Abstract
Backgrounds: Non-small cell lung cancer (NSCLC) is one of the most common malignancies with a high mortality level. Recently, a variety of studies explored the role of osteopontin (OPN) expression in the prognosis of NSCLC, but the results were controversial. Methods: We performed a meta-analysis of eligible studies to evaluate the prognostic significance of OPN expression in NSCLC patients. In order to assess the association between OPN and OS and DFS/PFS, hazard ratio (HR) with 95% confidence interval (CI) was calculated. Results: A total of ten studies comprising 1420 patients were included in the meta-analysis. The summary results indicated that high OPN expression was a poor predictor for OS (HR = 2.19, 95% CI: 1.6-2.98), and DFS/PFS (HR = 2, 95% CI: 1.66-2.41). Subgroup analysis revealed that high OPN expression was a negative prognostic marker for OS and DFS/PFS regardless of ethnicity background, treatment and OPN detection method. Conclusion: Our results showed that increased OPN expression significantly correlated with poor OS and DPS/PFS in NSCLC patients.
Keywords: Non-small cell lung cancer, osteopontin, prognosis, biomarker
Introduction
Lung cancer is one of the most common malignancies and the leading cause of cancer-related death in the world [1]. Non-small cell lung cancer (NSCLC) constitutes 85% of all lung cancer cases [2]. In spite of multiple treatment methods including surgery, chemotherapy, radiotherapy and targeted therapy have been applied in NSCLC therapies over the past few decades, the prognosis of NSCLC remains dismal with the 5-year survival rate of <15% after the initial diagnosis [3]. The conventional predictive factors such as TNM stage, age, sex, and histological type have limited prognostic values [4]. Therefore, it is necessary to exploit novel prognostic markers which could accurately predict the outcome so as to guide the clinical management of NSCLC patients.
Osteopontin (OPN) is a secreted phosphoglycoprotein that was originally isolated from bone. OPN is critical to control osteoclast differentiation, biomineralization and bone resorption [5]. Recently, accumulating evidence has showed that OPN is a key player in different pathological processes of tumor development and angiogenesis through modulation of expression of genes involved in invasion, migration, proliferation and vascularization [6,7]. A variety of studies have explored the association between OPN expression and the clinical outcome and prognosis of NSCLC patients, but the results emerged controversial [8-12]. Therefore, it is necessary to perform a meta-analysis to comprehensively and systematically clarify the prognostic significance of OPN in NSCLC patients. In the present study, we pooled results from the available data and made an evaluation of the prognostic significance of elevated serum-and tissue-based OPN levels for overall survival (OS) and disease-free survival (DFS)/progression-free survival (PFS) in NSCLC patients.
Materials and methods
Literature search
A comprehensive literature search was conducted in the databases of PubMed, EMBASE and Web of Science (last search updated to May 2015). The search strategy was based on the combinations of the following keywords: “Osteopontin”, “OPN”, “non-small cell lung cancer”, “NSCLC”, “lung cancer”, “lung neoplasm”, “prognosis”, “prognostic” and “survival”. Only papers published in English language were retrieved. All eligible studies were retrieved, and their bibliographies were manually checked for other potentially relevant publications.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) OPN expression was measured by reverse transcription-polymerase chain reaction (RT-PCR), immunohistochemistry (IHC) or enzyme linked immunosorbent assay (ELISA); (2) pathologically or histologically confirmed non-small cell lung cancer; (3) to evaluate the correlation between OPN expression and OS, DFS/PFS or to provided sufficient data to estimate hazard ratio (HR) and their 95% confidence intervals (CIs); (4) have a maximum follow-up time exceeding 3 years; (5) articles with full texts published in English. The studies were excluded based on the following criteria: (1) studies had duplicate data or repeat analysis; (2) meeting abstracts, letters, case reports, reviews; (3) studies in which necessary data were not provided; (4) published in non-English; (5) animal studies.
