Abstract
Patients with esophageal cancer are often diagnosed at advanced stages, leading to poor prognosis. Biomarkers are needed to enable earlier detection as well as to aid in the prediction of prognosis, but to date these tools remain scarce. Thymidine kinase (TK1) has been shown to exhibit altered expression levels in esophageal tumor cells, therefore this study sought to determine whether serum TK1 levels are also altered and, if so, to assess the utility of TK1 as a biomarker in esophageal squamous cell carcinoma. Eighty patients with esophageal squamous cell carcinoma were included as the case group and 80 healthy persons were selected as the control group. Serum TK1 levels, postoperatively for cancer patients, were detected by chemiluminescence. Follow-up was performed for cancer patients to determine the progression free survival (PFS) and overall survival (OS). Serum TK1 levels were significantly higher in cases of esophageal cancer than in healthy control individuals (t=7.235, P<0.05). When cancer cases were sub-divided into lower and higher serum TK1 levels, based on the mean level of 3.38 pmol/L, statistically significant differences in TNM stage, tumor differentiation, and lymph node metastasis were observed between patients with ≥3.38 pmol/L and <3.38 pmol/L (χ2=28.134, 3.187, 7.234, P<0.05). The average OS of all esophageal cancer patients was 30.13 months, and the average PFS was 24.73 months. However, when the cases were divided by serum TK1 level, average OS of those with higher serum TK1 (≥3.38 pmol/L) was significantly lower (23.98 mo) than those with lower serum TK1 (32.96 mo) (χ2=5.439, P<0.05). Similarly, average PFS was significantly lower in patients with higher serum TK1 (17.65 mo versus 27.62) (χ2=4.640, P<0.05). OS was correlated with TNM stage (hazard ratio, HR=3.116), degree of tumor differentiation (HR=0.427), lymph node metastasis (HR=0.535), and serum TK1 level (HR=1.913) (Wald χ2=6.782, 6.228, 4.562, 5.681, P<0.05). Similarly, PFS was correlated with TMN stage (HR=2.153), degree of tumor differentiation (HR=0.627), and serum TK1 level (HR=1.632) (Wald χ2=7.035, 5.335, 4.887, P<0.05). Thus, patients with esophageal squamous cell carcinoma exhibit higher circulating TK1 levels, consistent with findings of increased TK1 expression in tumor cells. Further, the correlation of serum TK1 levels with clinical features of esophageal cancer and with patient survival suggest that serum TK1 may serve as a valuable biomarker for predicting patient prognosis.
Keywords: Thymidine kinase 1, esophageal squamous cell carcinoma, prognosis, survival analysis, serological detection
Introduction
Esophageal cancer is a common malignancy of the digestive system that is clinically characterized by strong aggressiveness and high lethality. This cancer ranks ninth in malignant diseases worldwide and is eighth in China [1]. Indeed, the incidence of this malignancy is so high in China that the country’s cases of esophageal cancer account for more than half of the total cases worldwide [2]. Although recent advances have resulted in improved diagnostic and treatment approaches for esophageal cancer, the survival rate remains low and patient prognosis is often poor. Therefore, continued progress in diagnosis and treatment is needed to improve patient outcomes.
Most esophageal cancers are adenocarcinoma and squamous cell carcinoma histotypes. Esophageal adenocarcinoma is mostly found in North America and Western Europe; esophageal squamous cell carcinoma is mainly found in China (90% of patients with esophageal cancer in China) [3-5]. The significant difference in distribution of histotypes highlights the contribution of genetic factors to disease pathology. Indeed, esophageal cancer is a multi-factorial disease, and dietary habits, carcinogens, illnesses, and genetic factors all influence an individual’s risk of developing it [6]. Esophageal squamous cell carcinoma is characterized by invisible symptoms, a low rate of early diagnosis, strong metastatic potential, and rapid disease progression; most patients progress to middle-and late-stage disease before they are definitely diagnosed; this late diagnosis often leads to poor prognosis.
