Abstract
This meta-analysis aimed to identify the accuracy of contrast-enhanced ultrasonography (CEUS) on the diagnosis of thyroid nodules. PubMed, Chinese Biomedical Medical databases (CNKI), Wan Fang (Chinese), and EBSCO database were searched from inception through April 15, 2015 without language and geographic restrictions. MetaDisc version 1.4 software was applied for this meta-analysis. We calculated the summary statistics for sensitivity (Sen), specificity (Spe), positive and negative likelihood ratio (LR+/LR-), diagnostic odds ratio (DOR), and receiver operating characteristic (SROC) curve. Twenty-five eligible studies were included in this meta-analysis. A total of 424 in 1154 nodules is malignant thyroid tumors. After all thyroid lesions were histologically confirmed by CEUS, the pooled Sen was 0.88 (95% confidence interval [CI] 0.85-0.91); the pooled Spe was 0.90 (95% CI 0.88-0.92). The pooled positive LR+ was 8.69(95% CI 5.78-13.09); the pooled negative LR- was 0.15 (95% CI 0.12-0.19). The pooled DOR of CEUS in the diagnosis of thyroid nodules was 63.18 (95% CI 37.82-105.53). The area under the SROC curve was 0.946 (standard error [SE] = 0.010). Our meta-analysis indicates that CEUS may have high accuracy in diagnosis the difference between benign and malignant thyroid nodules. US is a traditional tool in the diagnosis thyroid nodules. However, with the development of science and technology, the emerging of CEUS significantly improve accuracy in the diagnosis thyroid nodules.
Keywords: Accuracy, ultrasound contrast imaging, CEUS, thyroid, nodules, meta-analysis
Introduction
Thyroid nodules are a common clinical problem. The prevalence of thyroid nodules is high in recent years, and approximately 8% of them may be an indicator of malignant nodules [1]. With the development of high-frequency ultrasound (US) imaging techniques, the detection rate of thyroid diseases has greatly been improved [2]. In spite of conventional US, as the preferred imaging method for diagnosing thyroid diseases, is inexpensive, worldwide available, non-invasive, no radiation and provides information regarding the characteristics associated with nature of thyroid nodules, such as margins, echogenicity, presence of microcalcification, and vascular flow. However, the US’s key limitation is weak in the differentiation benign from malignant nodules. In order to improve the accuracy in the diagnosis of thyroid nodules, a large number of methods for detection of thyroid nodules are assessed, such as Color-Doppler ultrasound (CDUS), Elastosonography, Contrast-enhanced ultrasound (CEUS) and so on. While CEUS has been used as an effective technique to improve the sonographic diagnosis of pseudocapsules in thyroid nodules, which is dynamic enhancement patterns of focal thyroid nodules immediately. CEUS has been introduced for diagnostic imaging in the renal, liver, heart, pancreas, trauma, and several other organs, which is based on employs microbubble contrast agents and complementary harmonic pulse sequences and ultrasound [3]. And CEUS can provide much better characterization of focal thyroid nodules than conventional US and the real-time of it has been widely applied to differentiate malignant from benign thyroid nodules in recent years. Some studies have proved the value in diagnosis of CEUS for thyroid nodules [4-6]. However, few studies have analyzed the accuracy of CEUS in differentiating benign and malignant thyroid nodules. Therefore, present meta-analysis was objeced to identify the accuracy of contrast-enhanced ultrasonography (CEUS) on the diagnosis of thyroid nodules.
Methods
Literature search
PubMed, Chinese Biomedical Medical databases (CNKI), Wan Fang (Chinese) and EBSCO databases were searched from inception through April 15, 2015 without language and geographic restrictions. The search terms include ultrasound, contrast-enhanced ultrasonography, ultrasound contrast imaging, CEUS, thyroid nodules, diagnostic accuracy, Meta-analysis or QUADAS. Literature review and manual search method were applied to prevent missing the literature. Two authors carried out the process of the literature retrieval. Any disagreement was resolved by a final consensus.