Data extraction and quality assessment
Eligible studies were reviewed by two investigators (XG and QL) and the following data was extracted: the author’s name, year of publication, ethnicity, and number of patients, follow-up, disease stage, treatment, detection method, cut-off value and survival analysis. Controversial problems were resolved through consensus with the third investigator (KF). Quality assessment was independently performed in each of the included studies by two reviewers (YL and TT) using the Newcastle-Ottawa Quality Assessment Scale (NOS) [13]. NOS scores of ≥6 were assigned as high-quality studies. Disagreements were resolved by discussion.
Statistical analysis
The impact of OPN expression on survival (OS, DFS/ PFS) was measured by the combined HRs and their 95% CIs extracted from each eligible study. The HR and its 95% CI in each eligible study was directly extracted from report, or indirectly estimated by methods described by Parmer et al [14]. Cochran’s Q test and Higgins I-squared statistic were used to evaluate the heterogeneity of pooled results. I2>50% is considered as a measure of severe heterogeneity. If P≥0.10 in the Q test or I2<50%, the fixed-effect model (Mantel-Haenszel method) was used. Otherwise, random effect model (DerSimonian-Laird method) was conducted. Subgroup analysis by different analytical factors was performed in the analysis of OS and DFS/PFS. Publication bias was evaluated using Begg’s funnel plot and the Egger’s linear regression test. Sensitivity analysis was also conducted by sequential omission of each study to evaluate stability of the results. All statistical tests were two-sided and the significance level was set at 5%. Analyses on main results were performed by using STATA 12.0 software (Stata Corporation, College Station, TX).
Results
Study selection and characteristics
Based on the search strategies given above, a total of 223 articles were found (Figure 1). After reviewing the titles and abstracts, 203 articles were excluded because they obviously did not meet our selection criteria. The remaining 20 articles were further checked by screening the full texts. 10 studies were excluded for the following reasons: insufficient data (n = 5), without outcome of interest (n = 3), data overlapping (n = 2). Finally, a total of 10 studies [12,15-23] including 1420 patients were qualified for our analysis. The NOS scores of the 10 studies ranged from 6 to 9.
Figure 1.
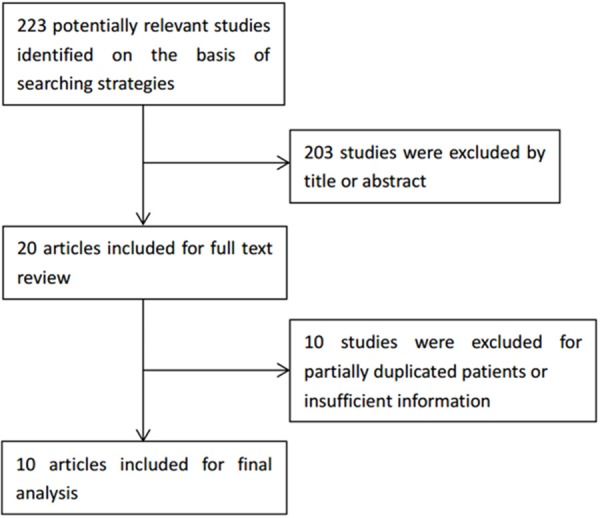
Flow diagram of studies selection procedure.
All the 10 studies investigated the impact of OPN expression on OS, and 6 of them [12,16,17,19,20,23] assessed the impact of OPN expression on DFS/ PFS. Among these 10 studies, five studies used ELISA, four used IHC and one used RT-PCR to detect serum-and tissue-based OPN expression. 6 studies evaluated patients in Caucasian, 4 studies evaluated patients in Asian. Six of these cohorts enrolled >100 patients and 4 cohorts had <100 patients. The main characteristics of included studies are summarized in Table 1.
Table 1.