Efforts to identify and utilize molecular markers for esophageal cancer have generally relied on immunohistochemistry and radioimmunoassays, though newer technologies combine proteomics with bioinformatics. However, few markers identified to date have clinical significance as diagnostic or prognostic indicators for esophageal cancer [7]. In particular, serum markers with the potential for clinical prognostic application are scarce [8]. As in most tumors, genetic factors play an important role in esophageal cancer etiology and may produce significant effects on disease progression in patients. The main genetic manifestations of patients are activation of proto-oncogenes and inactivation of tumor suppressor genes, as well as abnormalities of apoptosis-related genes and metastasis suppressor genes [9]. Genes associated with esophageal cancer encode proteins functioning in diverse pathways including carcinogen metabolism (GST, CYP, NAT2, ADH2, and ALDH2), DNA damage repair (MGMT), and cell cycle regulation (MTS1, cyclinD1, Rb, and p53), as well as other pathways (FzE3, FHIT, DMBT1, and FEZ1) [10].
Another potential player in esophageal cancer is thymidine kinase (TK), which acts in the pyrimidine nucleotide salvage pathway as well as in DNA synthesis [11,12]. TK works with ATP and Mg2+ to catalyze the conversion of deoxythymidine to dTMP. In humans, TK occurs as two isoenzymes, the cytoplasmic thymidine kinase (TK1) and the mitochondrial thymidine kinase (TK2) [13]. Both TKs are implicated in disease, including tumors, in humans and animals [14]. During DNA replication, TK1 expression is lower during the G1 phase of the cell cycle, rises gradually in the S phase, and reaches its peak in the G2 phase [15]. Tumor cells display higher TK1 expression; its expression is correlated with tumor proliferation, changes, progression, and staging [16].
Indeed, TK1 is considered a novel cell proliferation marker with potential application for early tumor detection, assessment of treatment efficacy, and prognosis [17]. Some evidence suggests that it may be applicable in esophageal cancer [18], but the evidence was based on detection of TK1 in tumor cells, thus limiting its utility as a biomarker. The current study sought to determine the value of detecting thymidine kinase 1 (TK1) levels in serum as a prognostic indicator in patients with esophageal squamous cell carcinoma.
Methods
Participants
The study prospectively assessed patients with esophageal squamous cell carcinoma who were admitted to Wuxi People’s Hospital Affiliated to Nanjing Medical University between January and December 2012. All patients had received radical surgery for esophageal cancer and a postoperative pathological examination confirmed each case as esophageal squamous cell carcinoma. No patients received radiochemotherapy before surgery. Patients with concurrent tumors in other regions were excluded, as were those with concurrent diseases including breast hyperplasia, prostatic hyperplasia, fatty liver, infectious diseases, and hemorrhagic trauma were ruled out. Other exclusion criteria included an inability to receive surgical therapy or palliative surgery and a presence of postoperative complications including anastomotic fistula, pleural effusion, and infections. Of the 80 included patients, 55 were males and 25 cases were females. The mean age was 56.4±8.3 years (range: 39 to 91 years). Additionally, 35 cases had a history of smoking and 13 cases had a family history of tumors. Tumor staging indicated that 14 cases were stage I, 38 were stage II, 23 were stage III, and 5 were stage IV. Further, 25 cases had poorly differentiated (G3) squamous cell carcinoma, 37 cases had moderately differentiated (G2) squamous cell carcinoma, and 18 cases had well differentiated (G1) squamous cell carcinoma. There were 29 cases with lymph node metastases. All participants provided informed consent. This study was approved by the Ethics Committee of Wuxi People’s Hospital Affiliated to Nanjing Medical University, Wuxi, Jiangsu Province, China.
The control group comprised 80 healthy persons who received physical examinations in our hospital during the same period. Each individual in the control group had esophageal cancer ruled out. All other exclusion criteria were the same as for the case group. The distributions of sex and age in the control group were similar to those of the case group (P>0.05). Specifically, 53 control individuals were males and 27 were females, and the mean age was 54.6±7.6 years (range: 37 to 78 years). Further, 32 had a history of smoking and 10 cases had a family history of tumors. Smoking and family history of tumors also did not differ from the case group (P>0.05).
