Abstract
Ischemia/reperfusion (I/R) induced spinal cord injury is an important pathologic mechanism leading to the paraplegia observed after surgery to repairaortic aneurysms. This study aims to investigate the neuroprotective effects of Lipoxin A4 and its potential mechanism in a rabbit model with I/R spinal cord injury. Forty-five rabbits were randomly divided into three groups: sham group, I/R group and Lipoxin A4 group. Rabbits were subject to 30 min aortic occlusion to induce transient spinal cord ischemia. All animals were sacrificed after neurological evaluation with modified Tarlov criteria at the 48th hour after reperfusion, and the spinal cord segments (L4-6) were harvested for histopathological examination, as well as local malondialdehyde (MDA) and total superoxide dismutase (SOD) activity analysis. All animals in the I/R group became paraplegic. While after 48-hour treatment, compared with I/R group, Lipoxin A4 significantly improved neurological function, reduced cell apoptosis and MDA levels as well as increased SOD activity (P < 0.05). These results suggest that Lipoxin A4 can ameliorate I/R induced spinal cord injury in Rabbit through its antiapoptosis and antioxidant activity.
Keywords: Lipoxin A4, spinal cord, ischemia/reperfusion injury, rabbits
Introduction
After surgical repair of thoracoabdominal aortic aneurysms, a major devastating and unpredictable complication is paraplegia [1]. Although this complication has been attributed to multiple factors, the principal root is thought to be related to ischemia/reperfusion (I/R) induced injury in spinal cord. Currently, numerous surgical techniques and pharmacologic interventions have been used to protect the spinal cord, but the complication still cannot be prevented completely [2-5]. Given its poor prognosis, more effective approaches to protect the spinal cord against I/R injury require further exploration. Lipoxins (LX) are a class of lipoxygenase products of arachidonic acid, which possess a wide spectrum of adjustment function for various inflammatory cells, including inhibition of the activation and aggregation of neutrophils, repression of proinflammatory factors expression such as tumor necrosis factor α and interleukin 1β, promotion of inflammatory resolution, and inhibition of transform from ischemia injury as well as inhibition of reactive oxygen species (ROS) production [6,7]. Recent studies showed that both Lipoxins and its analogues or Lipoxins receptor agonists could protect I/R injury in multiple organs, such as heart, brain, lung, and kidney et al. [8-11]. However, to our knowledge, little is known about its effect on spinal cord I/R injury.
Therefore, in this study we aimed to investigate the neuroprotective effect of Lipoxin A4 and its potential mechanism in a rabbit model with spinal cord I/R injury.
Methods
Animal and setting of groups
This study was performed in Jining Medical College. Forty-five New Zealand white healthy male rabbits were obtained from the center of experimental animals of Jining Medical College. The animals weighing 2.0 to 2.5 kg (mean 2.25 ± 0.25 kg) were divided into three groups: sham group (n = 15): the animals were surgical procedure but the aorta was not occluded; I/R group (n = 15): aorta was occluded for 30 min, followed by reperfusion for 48 h; Lipoxin A4 group (n = 15): same surgical procedure with I/R group, except for Lipoxin A4 (0.1 mg/kg) infusion 10 min before reperfusion. All procedures were approved by the animal care committee of Jining Medical College.
Surgery preparation
Rabbit model with spinal cord I/R injury was performed as we previously reported [4]. Briefly, rabbits were anesthetized with pentobarbital sodium (30 mg/kg, IV) and were placed in supine position. Then a catheter was inserted into the left femoral artery to measure the distal blood pressure by using pressure monitor. The arterial oxygen tension (PaO2), arterial carbon dioxide tension (PaCO2), and pH were monitored by means of OMNI Modular System. After that, a 10 cm midline incision was made to expose the abdominal aorta. Following anticoagulation with 400 unit’s heparin, the abdominal aorta was cross-clamped for 30 min using atraumatic vascular clamps at the level of the left renal artery. At the end of the occlusion period the clamp was removed, and the abdominal wall was subsequently carefully closed in layers using 4.0 nylon suture. Lipoxin A4 group animals were injected with 0.1 mg/kg Lipoxin A4 (dissolved in physiological saline) into a branch of glossopharyngeal vein 10 min before reperfusion, and the sham and I/R group was injected with the same volume of physiological saline. During the surgery, the animals were placed under an overhead lamp to maintain body temperature until recovery from anesthesia. Then they were returned to their home cage, and given prophylactic antibiotic (gentamicin; 40,000 IU) and saline (5 ml, twice a day for 5 days, to prevent dehydration). Bladders were voided manually twice a day until normal function returned.
