntyre E, Camfield DA. Plant-based medicines for anxiety disorders, part 2: a review of clinical studies with supporting preclinical evidence. CNS Drugs. 2013;27:301–319. doi: 10.1007/s40263-013-0059-9. [DOI] [PubMed] [Google Scholar]
- 6.Lakhan SE, Vieira KF. Nutritional and herbal supplements for anxiety and anxiety-related disorders: systematic review. Nutr J. 2010;9:42. doi: 10.1186/1475-2891-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perry NS, Houghton Pj Fau-Jenner P, Jenner P Fau-Keith A, Keith A Fau-Perry EK, Perry EK. Salvia lavandulaefolia essential oil inhibits cholinesterase in vivo. Phytomedicine. 2002;9:48–51. doi: 10.1078/0944-7113-00082. [DOI] [PubMed] [Google Scholar]
- 8.Savelev SU, Okello EJ, Perry EK. Butyryl- and acetyl-cholinesterase inhibitory activities in essential oils of Salvia species and their constituents. Phytother Res. 2004;18:315–24. doi: 10.1002/ptr.1451. [DOI] [PubMed] [Google Scholar]
- 9.Tildesley NT, Kennedy DO, Perry EK, Ballard CG, Wesnes KA, Scholey AB. Positive modulation of mood and cognitive performance following administration of acute doses of Salvia lavandulaefolia essential oil to healthy young volunteers. Physiol Behav. 2005;83:699–709. doi: 10.1016/j.physbeh.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Miroddi M, Navarra M, Quattropani MC, Calapai F, Gangemi S, Calapai G. Systematic review of clinical trials assessing pharmacological properties of Salvia species on memory, cognitive impairment and Alzheimer’s disease. CNS Neurosci Ther. 2014;20:485–495. doi: 10.1111/cns.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savelev S, Okello E, Perry NS, Wilkins RM, Perry EK. Synergistic and antagonistic interactions of anticholinesterase terpenoids in Salvia lavandulaefolia essential oil. Pharmacol Biochem Behav. 2003;75:661–668. doi: 10.1016/s0091-3057(03)00125-4. [DOI] [PubMed] [Google Scholar]
- 12.Perry NS, Houghton PJ, Sampson J, Theobald AE, Hart S, Lis-Balchin M, Hoult JR, Evans P, Jenner P, Milligan S, Perry EK. In-vitro activity of S. lavandulaefolia (Spanish sage) relevant to treatment of Alzheimer’s disease. J Pharm Pharmacol. 2001;53:1347–1356. doi: 10.1211/0022357011777846. [DOI] [PubMed] [Google Scholar]
- 13.Tildesley NT, Kennedy DO, Perry EK, Ballard CG, Savelev S, Wesnes KA, Scholey AB. Salvia lavandulaefolia (Spanish sage) enhances memory in healthy young volunteers. Pharmacol Biochem Behav. 2003;75:669–674. doi: 10.1016/s0091-3057(03)00122-9. [DOI] [PubMed] [Google Scholar]
- 14.Xia Z, Gu J, Ansley DM, Xia F, Yu J. Antioxidant therapy with Salvia miltiorrhiza decreases plasma endothelin-1 and thromboxane B2 after cardiopulmonary bypass in patients with congenital heart disease. J Thorac Cardiovasc Surg. 2003;126:1404–1410. doi: 10.1016/s0022-5223(03)00970-x. [DOI] [PubMed] [Google Scholar]
- 15.Wang G, Wen J, Wilbur RR, Wen P, Zhou SF, Xiao X. The effect of somatostatin, ulinastatin and Salvia miltiorrhiza on severe acute pancreatitis treatment. Am J Med Sci. 2013;346:371–376. doi: 10.1097/MAJ.0b013e31827aa2bc. [DOI] [PubMed] [Google Scholar]
- 16.Qian Q, Qian S, Fan P, Huo D, Wang S. Effect of Salvia miltiorrhiza hydrophilic extract on antioxidant enzymes in diabetic patients with chronic heart disease: a randomized controlled trial. Phytother Res. 2012;26:60–66. doi: 10.1002/ptr.3513. [DOI] [PubMed] [Google Scholar]
- 17.Jiang RW, Lau KM, Hon PM, Mak TC, Woo KS, Fung KP. Chemistry and biological activities of caffeic acid derivatives from Salvia miltiorrhiza. Curr Med Chem. 2005;12:237–246. doi: 10.2174/0929867053363397. [DOI] [PubMed] [Google Scholar]
- 18.Lei XL, Chiou GC. Studies on cardiovascular actions of Salvia miltiorrhiza. Am J Chin Med. 1986;14:26–32. doi: 10.1142/S0192415X86000053. [DOI] [PubMed] [Google Scholar]
- 19.Sung HJ, Choi SM, Yoon Y, An KS. Tanshinone IIA, an ingredient of Salvia miltiorrhiza BUNGE, induces apoptosis in human leukemia cell lines through the activation of caspase-3. Exp Mol Med. 1999;31:174–8. doi: 10.1038/emm.1999.28. [DOI] [PubMed] [Google Scholar]
- 20.Setzer WN. Essential oils and anxiolytic aromatherapy. Nat Prod Commun. 2009;4:1305–16. [PubMed] [Google Scholar]
- 21.Tsang HW, Ho TY. A systematic review on the anxiolytic effects of aromatherapy on rodents under experimentally induced anxiety models. Rev Neurosci. 2010;21:141–52. [PubMed] [Google Scholar]
- 22.Saiyudthong S, Pongmayteegul S, Marsden CA, Phansuwan-Pujito P. Anxiety-like behaviour and c-fos expression in rats that inhaled vetiver essential oil. Nat Prod Res. 2015;29:2141–4. doi: 10.1080/14786419.2014.992342. [DOI] [PubMed] [Google Scholar]