TK1 measurement
Fasting peripheral venous blood samples were collected in the morning from both groups. In the case group, blood was collected on the second day after admission, and the female patients were required to not in menstrual periods. In the control group, blood was collected on the day of physical examination. Samples were centrifuged at 3000 rpm for 10 min to separate the serum, and serum was transferred to EP tubes and stored at -20°C. Before detection, serum was defrosted at 2-8°C. TK1 levels in serum samples were quantitatively detected using chemiluminescent enzyme immunoassay (Baoman Biological Technology Co., Ltd, Shanghai, China). In the case group, postoperative follow-up was performed by telephone calls in combination with visits. Comparisons were performed for the progression-free survival (PFS) and overall survival (OS) of the patients.
Statistical methods
Data analysis was performed using SPSS 13.0. Measurement data are expressed as mean ± standard deviation. The two groups were compared using the independent samples t-test. Numerical data without hierarchical relationships were compared using the chi-square test, while numerical data with hierarchical relationships were compared using the rank-sum test (Mann-Whitney U method). The OS and PFS were compared using Kaplan-Meier survival analysis. Differences in survival were tested with a log-rank test. Factors related to the OS and PFS were analyzed by using the multivariate Cox regression analysis. In all above statistical tests, P<0.05 was considered to indicate that a difference was statistically significant.
Results
Serum TK1 levels in esophageal cancer
To determine whether circulating TK1 levels are higher in patients with esophageal cancer, similar to the increased TK1 reported for tumor cells, serum TK1 was compared between individuals with and without esophageal squamous cell carcinoma. Serum TK1 was significantly higher in the case group than in the control group (t=7.235, P<0.05; see Table 1).
Table 1.
Serum TK1 levels in individuals with and without esophageal cancer
| Group | N | TK1 (pmol/L) |
|---|---|---|
| Case group | 80 | 3.38±3.05 |
| Control group | 80 | 1.41±0.32 |
| t value | 7.235 | |
| P value | <0.05 |
Correlation between serum TK1 and clinical features of esophageal cancer
In patients with esophageal cancer, 3.38 pmol/L-the mean value of the serum TK1 levels-was used as a critical value; the group was then sub-divided into 42 cases with TK1 ≥3.38 pmol/L and 38 cases with TK1 <3.38 pmol/L. These sub-groups were not statistically different in age, sex, history of smoking, or family history of tumors (χ2=0.002-1.054, P>0.05). In contrast, the sub-groups were statistically different regarding the TNM classification, the degree of tumor differentiation, and the status of lymph node metastasis (χ2=28.134, 3.187, 7.234; P<0.05; Table 2).
Table 2.