Neurological evaluation
Neurological status of the animals was assessed at 48 h after reperfusion by an independent observer who had no prior knowledge of the experimental protocol and grouping. Modified Tarlov criteria were used to grade the motor function of hind limbs as previously described [12]: grade 0, no movement of the lower limbs; grade 1, minimal movement; grade 2, good movement but unable to stand; grade 3, able to stand and walk but unable to hop normally; and grade 4, normal recovery.
Histological study
Forty-eight hours after I/R injury, 10 rabbits from each group were reanesthetized with pentobarbital sodium (40 mg/kg). Then intracardiac perfusion was performed with 2000 ml heparinized saline and 1000 ml 10% buffered formalin. After perfusion, the lumbar spinal cord (L4-6 segments) was removed immediately, immersed in 10% formaldehyde and stored at 4°C for 48 h. After dehydration in graded ethanol, the spinal cord specimens were embedded in paraffin. Coronal sections of the spinal cord segment were cut at a thickness of 5 μm and stained with hematoxylin and eosin (HE) for evaluation of structural changes. Injured neurons were identified by intensely eosinophilic cytoplasm, loss of Nissl substance and pyknotic nuclei. The remaining normal neurons in the ischemic ventral spinal cord in each animal, judged by their morphological appearance, were counted in three sections selected randomly from the rostral, middle, and caudal levels of the L5 segment and then averaged. The numbers of normal neurons per section were compared among these three groups.
TUNEL assay
Sections were prepared as described for HE staining, and the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was performed according to the manufacturer’s instructions. The results were observed under a Leica photograph microscope. The number of apoptotic cells in five unfolded continuous fields in each section was counted by the same observer, who was blinded to the protocol. The total number of apoptotic cells was expressed as the average percentage of TUNEL positive cells.
Biochemical analysis
Five rabbits from each group were reanesthetized with pentobarbital sodium (40 mg/kg) 48 h after I/R injury. The lumbar spinal cord was immediately removed, washed twice with cold saline solution, placed into glass bottles, labeled and stored at -70°C until processing (maximum 10 h). Spinal cord tissue samples were cut into small pieces, weighed, and homogenized in ice-cold Tris-HCl buffer (50 mmol, pH 7.4). The homogenate was then centrifuged at 5000 g for 30 min to remove debris. For further extraction, the supernatant solution was extracted with an equal volume of an ethanol/chloroform mixture (5/3, volume per volume [v/v]). After the second centrifugation at 5000 g for 60 min, MDA levels were determined as we previously described [4]. Briefly, MDA was reacted with thiobarbituric acid by incubating for 1 h at 95-100°C. Following the reaction, fluorescence intensity was measured in the n-butanol phase with a fluorescence spectrophotometry (Hitachi, Model F-4010, Japan), and by comparing with a standard solution of 1, 1, 3, 3 tetramethoxypropane, results were expressed in terms of nmol/g wet tissue.
Total (Cu-Zn and Mn) SOD activity was measured by reduction of nitrobluetetrazolium (NBT) and xanthine-xanthine oxidase system. Enzyme activity leading to 50% inhibition was accepted as one unit. Results were expressed as U/mg protein. Protein concentrations were determined according to Lowry’s method.
Statistical analysis
Statistical analysis was performed using SPSS 10.0 software. Physiological parameters, MDA levels, SOD activity, and apoptotic levels were performed with a one-way analysis of variance followed by least significant difference test. Neurological scores were analyzed with non-parametric method (Kruskal-Wallis test) followed by the Mann-Whitney U test with Bonferroni correction. Data were expressed as mean ± SD and P < 0.05 was considered as statistically significant.