Main characteristics of all studies included in the meta-analysis
| Study | Year | Ethnicity | No. of patients | Follow-up (months) | Stage | Treatment | Method | Cut-off value | Survival analysis | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|
| Schneider | 2004 | Caucasian | 82 | 63-105 | I | Surgery | RT-PCR | 4.1 | OS | 9 |
| Donati | 2005 | Caucasian | 163 | 72-137 | I-IIIA | Surgery | IHC | 20% | OS/PFS | 7 |
| Mack | 2008 | Caucasian | 156 | 3-80 | IIIB-IV | Chemotherapy | ELISA | Median | OS/PFS | 6 |
| Isa | 2009 | Asian | 67 | 2-46 | IIIB-IV | Chemotherapy | ELISA | Median | OS/PFS | 6 |
| Oberije | 2010 | Caucasian | 158 | 1-71 | I-IIIB | Radiotherapy | ELISA | Median | OS | 7 |
| Rud | 2013 | Caucasian | 244 | 1-60 | I-III | Surgery | IHC | Score 2 | OS/PFS | 8 |
| Sun | 2013 | Asian | 159 | 2-120 | I-III | Surgery | IHC | 20% | OS/PFS | 9 |
| Takenaka | 2013 | Asian | 244 | 48 (median) | I-III | Surgery | ELISA | ROC curve | OS | 7 |
| Ostheimer | 2014 | Caucasian | 55 | 37 (median) | I-III | Radiotherapy | ELISA | Median | OS | 7 |
| Zhang | 2014 | Asian | 92 | 2-50 | III-IV | Chemotherapy | IHC | Score 1 | OS/PFS | 6 |
OPN and OS in NSCLC
All the 10 studies reported the relationship between OPN and OS in NSCLC. Our analysis suggested that increased OPN expression was significantly associated with poor OS with a pooled HR estimate of 2.19 (95% CI: 1.6-2.98, P<0.001; Figure 2), though with heterogeneity (I2 = 72.9%, P het<0.001). Subgroup analysis by ethnicity suggested that both Caucasian and Asian patients with increased OPN had a significant impact on OS (Caucasian: HR = 1.71, 95% CI = 1.42-2.05, P<0.001, I2 = 0%, P het = 0.829; Asian: HR = 3.25, 95% CI = 1.51-6.98, P = 0.003, I2 = 72.9%, P het<0.001). When grouped according to tumor stage of patients, both patients of early stage and advanced stage suggested the significant results (early stage: HR = 1.87, 95% CI = 1.54-2.26, P<0.001, I2 = 0%, P het = 0.632; advanced stage: HR = 3.17, 95% CI = 1.08-9.34, P = 0.036, I2 = 92.7%, P het<0.001). Further subgroup analysis also indicated that high OPN expression remained to be a worse prognostic marker regardless of sample size, treatment and OPN detection method (Table 2).
Figure 2.
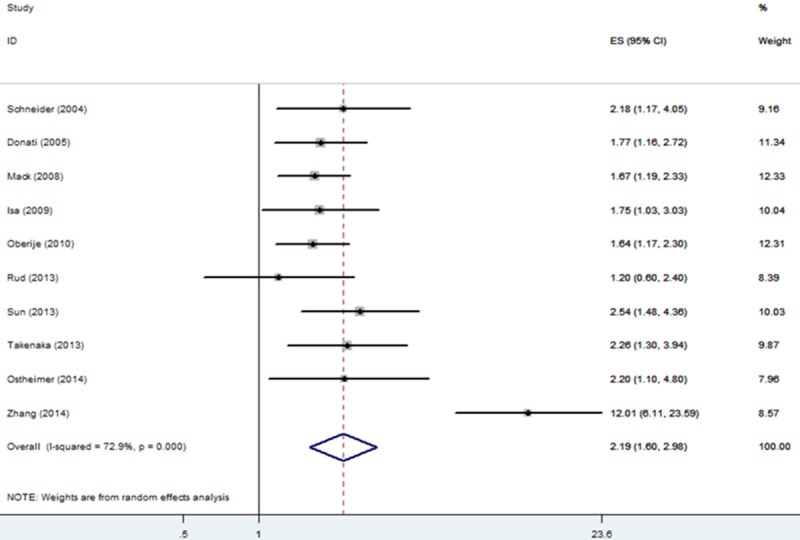
Forrest plot of HR for the association of OPN expression with OS in patients with NSCLC.
Table 2.