Clinical characteristics and serum TK1 levels in patients with esophageal squamous cell carcinoma
| Characteristic | N | TK1 (pmol/L) | χ2/U | P | ||
|---|---|---|---|---|---|---|
|
| ||||||
| ≥3.38 (N=42) | <3.38 (N=38) | |||||
| Gender | Male | 55 | 31 (56.4) | 24 (43.6) | 1.054 | >0.05 |
| Female | 25 | 11 (44.0) | 14 (56.0) | |||
| Age (years) | <55 | 36 | 19 (52.8) | 17 (47.2) | 0.002 | >0.05 |
| ≥55 | 44 | 23 (52.3) | 21 (47.7) | |||
| Smoking history | Ever | 35 | 19 (54.3) | 16 (45.7) | 0.080 | >0.05 |
| Never | 45 | 23 (51.1) | 22 (48.9) | |||
| TNM staging | I~II | 52 | 16 (30.8) | 36 (69.2) | 28.134 | <0.05 |
| III~IV | 28 | 26 (92.9) | 2 (7.1) | |||
| Family history of carcinoma | Yes | 13 | 8 (61.5) | 5 (38.5) | 0.509 | >0.05 |
| No | 67 | 34 (50.7) | 33 (49.3) | |||
| Degree of tumor differentiation | G3 | 25 | 22 (88.0) | 3 (12.0) | 3.187 | <0.05 |
| G2 | 37 | 16 (43.2) | 21 (56.8) | |||
| G1 | 18 | 4 (22.2) | 14 (77.8) | |||
| Lymph node metastases | Yes | 29 | 21 (72.4) | 8 (27.6) | 7.234 | <0.05 |
| No | 51 | 21 (41.2) | 30 (58.8) | |||
Postoperative survival and serum TK1 levels
The mean follow-up period for patients with esophageal cancer was 26 months (range: 5 to 36 months), and the follow-up ended on Dec 30, 2014. The mean OS of patients was 30.129 months (95% confidence interval: 27.793-32.465); the Kaplan-Meier curve for OS is shown in Figure 1. The mean PFS of patients with esophageal cancer was expected to be 24.732 months (95% confidence interval: 21.691-27.773); the Kaplan-Meier curve for PFS is shown in Figure 2. The mean OS of the sub-group of patients with TK1 ≥3.38 pmol/L was expected to be 23.977 months, while for the sub-group of patients with TK1 <3.38 pmol/L it was 32.963 months (95% confidence interval: 21.439-26.514 and 30.428-35.497, respectively); this difference is statistically significant (χ2=5.439, P<0.05). The Kaplan-Meier curves for OS of the two sub-groups are shown in Figure 3. The mean PFS of the sub-group with TK1 ≥3.38 pmol/L was expected to be 17.646 months, while for the sub-group with TK1 <3.38 pmol/L it was 27.622 months (95% confidence interval: 14.488-20.803 and 23.944-31.299, respectively); this difference is statistically significant (χ2=4.640, P<0.05). The Kaplan-Meier curves for PFS of the two sub-groups are shown in Figure 4.
Figure 1.
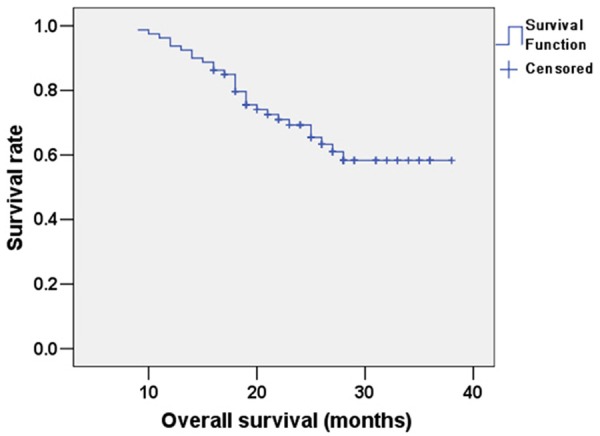
Kaplan-Meier survival analysis curve for OS of patients with esophageal cancer.
Figure 2.
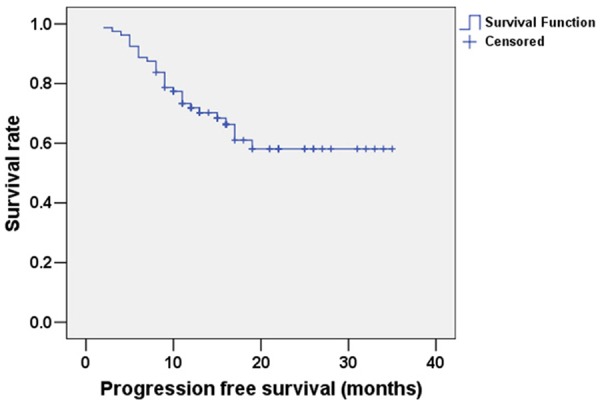
Kaplan-Meier survival analysis curve for PFS of patients with esophageal cancer.
Figure 3.