Results
Physical parameters
The hemodynamics, rectal temperature, arterial pH, PaCO2, and PaO2 were no difference in all three groups at any time point (data not shown). Before blocking the abdominal aorta, the distal blood pressure was about 75 to 85 mmHg and decreased to 15 to 20 mmHg during the period of ischemia.
Neurological function evaluation
Neurological evaluation was presented in Table 1. There were no neurological abnormalities at the 48th hour after reperfusion in sham group. The scores in both I/R group and Lipoxin A4 group were lower than those in sham group. While the neurological outcomes in Lipoxin A4 group exhibited better than those in I/R group at the 48th hour after reperfusion (P < 0.05).
Table 1.
Neurological scores of rabbits 48 h after reperfusion
| Groups | N | Motor score | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 0 | 1 | 2 | 3 | 4 | ||
| Sham Group | 15 | 0 | 0 | 0 | 0 | 15 |
| I/R Group | 15 | 6 | 6 | 3 | 0 | 0 |
| Lipoxin A4 Group | 15 | 4 | 6 | 4 | 1 | 0 |
Neurological scores were analyzed with non-parametric method (Kruskal-Wallis test) followed by the Mann-Whitney U test with Bonferroni correction. Compared with the scores in Sham group, those in both I/Rgroup and Lipoxin A4 group were significantly lower (P < 0.05). While compared with I/R group, Lipoxin A4 treatment improved the rabbits’ neurological outcomes (P < 0.05).
Histological study
Representative histopathological photographs of sections are shown in sham group which showed no signs of histopathological abnormalities (Figure 1A). In contrast, the image from I/R group exhibited necrotic changes with pronounced vacuolization, intensely eosinophilic cytoplasm, Nissl granule loss, and pyknosis (Figure 1B). Representative images from Lipoxin A4 group exhibited mild destruction with significantly more normal neurons (Figure 1C).
Figure 1.

Micrographs of Spinal cord histopathology at the 48th hour reperfusion. Histological changes of spinal cord segment stained with HE after Lipoxin A4 treatment. A. Sham group: No abnormalities were seen; B. I/R group: significant necrotic changes with pronounced vacuolization, intensely eosinophilic cytoplasm, loss of Nissl granule, and pyknosis; C. Lipoxin A4 group: mild destruction with significantly more normal neurons (magnification, × 400).
As shown in Figure 2, TUNEL staining identified a few dead cells in the cord sections of sham-operated animals. In the spinal cords of I/R group, numerous cells were strongly positive for TUNEL staining. However, in Lipoxin A4 treated group, some cells were positive for TUNEL staining but less than those in I/R group. Using quantitative analysis, both positive and negative cells for TUNEL staining were counted and results were also shown in Figure 2. It was showed that Lipoxin A4 treated group has much lower positive cells than those in I/R group (P < 0.05), which suggested Lipoxin A4 may protect spinal cord from I/R injury through its antiapoptosis activity.
Figure 2.