Main meta-analysis results
| Analysis | No. of studies | No. of patients | Model | HR (95% CI) | P | Heterogeneity | Publication bias | ||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Ieteroge | P het | Begg’s P | Egger’s P | ||||||
| OS | 10 | 1420 | Random | 2.19 (1.6-2.98) | <0.001 | 72.9 | <0.001 | 0.281 | 0.181 |
| Subgroup 1: ethnicity | |||||||||
| Caucasian | 6 | 858 | Fixed | 1.71 (1.42-2.05) | <0.001 | 0 | 0.829 | 0.573 | 0.682 |
| Asian | 4 | 562 | Random | 3.25 (1.51-6.98) | 0.003 | 72.9 | <0.001 | 0.174 | 0.023 |
| Subgroup 2: stage | |||||||||
| Early stage | 7 | 1105 | Fixed | 1.87 (1.54-2.26) | <0.001 | 0 | 0.632 | 0.881 | 0.509 |
| Advanced stage | 3 | 315 | Random | 3.17 (1.08-9.34) | 0.036 | 92.7 | <0.001 | 0.117 | 0.424 |
| Subgroup 3: treatment | |||||||||
| Surgery | 5 | 892 | Fixed | 1.97 (1.54-2.15) | <0.001 | 0 | 0.493 | 0.327 | 0.77 |
| Chemotherapy | 3 | 315 | Random | 3.17 (1.08-9.34) | 0.036 | 92.7 | <0.001 | 0.117 | 0.424 |
| Radiotherapy | 2 | 213 | Fixed | 1.73 (1.27-2.35) | <0.001 | 0 | 0.477 | 0.317 | - |
| Subgroup 4: sample size | |||||||||
| >100 | 6 | 1124 | Fixed | 1.77 (1.48-2.12) | <0.001 | 0 | 0.551 | 0.851 | 0.689 |
| ≤100 | 4 | 296 | Random | 3.14 (1.33-7.41) | 0.009 | 86.2 | <0.001 | 0.174 | 0.544 |
| Subgroup 5: method | |||||||||
| RT-PCR | 1 | 82 | - | 2.18 (1.17-4.05) | 0.014 | - | - | - | - |
| IHC | 4 | 658 | Random | 2.81 (1.18-6.66) | 0.019 | 89.1 | <0.001 | 1 | 0.583 |
| ELISA | 5 | 680 | Fixed | 1.77 (1.45-2.15) | <0.001 | 0 | 0.845 | 0.142 | 0.072 |
| DFS/PFS | 6 | 881 | Fixed | 2 (1.66-2.41) | <0.001 | 39.8 | 0.14 | 0.452 | 0.163 |
| Subgroup 1: ethnicity | |||||||||
| Caucasian | 3 | 563 | Fixed | 1.64 (1.29-2.08) | <0.001 | 0 | 0.534 | 0.602 | 0.505 |
| Asian | 3 | 318 | Fixed | 2.7 (2.01-3.63) | <0.001 | 0 | 0.819 | 0.602 | 0.706 |
| Subgroup 2: treatment | |||||||||
| Surgery | 3 | 566 | Fixed | 2.14 (1.58-2.9) | <0.001 | 0 | 0.545 | 0.602 | 0.817 |
| Chemotherapy | 3 | 315 | Random | 2.11 (1.33-3.35) | 0.002 | 70.5 | 0.034 | 0.602 | 0.228 |
| Subgroup 3: method | |||||||||
| IHC | 4 | 658 | Fixed | 2.35 (1.82-3.04) | <0.001 | 0 | 0.473 | 1 | 0.865 |
| ELISA | 2 | 223 | Random | 1.78 (1.1-2.87) | 0.018 | 61 | 0.11 | 0.317 | - |
OPN and DFS/PFS in NSCLC
Six studies including 881 patients investigated the association between OPN expression and DFS/PFS in NSCLC [12,16,17,19,20,23].Two studies evaluated DFS [12,20] and the remaining four studies evaluated PFS. Our analysis suggested that high OPN expression significantly associated with unfavorable DFS/PFS (HR = 2.95% CI = 1.66-2.41, P<0.001) without significant heterogeneity in the data (I2 = 39.8%, P het = 0.14). (Figure 3; Table 2).
Figure 3.