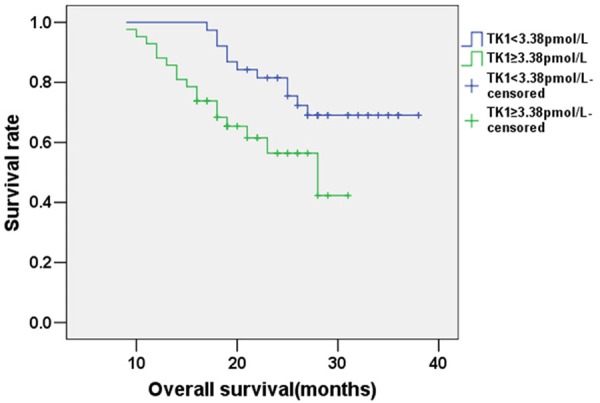
Kaplan-Meier survival analysis curve for OS of patients with esophageal cancer sub-divided by serum TK1 level.
Figure 4.
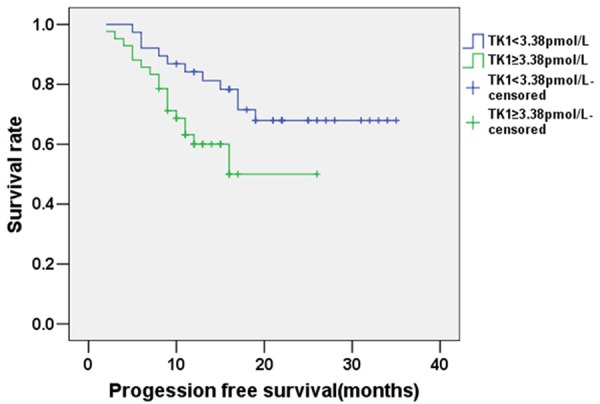
Kaplan-Meier survival analysis curve for PFS of the patients with esophageal cancer sub-divided by serum TK1 level.
Analysis of related factors that influenced the postoperative survivals of the patients with esophageal squamous cell carcinoma
The results of the multivariate Cox regression analysis showed that the OS of the case group was correlated with the TNM classification [hazard ration (HR)=3.116], the degree of tumor differentiation (HR=0.427) , the status of lymph node metastasis (HR=0.535), and the serum TK1 level (HR=1.913) (Wald χ2=6.782, 6.228, 4.562, 5.681; P<0.05; Table 3). The PFS was correlated with the TNM classification (HR=2.153), the degree of tumor differentiation (HR=0.627), and the serum TK1 level (HR=1.632) (Wald χ2=7.035, 5.335, 4.887; P<0.05; Table 4).
Table 3.
Cox multivariate analysis of OS of patients with esophageal cancer
| Variable | Reference | HR | Wald χ2 | P |
|---|---|---|---|---|
| Gender | Male | 1.635 | 1.023 | >0.05 |
| Age | <55 | 1.835 | 2.145 | >0.05 |
| Smoking history | Never | 0.973 | 0.547 | >0.05 |
| TNM staging | I~II | 3.116 | 6.782 | <0.05 |
| Family history of carcinoma | Yes | 1.307 | 1.692 | >0.05 |
| Degree of tumor differentiation | G3 | 0.427 | 6.228 | <0.05 |
| Lobe metastases | Yes | 0.535 | 4.562 | <0.05 |
| Serum TK1 level | <3.38 pmol/L | 1.913 | 5.681 | <0.05 |
Table 4.