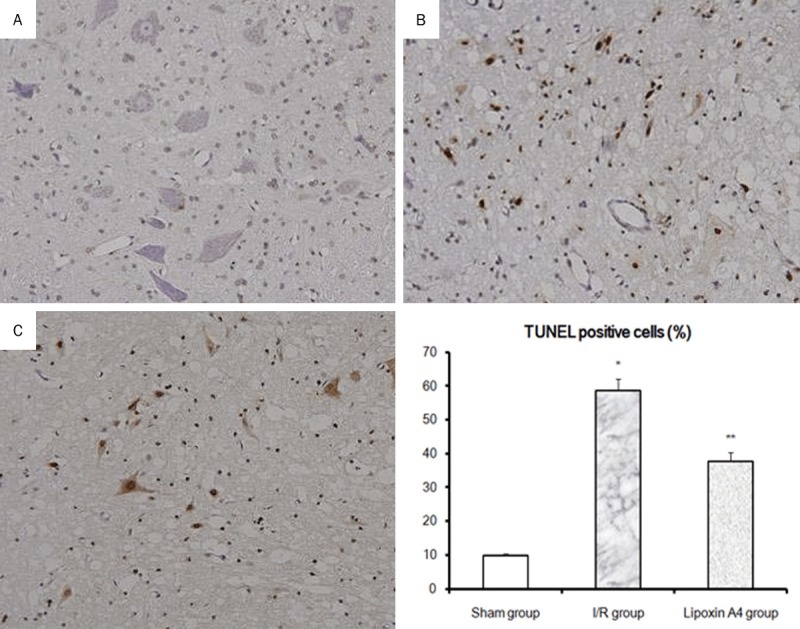
Effects of Lipoxin A4 on apoptosis in spinal cord after 48 hours reperfusion. TUNEL staining was performed to detect the cell apoptosis in spinal cord. A. Sham group; B. I/R group; C. Lipoxin A4 group. Cell apoptosis rate is expressed as the mean ± S.D. from three experiments. *P < 0.01, vs sham group; **P < 0.01, vs I/R group.
Biochemical analysis
Compared with sham group, SOD activities were significantly decreased in I/R group rabbits (P < 0.01). While after Lipoxin A4 treatment, although the SOD activities were still lower than those in sham group, they were significantly higher than those in I/R group (Figure 3). Moreover, MDA Levels were also measured after 48 hour reperfusion. As shown in Figure 4, significantly higher MDA levels were found in the I/R group compared with those in the sham group (P < 0.01). Similar to those in SOD activities, Lipoxin A4 treatment, although failed to recover thoroughly, it indeed reduced the local MDA levels. Taken together, Lipoxin A4 protected spinal cord from I/R induced oxidative stress as shown by decreased MDA levels and increased SOD activities.
Figure 3.
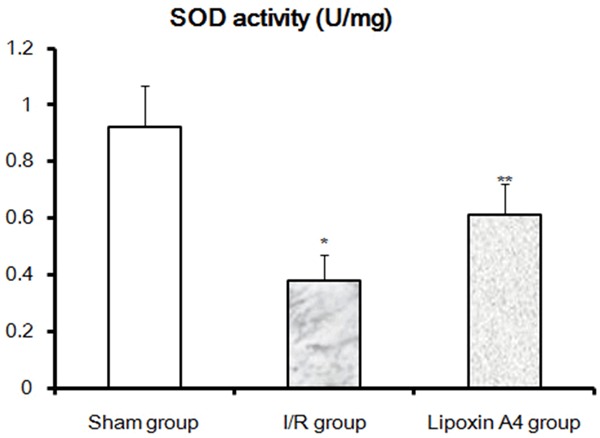
Effects of Lipoxin A4 on SOD Activities at the 48th hour reperfusion. SOD activities in spinal cord of different groups were performed as described in methods. *P < 0.01, vs sham group; **P < 0.01, vs I/R group.
Figure 4.
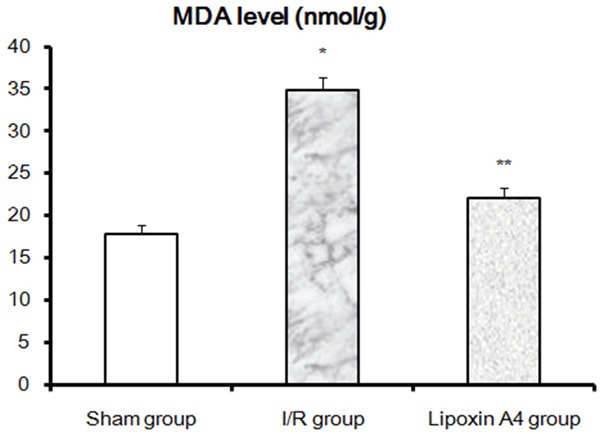
Effects of Lipoxin A4 on MDA Levels at the 48th hour reperfusion. Spinal cord MDA levels in all three groups were determined as described in methods. *P < 0.01, vs sham group; **P < 0.01, vs I/R group.