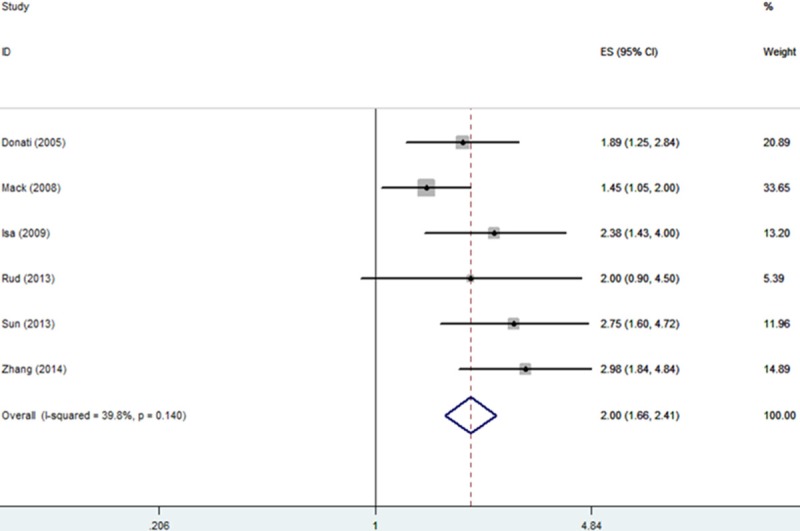
Forrest plot of HR for the association of OPN expression with DFS/PFS in patients with NSCLC.
Heterogeneity
Meta-regression was conducted by using variables as ethnicity, sample size, tumor stage, treatment and OPN detection method for OS analysis. The results showed that ethnicity (P = 0.439), sample size (P = 0.176), tumor stage (P = 0.298), treatment (P = 0.86) and OPN detection method (P = 0.387) did not contribute to the source of heterogeneity. Then we conducted sensitivity analysis, the result showed that the pooled HRs were significantly influenced by omitting Zhang’s study [2 3]. However, after omitting Zhang’s study, the correlation between high OPN expression and poor OS in NSCLC patients remained significant (HR = 1.81, 95% CI = 1.55-2.12, P<0.001) and without heterogeneity (I2 = 0%, P het = 0.792).
Publication bias
To assess the publication bias of studies, Begg’s funnel plot and Egger’s test were performed. As showed in Table 2, no publication bias was detected in all comparisons. The funnel plot in the comparison of OS in patients with NSCLC was shown in Figure 4.
Figure 4.
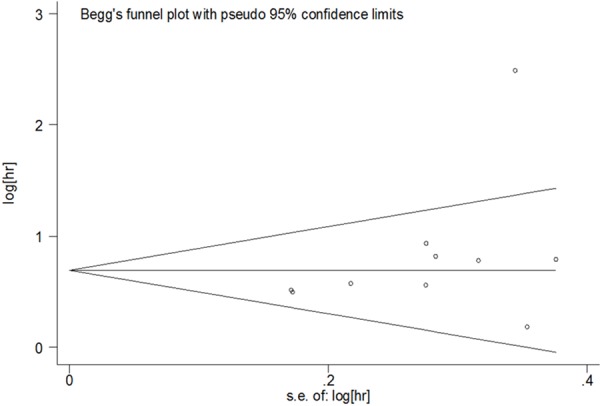
The funnel plot of the meta-analysis of the impact of OPN expression on OS in patients with NSCLC.
Discussion
The advanced tumor grade, metastasis, and elevated levels of serum biomarkers are often associated with poor prognosis of NSCLC patients [24]. However, the prognostic efficiency of traditional tumor markers is inadequate due to lack of accuracy. From the clinical view, therefore, it is of great significance to identify the most useful biomarkers predicting patient outcome so as to help guide decision-making with regard to NSCLC treatment. Recently, OPN was reported as one candidate marker for the prognosis of NSCLC [12,25,26]. However, the results were inconsistent and the available data regarding the impact of OPN on OS, DFS/PFS have not been analyzed comprehensively till now. Consequently, we carried out a meta-analysis to investigate the correlation of OPN expression in patients with NSCLC.