Cox multivariate analysis on PFS of the patients with esophageal cancer
| Variable | Reference | HR | Wald χ2 | P |
|---|---|---|---|---|
| Gender | Male | 1.152 | 0.765 | >0.05 |
| Age | <55 | 1.638 | 1.369 | >0.05 |
| Smoking history | Never | 1.337 | 0.923 | >0.05 |
| TNM staging | I~II | 2.153 | 7.035 | <0.05 |
| Family history of carcinoma | Yes | 1.381 | 1.755 | >0.05 |
| Degree of tumor differentiation | G3 | 0.627 | 5.335 | <0.05 |
| Lobes metastases | Yes | 0.774 | 2.151 | >0.05 |
| Serum TK1 level | <3.38 pmol/L | 1.632 | 4.887 | <0.05 |
Discussion
Serum TK1 level can be used for early diagnosis of multiple malignant tumors including prostate cancer, bladder cancer, kidney cancer, gastric cancer, liver cancer, and non-small cell lung cancer [19]. The results of this study, in which serum TK1 was significantly higher in individuals with esophageal cancer than in healthy individuals, suggest that serum TK1 may serve as a clinically useful indicator in esophageal cancer as well. Indeed, patients with higher levels of serum TK1 had significant differences in TNM classification, degree of tumor differentiation, and lymph node metastasis than patients with lower serum TK1. Thus, patients with esophageal squamous cell carcinoma exhibit a significant rise in the serum TK1 level and the level correlates with certain clinical features of the disease.
The development of anti-human TK1 monoclonal antibodies and polyclonal antibodies has made it possible to detect traces of TK1 in the serum, using sensitive enhanced chemiluminescence and dot blot methods. Serum TK1 is being widely used for judging the prognosis of various solid tumors and hematological malignancies [20]. According to a serum detection of lung cancer, serum TK1 levels in patients with tumors fluctuated between less than 1 pmol/L and more than 40 pmol/L; in contrast, those in patients with benign diseases were mostly less than 10 pmol/L, and those in healthy populations were generally less than 2 pmol/L. Similarly, in a screen for serum TK1 levels in 26,055 persons who received physical examinations, using 2 pmol/L as a normal critical value enabled the detection of precancerous lesions or pathological changes related to tumorigenesis in over 60% of those with elevated serum TK1 levels [21]. Clinically, 2 pmol/L also serves as a positive critical value for detecting the serum TK1 level.
Survival analyses indicated that OS and PFS differed by several features, including TK1 levels. When patients were subdivided by TK1 level, those with higher TK1 in serum had lower OS and PFS. Thus, the prognosis of patients with esophageal squamous cell carcinoma appears to be related to serum TK1 levels; therefore, the serum TK1 level could serve as an auxiliary prognostic indicator. Further, OS was correlated with the TNM classification, the degree of tumor differentiation, the status of lymph node metastasis, and the serum TK1 level, while PFS was correlated with the TNM classification, the degree of tumor differentiation, and the serum TK1 level.
Compared with esophageal adenocarcinoma, esophageal squamous cell carcinoma has different biological characteristics and higher aggressiveness. Clinically, a comprehensive treatment approach by surgery in combination with radiotherapy and chemotherapy is usually used in patients with esophageal squamous cell carcinoma [22]. The prognosis of patients is closely related to the tumor classification. The 5-year OS of patients with esophageal squamous cell carcinoma is reportedly less than 20%; patients treated at early stages have OS of over 90%, but patients with mid- or late-stage esophageal cancer have OS of only approximately 10-15% [23]. Although prognosis is associated with age, red blood cell levels before treatment, number of lymph node metastases, clinical staging, degree of tumor invasion or differentiation, and surgical approaches [24], prognosis is generally assessed using traditional indexes like tumor staging and histopathological grading. However, the degree of tumor differentiation is often inconsistent with the prognosis, and the prognosis of patients with well differentiated tumors is not necessarily better than that of those with poorly differentiated tumors; thus, when changes in tumor morphology serve as the only basis for judging the prognosis of esophageal cancer, certain limitations exist [25]. Therefore, the identification and translation of multiple indexes will enable a more comprehensive analysis of the prognosis of patients with esophageal cancer that can fully and accurately reflect the condition of patients [26].
The results of this study suggests that serum TK1 level, as a serological marker that is sampled and measured rapidly, offers clinical utility for predicting the prognosis of patients with esophageal cancer. When combined with traditional indexes for assessing the prognosis, it will help improve the accuracy of the prediction.