Discussion
The current study demonstrated that treatment of Lipoxin A4 showed a neuroprotective effect in a rabbit model with spinal cord I/R injury, accompanied by an increase in activity of the major antioxidant enzyme SOD, and inhibition of apoptosis in local spinal cord tissue, as well as a decrease of the major oxidative damage marker MDA.
Paraplegia, a major unpredictable complication after surgical repair of thoracoabdominal aortic aneurysms, is thought to be mainly related to the ischemia/reperfusion injury of the spinal cord [1]. Although great progress has been made in the basic research about the mechanism of spinal cord I/R injury, symptomatic treatment still remains as the primary therapeutic plan. Lipoxins, the most recent addition to the family of bioactive products generated from arachidonic acid [6,7], have been gained more attention as a therapeutic agent for a variety of diseases, especially for I/R related injury in multiple organs [8-11]. For example, just recently, Wu et al. reported that Lipoxin A4 effectively reduced brain infarct volumes and improved neurological scores in a rat model with cerebral ischaemia/reperfusion injury [13]. And their results also showed Lipoxin A4 can induce nuclear factor erythroid 2-related factor 2 expression and its nuclear translocation, as well as haeme oxygenase-1 and glutathione expression to combat increased oxidative stress [13]. However, there has been little research about their effect on I/R injury in spinal cord. In this study, we found that Lipoxin A4 treatment significantly improved I/R injured neurological function. Furthermore, as noted, numerous dead cells were observed in the spinal cords of the I/R model animals, while the total number of TUNEL positive cells was significantly reduced in the Lipoxin A4 group, which suggested that Lipoxin A4 can further provide neuronal protection through antiapoptotic mechanisms to prevent cell apoptosis and improve physical and neurological function. Meanwhile, during ischemia, a burst of ROS production may lead to tissue damage and even subsequent cell death [14]. Thus, oxidative stress, caused by imbalance between ROS production and scavenging, plays an important role in the spinal cord injury induced by I/R [15]. To determine the local oxidant/antioxidant levels, we further measured the MDA and SOD activities in spinal cord from all three groups. Our results showed that Lipoxin A4 treatment significantly reduced MDA levels and improved SOD activities in local spinal cord after I/R. Since both MDA (the product of peroxidation of lipids) and SOD (an oxygen radical scavenger) are often used to evaluate the extent of oxidative stress, our results provided new evidence that Lipoxin A4 can also significantly decrease I/R induced oxidative stress in spinal cords. Our results suggest that Lipoxin A4 may protect against neuronal injury and has a potential clinical benefit in the future.
One should be noted that, in this study, we only detected the neuroprotective effect of Lipoxin A4 postconditioning. As we all known, after the concept of attenuating reperfusion injury was introduced, ischemic preconditioning was first proposed in 1986 [16]. However, although ischemic preconditioning showed beneficial effects in enhancing the tolerance against ischemia in many organs including spinal cord, mainly due to the unpredictable onset of ischemia and potential risk of ischemic preconditioning implementation to patients, it is unfeasible in clinical situations. Thus, researchers paid more attention to the novel approach termed postconditioning. In myocardial I/R injury, postconditioning was firstly proven as effective as preconditioning in reducing infarct size and preserving endothelial function [17]. And shortly after that, postconditioning has been successfully used in clinical procedures [18]. Ischemic postconditioning also showed attractive effect in protecting the spinal cord from I/R injury. For example, just currently, Song and coauthors showed ischemic postconditioning significantly preserved locomotor function and decreased the paraplegia rate in rabbits subjected to 20 minutes of spinal cord ischemia, and the authors further proved that antioxidant mechanism is associated with the mechanism of production of neuroprotection of ischemic postconditioning [19]. In this study, our results firstly showed a neuroprotective effect of Lipoxin A4 postconditioning on spinal cord I/R injury in rabbits, and at least partially, this effect depends on its antioxidant capacity. One limitation is that we did not compare the different effects of Lipoxin A4 preconditioning and postconditioning. However, one recent study showed both Lipoxin A4 preconditioning and postconditioning can protect the ultrastructure of the myocardium by inhibiting the inflammatory reaction and oxidative stress in a rat model with myocardial I/R injury [20]. And most interestingly, their results also showed that Lipoxin A4 postconditioning had more effective than Lipoxin A4 preconditioning in enhancing SOD activity and inhibition of MDA levels, indicating that Lipoxin A4 postconditioning had better antioxidant activity than Lipoxin A4 preconditioning [20].