- 23.Cayuela Sanchez JA, Elamrani A. Nutrigenomics of essential oils and their potential domestic use for improving health. Nat Prod Commun. 2014;9:1641–1648. [PubMed] [Google Scholar]
- 24.Lee MS, Choi J, Posadzki P, Ernst E. Aromatherapy for health care: an overview of systematic reviews. Maturitas. 2012;71:257–60. doi: 10.1016/j.maturitas.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 25.Tao L, Hu HS, Shen XC. Endothelium-dependent vasodilatation effects of the essential oil from Fructus alpiniae zerumbet (EOFAZ) on rat thoracic aortic rings in vitro. Phytomedicine. 2013;20:387–393. doi: 10.1016/j.phymed.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Wangchuk P, Keller PA, Pyne SG, Taweechotipatr M, Kamchonwongpaisan S. GC/GC-MS analysis, isolation and identification of bioactive essential oil components from the Bhutanese medicinal plant, Pleurospermum amabile. Nat Prod Commun. 2013;8:1305–1308. [PubMed] [Google Scholar]
- 27.Yang BB, Rong R, Hu JF, Yang Y, Lv QT, Jiang HQ. [GC-MS analysis for volatile components from alpiniae katsumadai semen by three extraction methods] . Zhong Yao Cai. 2014;37:443–447. [PubMed] [Google Scholar]
- 28.Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–5. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 29.Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3alpha,5alpha-THP. Pharmacol Biochem Behav. 2000;67:587–96. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 30.Nagai H, Egashira N, Sano K, Ogata A, Mizuki A, Mishima K, Iwasaki K, Shoyama Y, Nishimura R, Fujiwara M. Antipsychotics improve Delta9-tetrahydrocannabinol-induced impairment of the prepulse inhibition of the startle reflex in mice. Pharmacol Biochem Behav. 2006;84:330–6. doi: 10.1016/j.pbb.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Bao L, Yao XS, Yau CC, Tsi D, Chia CS, Nagai H, Kurihara H. Protective effects of bilberry (Vaccinium myrtillus L. ) extract on restraint stress-induced liver damage in mice. J Agric Food Chem. 2008;56:7803–7. doi: 10.1021/jf800728m. [DOI] [PubMed] [Google Scholar]
- 32.Woodward CJ, Emery PW. Determination of plasma corticosterone using high-performance liquid chromatography. J Chromatogr. 1987;419:280–4. doi: 10.1016/0378-4347(87)80287-6. [DOI] [PubMed] [Google Scholar]
- 33.Yoon SY, dela Peña IC, Shin CY, Son KH, Lee YS, Ryu JH, Cheong JH, Ko KH. Convulsion-related activities of Scutellaria flavones are related to the 5,7-dihydroxyl structures. Eur J Pharmacol. 2011;659:155–60. doi: 10.1016/j.ejphar.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Werner FM, Covenas R. Safety of antipsychotic drugs: focus on therapeutic and adverse effects. Expert Opin Drug Saf. 2014;13:1031–1042. doi: 10.1517/14740338.2014.935761. [DOI] [PubMed] [Google Scholar]
- 35.Divac N, Prostran M, Jakovcevski I, Cerovac N. Second-generation antipsychotics and extrapyramidal adverse effects. Biomed Res Int. 2014;2014:656370. doi: 10.1155/2014/656370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rybakowski JK, Vansteelandt K, Remlinger-Molenda A, Fleischhacker WW, Kahn RS, Peuskens J EUFEST Study Group. Extrapyramidal symptoms during treatment of first schizophrenia episode: results from EUFEST. Eur Neuropsychopharmacol. 2014;24:1500–1505. doi: 10.1016/j.euroneuro.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Lehrner J, Marwinski G, Lehr S, Johren P, Deecke L. Ambient odors of orange and lavender reduce anxiety and improve mood in a dental office. Physiol Behav. 2005;86:92–5. doi: 10.1016/j.physbeh.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 38.Buchbauer G, Jirovetz L, Jäger W, Plank C, Dietrich H. Fragrance compounds and essential oils with sedative effects upon inhalation. J Pharm Sci. 1993;82:660–4. doi: 10.1002/jps.2600820623. [DOI] [PubMed] [Google Scholar]
- 39.Handley SL, McBlane JW. An assessment of the elevated X-maze for studying anxiety and anxiety-modulating drugs. J Pharmacol Toxicol Methods. 1993;29:129–38. doi: 10.1016/1056-8719(93)90063-k. [DOI] [PubMed] [Google Scholar]
- 40.File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- 41.Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res. 2005;29:1351–5. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kent JM, Mathew SJ, Gorman JM. Molecular targets in the treatment of anxiety. Biol Psychiatry. 2002;52:1008–30. doi: 10.1016/s0006-3223(02)01672-4. [DOI] [PubMed] [Google Scholar]
- 43.Voigt JP, Rex A, Sohr R, Fink H. Hippocampal 5-HT and NE release in the transgenic rat TGR(mREN2)27 related to behavior on the elevated plus maze. Eur Neuropsychopharmacol. 1999;9:279–285. doi: 10.1016/s0924-977x(98)00031-5. [DOI] [PubMed] [Google Scholar]