Disclosure of conflict of interest
None.
References
- 1.Almhanna K, Hoffe S. Esophageal cancer 2015, more questions than answers. J Gastrointest Oncol. 2015;6:1–2. doi: 10.3978/j.issn.2078-6891.2014.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamrah MS, Hamrah MH, Rabi M, Wu HX, Hao CN, Harun-Or-Rashid M, Sakamoto J, Ishii H. Prevalence of esophageal cancer in the northern part of afghanistan. Asian Pac J Cancer Prev. 2014;15:10981–10984. doi: 10.7314/apjcp.2014.15.24.10981. [DOI] [PubMed] [Google Scholar]
- 3.Edgren G, Adami HO, Weiderpass E, Nyrén O. A global assessment of the oesophageal adenocarcinoma epidemic. Gut. 2013;62:1406–1414. doi: 10.1136/gutjnl-2012-302412. [DOI] [PubMed] [Google Scholar]
- 4.Zhang YW. Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19:5598–5606. doi: 10.3748/wjg.v19.i34.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt T, Lordick F, Herrmann K, Ott K. Value of Functional Imaging by PET in Esophageal Cancer. J Natl Compr Canc Netw. 2015;13:239–247. doi: 10.6004/jnccn.2015.0030. [DOI] [PubMed] [Google Scholar]
- 7.Chang H, Shi YH, Talaf TK, Lin C. Aquaporin-8 mediates human esophageal cancer Eca-109 cell migration via the EGFR-Erk1/2 pathway. Int J Clin Exp Pathol. 2014;7:7663–7671. [PMC free article] [PubMed] [Google Scholar]
- 8.Wu AJ, Goodman KA. Clinical tools to predict outcomes in patients with esophageal cancer treated with definitive chemoradiation: are we there yet? J Gastrointest Oncol. 2015;6:53–59. doi: 10.3978/j.issn.2078-6891.2014.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi AR, Chon NR, Youn YH, Paik HC, Kim YH, Park H. Esophageal cancer in esophageal diverticula associated with achalasia. Clin Endosc. 2015;48:70–73. doi: 10.5946/ce.2015.48.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreno Racionero F, de Andres Asenjo B, Bedate Nuñez M, Legido Moran P, Ortega Loubon C, Rabadán Jimenez J, Beltran de Heredía Y Rentería J. Unusual relationship between skin lesions and esophageal cancer: a case report and review of literature. Z Gastroenterol. 2015;53:115–119. doi: 10.1055/s-0034-1398791. [DOI] [PubMed] [Google Scholar]
- 11.Thamm DH, Kamstock DA, Sharp CR, Johnson SI, Mazzaferro E, Herold LV, Barnes SM, Winkler K, Selting KA. Elevated serum thymidine kinase activity in canine splenic hemangiosarcoma. Vet Comp Oncol. 2012;10:292–302. doi: 10.1111/j.1476-5829.2011.00298.x. [DOI] [PubMed] [Google Scholar]
- 12.Xu XH, Zhang YM, Shu XH, Shan LH, Wang ZW, Zhou YL, Wen HK, He F, He E, Skog S. Serum thymidine kinase 1 reflects the progression of premalignant and malignant tumors during therapy. Mol Med Rep. 2008;1:705–711. doi: 10.3892/mmr_00000017. [DOI] [PubMed] [Google Scholar]
- 13.Lagunoff M. KSHV-TK: thymidine kinase or tyrosine kinase? EMBO J. 2015;34:427–429. doi: 10.15252/embj.201490843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Topolcan O, Holubec L Jr. The role of thymidine kinase in cancer diseases. Expert Opin Med Diagn. 2008;2:129–141. doi: 10.1517/17530059.2.2.129. [DOI] [PubMed] [Google Scholar]