Taken together, our studies provide evidence that Lipoxin A4, at least in an animal model, can reduce MDA levels and restore SOD activity, as well as inhibition of cell apoptosis in spinal cord. And the neuroprotective effects of Lipoxin A4 in spinal cord I/R injury may due to its antioxidant and antiapoptosis capacities, which may provide a novel approach in the treatment of spinal cord I/R injury. Further studies are required to examine the safety and therapeutic potency of Lipoxin A4 in patients, especially those with high risk of I/R injury.
Acknowledgements
This work was funded by the Science and Technology Bureau Funded Projects (to L. Z.), Natural Science Foundation (No. ZR2009CQ023) and Medical Science Development Plan (No. 2009QZ025) of Shandong Province (both to Z. W.).
Disclosure of conflict of interest
None.
References
- 1.Etz DC, Luehr M, Aspern KV, Misfeld M, Gudehus S, Ender J, Koelbel T, Debus ES, Mohr FW. Spinal cord ischemia in open and endovascular thoracoabdominal aortic aneurysm repair: new concepts. J Cardiovasc Surg (Torino) 2014;55:159–168. [PubMed] [Google Scholar]
- 2.Sahin MA, Onan B, Guler A, Oztas E, Uysal B, Arslan S, Demirkilic U, Tatar H. Cilostazol, a type III phosphodiesterase inhibitor, reduces ischemia/reperfusion-induced spinal cord injury. Heart Surg Forum. 2011;14:E171–7. doi: 10.1532/HSF98.20101126. [DOI] [PubMed] [Google Scholar]
- 3.Ucak A, Onan B, Güler A, Sahin MA, Kılıçkaya O, Oztaş E, Uysal B, Arslan S, Yılmaz AT. Rosuvastatin, a new generation 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor, reduces ischemia/reperfusion-induced spinal cord tissue injury in rats. Ann Vasc Surg. 2011;25:686–695. doi: 10.1016/j.avsg.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 4.Liu ZQ, Xing SS, Zhang W. Neuroprotective effect of curcumin on spinal cord in rabbit model with ischemia/reperfusion. J Spinal Cord Med. 2013;36:147–152. doi: 10.1179/2045772312Y.0000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reece TB, Okonkwo DO, Ellman PI, Warren PS, Smith RL, Hawkins AS, Linden J, Kron IL, Tribble CG, Kern JA. The evolution of ischemic spinal cord injury in function, cytoarchitecture, and inflammation and the effects of adenosine A2A receptor activation. J Thorac Cardiovasc Surg. 2004;128:925–932. doi: 10.1016/j.jtcvs.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Souza DG, Fagundes CT, Amaral FA, Cisalpino D, Sousa LP, Vieira AT, Pinho V, Nicoli JR, Vieira LQ, Fierro IM, Teixeira MM. The required role of endogenously produced lipoxin A4 and annexin-1 for the production of IL-10 and inflammatory hyporesponsiveness in mice. J Immunol. 2007;179:8533–8543. doi: 10.4049/jimmunol.179.12.8533. [DOI] [PubMed] [Google Scholar]
- 7.Bannenberg G, Moussignac RL, Gronert K, Devchand PR, Schmidt BA, Guilford WJ, Bauman JG, Subramanyam B, Perez HD, Parkinson JF, Serhan CN. Lipoxins and novel 15-epi-lipoxin analogs display potent anti-inflammatory actions after oral administration. Br J Pharmacol. 2004;143:43–52. doi: 10.1038/sj.bjp.0705912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Z, Wu Z, Huang C, Zhao Y, Zhou Y, Zhou X, Lu X, Mao L, Li S. Effect of lipoxin A4 on myocardial ischemia reperfusion injury following cardiac arrest in a rabbit model. Inflammation. 2013;36:468–475. doi: 10.1007/s10753-012-9567-x. [DOI] [PubMed] [Google Scholar]