Our results suggested that increased OPN expression was associated with poor OS and DFS/PFS in patients with NSCLC. Subgroup analysis showed that the prognostic efficiency of high OPN expression for poor OS in NSCLC was not influenced by ethnicity background, sample size, tumor stage, and treatment or OPN detection method. OPN expression was also a significant predictive factor for worse DFS/PFS regardless of ethnicity, treatment and OPN detection method. To investigate the source of heterogeneity, meta-regression was utilized. However, none of the variables listed above contributed to the heterogeneity. Sensitivity analysis indicated that Zhang’s study [23] lead to heterogeneity for the analysis of OPN and OS when the data was pooled. After omitting Zhang’s study, heterogeneity disappeared and the main results did not change substantially, which demonstrated the intrinsically prognostic value of OPN in NSCLC. In addition, there was no significant publication bias in our analysis.
Accumulating evidence showed the connection between OPN and cancer and mechanistic studies have presented solid evidence to support the biological and prognostic importance OPN overexpression in cancer progression. OPN is a ma-tricellular protein which is produced by multiple tissues and is most abundant in bone [5]. OPN plays pivotal roles in tumor progression and metastasis. OPN can activate expression of genes and functions that contributeto metastasis of tumor, thus conferring it increased aggressiveness [27]. OPN could also prevent apoptosis by activating the Akt pathway under experimental conditions [28]. OPN can mediate the motility of tumor cells through the interaction and involvement of numerous integrins [29]. Furthermore, OPN has also been reported to facilitate tumor neovascularization and progression [30]. These may account for the poor prognosis of NSCLC patients with high OPN expression.
Notably, a recent meta-analysis conducted by Zhang et al. [31] explored prognostic value of OPN expression in NSCLC. However, their study merely investigated the association between osteopontin expression and clinicopathological features such as tumor stage, lymph node metastasis, tumor size and pathological types in NSCLC. Their report had no consideration of survival rates in NSCLC patients when stratified by OPN expression. Nevertheless, survival rates are the most valuable parameters for cancer patients. Therefore, our results may have more reference value for clinical practice.
However, some limitations need to be interpreted cautiously for further consideration in this meta-analysis. First, significant heterogeneity among the groups was observed when OPN and OS analysis. Though the heterogeneity could be removed by exclusion of Zhang’s study. Second, studies performed with positive results are easier to be published than studies with negative results, suggesting potential publication bias. Third, we did not investigate the correlations between OPN expression and clinical features such as tumor stage and metastasis, because limited primary studies provided the corresponding data in our analysis. Fourth, studies in other languages than English were excluded, which may result in language bias in our meta-analysis. Fifth, the detection of OPN expression by different methods (IHC, RT-PCR and ELISA) in the primary studies may lead to bias. Further investigations are needed to in determine whether these factors could influence the results of the meta-analysis.
In summary, this meta-analysis revealed that increased OPN expression was significantly associated with poor OS and DPS/PFS in NSCLC. OPN could serve as a biomarker for prognosis of NSCLC patients, which should be validated by performing more studies in the future.
Acknowledgements