- 15.Gasparri F, Wang N, Skog S, Galvani A, Eriksson S. Thymidine kinase 1 expression defines an activated G1 state of the cell cycle as revealed with site-specific antibodies and ArrayScan assays. Eur J Cell Biol. 2009;88:779–785. doi: 10.1016/j.ejcb.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Jeong MH, Jin YH, Kang EY, Jo WS, Park HT, Lee JD, Yoo YJ, Jeong SJ. The modulation of radiation-induced cell death by genistein in K562 cells: activation of thymidine kinase 1. Cell Res. 2004;14:295–302. doi: 10.1038/sj.cr.7290230. [DOI] [PubMed] [Google Scholar]
- 17.Nisman B, Nechushtan H, Biran H, Gantz-Sorotsky H, Peled N, Gronowitz S, Peretz T. Serum thymidine kinase 1 activity in the prognosis and monitoring of chemotherapy in lung cancer patients: a brief report. J Thorac Oncol. 2014;9:1568–1572. doi: 10.1097/JTO.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 18.He E, Xu XH, Guan H, Chen Y, Chen ZH, Pan ZL, Tang LL, Hu GZ, Li Y, Zhang M, Zhou J, Eriksson S, Fornander T, Skog S. Thymidine kinase 1 is a potential marker for prognosis and monitoring the response to treatment of patients with breast, lung, and esophageal cancer and non-Hodgkin’s lymphoma. Nucleosides Nucleotides Nucleic Acids. 2010;29:352–358. doi: 10.1080/15257771003738535. [DOI] [PubMed] [Google Scholar]
- 19.Xu Y, Liu B, Shi QL, Huang PL, Zhou XJ, Ma HH, Lu ZF, Bo Y, Eriksson S, He E, Skog S. Thymidine kinase 1 is a better prognostic marker than Ki-67 for pT1 adenocarcinoma of the lung. Int J Clin Exp Med. 2014;7:2120–2128. [PMC free article] [PubMed] [Google Scholar]
- 20.Nakajo M, Nakajo M, Kajiya Y, Goto Y, Jinguji M, Tanaka S, Fukukura Y, Tani A, Higashi M. Correlations of (18) F-fluorothymidine uptake with pathological tumour size, Ki-67 and thymidine kinase 1 expressions in primary and metastatic lymph node colorectal cancer foci. Eur Radiol. 2014;24:3199–3209. doi: 10.1007/s00330-014-3379-3. [DOI] [PubMed] [Google Scholar]
- 21.Alegre MM, Weyant MJ, Bennett DT, Yu JA, Ramsden MK, Elnaggar A, Robison RA, O’Neill KL. Serum detection of thymidine kinase 1 as a means of early detection of lung cancer. Anticancer Res. 2014;34:2145–2151. [PubMed] [Google Scholar]
- 22.Vidović V, Nikolić I, Vukojević J, Samardžija G, Kukić B, Bogdanović B, Petrović N. Unusual metastasis of esophageal cancer. Vojnosanit Pregl. 2014;71:975–977. [PubMed] [Google Scholar]
- 23.Purwar P, Bambarkar S, Jiwnani S, Karimundackal G, Laskar SG, Pramesh CS. Multimodality management of esophageal cancer. Indian J Surg. 2014;76:494–503. doi: 10.1007/s12262-014-1163-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shapiro J, van Lanschot JJ, Hulshof MC, van der Gaast A. Effectiveness of neoadjuvant chemoradiotherapy for early-stage esophageal cancer. J. Clin. Oncol. 2015;33:288–289. doi: 10.1200/JCO.2014.59.2428. [DOI] [PubMed] [Google Scholar]
- 25.Lin D, Leichman L. The current status of neoadjuvant therapy for esophageal cancer. Semin Thorac Cardiovasc Surg. 2014;26:102–109. doi: 10.1053/j.semtcvs.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Kunisaki C, Makino H, Kimura J, Ota M, Takagawa R, Kosaka T, Ono HA, Akiyama H, Endo I. Postoperative surveillance and prognostic factors in patients with esophageal cancer. Hepatogastroenterology. 2014;61:1262–1273. [PubMed] [Google Scholar]