- 9.Wu Y, Wang YP, Guo P, Ye XH, Wang J, Yuan SY, Yao SL, Shang Y. A lipoxin A4 analog ameliorates blood-brain barrier dysfunction and reduces MMP-9 expression in a rat model of focal cerebral ischemia-reperfusion injury. J Mol Neurosci. 2012;46:483–491. doi: 10.1007/s12031-011-9620-5. [DOI] [PubMed] [Google Scholar]
- 10.Scully M, Gang C, Condron C, Bouchier-Hayes D, Cunningham AJ. Protective role of cyclooxygenase (COX)-2 in experimental lung injury: evidence of a lipoxin A4-mediated effect. J Surg Res. 2012;175:176–184. doi: 10.1016/j.jss.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Kieran NE, Doran PP, Connolly SB, Greenan MC, Higgins DF, Leonard M, Godson C, Taylor CT, Henger A, Kretzler M, Burne MJ, Rabb H, Brady HR. Modification of the transcriptomic response to renal ischemia/reperfusion injury by lipoxin analog. Kidney Int. 2003;64:480–492. doi: 10.1046/j.1523-1755.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 12.Shi E, Jiang X, Wang L, Akuzawa S, Nakajima Y, Kazui T. Intrathecal injection of hepatocyte growth factor gene-modified marrow stromal cells attenuates neurologic injury induced by transient spinal cord ischemia in rabbits. Anesthesiology. 2010;113:1109–1117. doi: 10.1097/ALN.0b013e3181f6970d. [DOI] [PubMed] [Google Scholar]
- 13.Wu L, Liu ZJ, Miao S, Zou LB, Cai L, Wu P, Ye du Y, Wu Q, Li HH. Lipoxin A4 ameliorates cerebral ischaemia/reperfusion injury through upregulation of nuclear factor erythroid 2-related factor 2. Neurol Res. 2013;35:968–975. doi: 10.1179/1743132813Y.0000000242. [DOI] [PubMed] [Google Scholar]
- 14.Liu PK. Ischemia-reperfusion-related repair deficit after oxidative stress: implications of faulty transcripts in neuronal sensitivity after brain injury. J Biomed Sci. 2003;10:4–13. doi: 10.1159/000068080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong HL, Zhang Y, Su BX, Zhu ZH, Gu QH, Sang HF, Xiong L. Limb remote ischemic preconditioning protects the spinal cord from ischemia-reperfusion injury: a newly identified nonneuronal but reactive oxygen species-dependent pathway. Anesthesiology. 2010;112:881–891. doi: 10.1097/ALN.0b013e3181d0486d. [DOI] [PubMed] [Google Scholar]
- 16.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 17.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 18.Staat P, Rioufol G, Piot C, Cottin Y, Cung TT, L’Huillier I, Aupetit JF, Bonnefoy E, Finet G, André-Fouët X, Ovize M. Postconditioning the human heart. Circulation. 2005;112:2143–2148. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- 19.Song W, Sun J, Su B, Yang R, Dong H, Xiong L. Ischemic postconditioning protects the spinal cord from ischemia-reperfusion injury via modulation of redox signaling. J Thorac Cardiovasc Surg. 2013;146:688–695. doi: 10.1016/j.jtcvs.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Q, Shao L, Hu X, Wu G, Du J, Xia J, Qiu H. Lipoxin a4 preconditioning and postconditioning protect myocardial ischemia/reperfusion injury in rats. Mediators Inflamm. 2013;2013:231351. doi: 10.1155/2013/231351. [DOI] [PMC free article] [PubMed] [Google Scholar]