This work was supported by grants from the Ministry of Science and Technology of China (973 projects) (No. 2012CB917104) and the National Natural Science Foundation of China (No. 81101833 and 81201605), Shanghai Municipal Commission of Health and Family Planning (No. XYQ2011040), and natural science foundation of Fujian province (No. 2013J01390). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global Cancer Statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 4.Chansky K, Sculier JP, Crowley JJ, Giroux D, Van Meerbeeck J, Goldstraw P. The International Association for the Study of Lung Cancer Staging Project: prognostic factors and pathologic TNM stage in surgically managed non-small cell lung cancer. J Thorac Oncol. 2009;4:792–801. doi: 10.1097/JTO.0b013e3181a7716e. [DOI] [PubMed] [Google Scholar]
- 5.Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11:279–303. doi: 10.1177/10454411000110030101. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed M, Behera R, Chakraborty G, Jain S, Kumar V, Sharma P, Bulbule A, Kale S, Kumar S, Mishra R, Raja R, Saraswati S, Kaur R, Soundararajan G, Kumar D, Thorat D, Sanyal M, Ramdasi A, Ghosh P, Kundu GC. Osteopontin: a potentially important therapeutic target in cancer. Expert Opin Ther Targets. 2011;15:1113–1126. doi: 10.1517/14728222.2011.594438. [DOI] [PubMed] [Google Scholar]
- 7.Chakraborty G, Jain S, Behera R, Ahmed M, Sharma P, Kumar V, Kundu GC. The multifaceted roles of osteopontin in cell signaling, tumor progression and angiogenesis. Curr Mol Med. 2006;6:819–830. doi: 10.2174/156652406779010803. [DOI] [PubMed] [Google Scholar]
- 8.Shijubo N, Uede T, Kon S, Maeda M, Segawa T, Imada A, Hirasawa M, Abe S. Vascular endothelial growth factor and osteopontin in stage I lung adenocarcinoma. Am J Respir Crit Care Med. 1999;160:1269–1273. doi: 10.1164/ajrccm.160.4.9807094. [DOI] [PubMed] [Google Scholar]
- 9.Guldur ME, Kibar Y, Deniz H, Bakir K. Comparison of osteopontin, beta-catenin and hnRNP B1 expression in lung carcinomas. Pathol Oncol Res. 2010;16:55–59. doi: 10.1007/s12253-009-9187-4. [DOI] [PubMed] [Google Scholar]
- 10.Jin Y, Tong DY, Tang LY, Chen JN, Zhou J, Feng ZY, Shao CK. Expressions of osteopontin (OPN), (alpha)(nu)(beta)3 and Pim-1 associated with poor prognosis in non-small cell lung cancer (NSCLC) Chin J Cancer Res. 2012;24:103–108. doi: 10.1007/s11670-012-0103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu TT, Han ZG, Shan L, Tao J, Zhang T, Yuan SF, Shen HL. Expression of osteopontin in non-small cell lung cancer and correlative relation with microvascular density. Asian Pac J Cancer Prev. 2014;15:29–32. doi: 10.7314/apjcp.2014.15.1.29. [DOI] [PubMed] [Google Scholar]
- 12.Donati V, Boldrini L, Dell’Omodarme M, Prati MC, Faviana P, Camacci T, Lucchi M, Mussi A, Santoro M, Basolo F, Fontanini G. Osteopontin expression and prognostic significance in non-small cell lung cancer. Clin Cancer Res. 2005;11:6459–6465. doi: 10.1158/1078-0432.CCR-05-0541. [DOI] [PubMed] [Google Scholar]
- 13.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. PLoS Negl Trop Dis. 2013;7:e2195. [Google Scholar]
- 14.Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Schneider S, Yochim J, Brabender J, Uchida K, Danenberg KD, Metzger R, Schneider PM, Salonga D, Holscher AH, Danenberg PV. Osteopontin but not Osteonectin Messenger RNA Expression is a Prognostic Marker in Curatively Resected Non-Small Cell Lung Cancer. Clin Cancer Res. 2004;10:1588–1596. doi: 10.1158/1078-0432.ccr-0565-3. [DOI] [PubMed] [Google Scholar]
- 16.Mack PC, Redman MW, Chansky K, Williamson SK, Farneth NC, Lara PN Jr, Franklin WA, Le QT, Crowley JJ, Gandara DR. Lower osteopontin plasma levels are associated with superior outcomes in advanced non-small-cell lung cancer patients receiving platinum-based chemotherapy: SWOG Study S0003. J. Clin. Oncol. 2008;26:4771–4776. doi: 10.1200/JCO.2008.17.0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isa SI, Kawaguchi T, Teramukai S, Minato K, Ohsaki Y, Shibata K, Yonei T, Hayashibara K, Fukushima M, Kawahara M, Furuse K, Mack PC. Serum osteopontin levels are highly prognostic for survival in advanced non-small cell lung cancer: Results from JMTO LC 0004. J Thorac Oncol. 2009;4:1104–1110. doi: 10.1097/JTO.0b013e3181ae2844. [DOI] [PubMed] [Google Scholar]
- 18.Dehing-Oberije C, Aerts H, Yu S, De Ruysscher D, Menheere P, Hilvo M, Van Der Weide H, Rao B, Lambin P. Development and validation of a prognostic model using blood biomarker information for prediction of survival of non-small-cell lung cancer patients treated with combined chemotherapy and radiation or radiotherapy alone (NCT00181519, NCT00573040, and NCT00572325) Int J Radiat Oncol Biol Phys. 2011;81:360–368. doi: 10.1016/j.ijrobp.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Rud AK, Boye K, Oijordsbakken M, Lund-Iversen M, Halvorsen AR, Solberg SK, Berge G, Helland A, Brustugun OT, Maelandsmo GM. Osteopontin is a prognostic biomarker in non-small cell lung cancer. BMC Cancer. 2013;13:540. doi: 10.1186/1471-2407-13-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun BS, Li Y, Zhang ZF, You J, Wang CL. Osteopontin combined with CD44v6, a novel prognostic biomarker in non-small cell lung cancer undergoing curative resection. Ann Thorac Surg. 2013;96:1943–1951. doi: 10.1016/j.athoracsur.2013.07.089. [DOI] [PubMed] [Google Scholar]
- 21.Takenaka M, Hanagiri T, Shinohara S, Yasuda M, Chikaishi Y, Oka S, Shimokawa H, Nagata Y, Nakagawa M, Uramoto H, So T, Yamada S, Tanaka F. Serum Level of Osteopontin as a Prognostic Factor in Patients Who Underwent Surgical Resection for Non-Small-Cell Lung Cancer. Clin Lung Cancer. 2013;14:288–294. doi: 10.1016/j.cllc.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Ostheimer C, Bache M, Guttler A, Kotzsch M, Vordermark D. A pilot study on potential plasma hypoxia markers in the radiotherapy of non-small cell lung cancer: Osteopontin, carbonicanhydrase IX and vascular endothelial growth factor. Strahlenther Onkol. 2014;190:276–282. doi: 10.1007/s00066-013-0484-1. [DOI] [PubMed] [Google Scholar]
- 23.Zhang T, Zhang DM, Zhao D, Hou XM, Yang TN. Osteopontin expression is associated with platinum-based chemotherapy response and prognosis of patients with advanced non small cell lung cancer. J BUON. 2014;19:742–748. [PubMed] [Google Scholar]
- 24.Alberg AJ, Brock MV, Ford JG, Samet JM, Spivack SD. Epidemiology of Lung Cancer Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2013;143:e1S–29S. doi: 10.1378/chest.12-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rittling SR, Chambers AF. Role of osteopontin in tumour progression. Br J Cancer. 2004;90:1877–1881. doi: 10.1038/sj.bjc.6601839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber GF. The Cancer Biomarker Osteopontin: Combination with Other Markers. Cancer Genomics Proteomics. 2011;8:263–288. [PubMed] [Google Scholar]
- 27.Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 2006;16:79–87. doi: 10.1016/j.tcb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Cook AC, Tuck AB, McCarthy S, Turner JG, Irby RB, Bloom GC, Yeatman TJ, Chambers AF. Osteopontin induces multiple changes in gene expression that reflect the six “hallmarks of cancer” in a model of breast cancer progression. Mol Carcinog. 2005;43:225–236. doi: 10.1002/mc.20105. [DOI] [PubMed] [Google Scholar]
- 29.Denda S, Reichardt LF, Muller U. Identification of osteopontin as a novel ligand for the integrin alpha 8 beta 1 and potential roles for this integrin-ligand interaction in kidney morphogenesis. Mol Biol Cell. 1998;9:1425–1435. doi: 10.1091/mbc.9.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chakraborty G, Jain S, Kundu GC. Osteopontin promotes vascular endothelial growth factor-dependent breast tumor growth and angiogenesis via autocrine and paracrine mechanisms. Cancer Res. 2008;68:152–161. doi: 10.1158/0008-5472.CAN-07-2126. [DOI] [PubMed] [Google Scholar]
- 31.Zhang T, Zhang DM, Zhao D, Hou XM, Liu XJ, Ling XL, Ma SC. The prognostic value of osteopontin expression in non-small cell lung cancer: a meta-analysis. J Mol Histol. 2014;45:533–540. doi: 10.1007/s10735-014-9574-3. [DOI] [PubMed] [Google Scholar]