Selection criteria
The inclusion criteria for the current meta-analysis were as follows: (1) The clinical cohort study or diagnostic test was designed in the studies; (2) CEUS was used in differential diagnosis between malignant and benign thyroid nodules; (3) All thyroid nodules were diagnosed by CEUS firstly, then by pathologic diagnosis; and (4) Studies must conclude data for estimating sensitivity (Sen.), specificity (Spe), positive and negative likelihood ratio (LR+/LR-), diagnostic odds ratio (DOR), and receiver operating characteristic (SROC) curve. If the study could not meet the above following inclusion criteria, it would be excluded. We chose the latest and largest data when the studies existed repeat data.
Data extraction
Two observers were extracted data carefully from all included studies by using a standardized form. The following information was extracted: the first author’s surname, publication year of article, geographical location, language of publication, ethnicity, sample size, gender (males/females), mean age, instrument, contrast agent, “gold standard”, etc. And we have collected True positive (TP), true negative (TN), false positive (FP), and false negative (FN) in the fourfold (2×2) tables. The group of ethnicity was classified as Asian, European, Oceania.
Quality assessment
The quality of diagnostic accuracy studies (QUADAS) tool [7] was applied to evaluate the quality of the studies included in this meta-analysis by two researchers assess independently. Study design-related issues and the validity of the study results are assessed by the QUADAS tool which contained 14 items. And scored as following: “yes” = 2, “no” = 0, “unclear” = 1. Range of QUADAS score was 0 to 28; and when score over 22 presents a good quality.
Statistical analysis
We analyzed the forest plots and summary receiver operating characteristic (SROC) curves using freeware Meta-DiSc, version 1.4 (Universidad Complutense, Madrid, Spain) software. The pooled summary statistics for sensitivity (Sen.), specificity (Spe), positive and negative likelihood ratio (LR+/LR-), and diagnostic odds ratio (DOR) with their 95% confidence intervals (CIs) was calculated. We obtained the summary receiver operating characteristic (SROC) curve and the corresponding area under the curve (AUC) [8]. The Spearman correlation coefficients were assessed to test the threshold effect. And we used the Cochran’s Q-statistic and I2 test to judge potential heterogeneity between studies [9]. The I2 statistic represented the percentage of total variation contributed by a between-study variation ranging from 0% to 100%. If I2>50% was indicated to be representative of statistically significant heterogeneity, then random effects model (the Mantel-Haenszel method) was used. If not, fixed-effects model (the Der-Simonian and Laird method) was applied [10]. For our original analysis, we limited meta-analysis to trials judged to be of low risk of bias.
Results
Study characteristics
Initially, one hundred and thirty potentially relevant articles were identified. After reviewing these articles, 22 repeated articles were excluded, 4 articles were excluded for animal study, 5 systematic reviews were excluded, 3 studies did not establish control group, and 20 articles were excluded because they did not provide sufficient data for this study. Finally, 25studies included in our study, and a total of 12 case-control studies’ data were identified, including 1154 samples (424 cases and 730 controls). All these studies were published from 1986 to 2014. The characteristics of included studies are showed in Table 1. The selection process of eligible articles are showed in Figure 1.
Table 1.
Characteristic of case-control studies included in the study
| Author | Year | Country | Language | Ethnicity | Sample size | Gender (M/F) | Age (years) | Instrument | Contrast-agent | 2×2 table | QUADAS score | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||||
| Malignant | Benign | TP | FP | TN | FN | ||||||||||
| Jiang T [11] | 2013 | China | Chinese | Asian | 31 | 64 | 4/38 | 45 | Mylab | SonoVue | 28 | 5 | 3 | 59 | 22 |
| Li M [12] | 2011 | China | Chinese | Asian | 34 | 48 | 27/55 | 42 | Siemens | SonoVue | 31 | 3 | 3 | 45 | 21 |
| Lin SB [13] | 2012 | China | Chinese | Asian | 68 | 96 | 54/110 | 42 | Siemens | SonoVue | 62 | 6 | 6 | 90 | 21 |
| Zhang HL [14] | 2013 | China | Chinese | Asian | 12 | 85 | 14/83 | 51.2 | Siemens | SonoVue | 11 | 2 | 1 | 83 | 23 |
| Chen LB [15] | 2013 | China | Chinese | Asian | 47 | 53 | 8/34 | 50.3 | Siemens | SonoVue | 43 | 15 | 4 | 38 | 22 |
| Xu YB [16] | 2013 | China | Chinese | Asian | 59 | 33 | 27/65 | 45.4 | Siemens | SonoVue | 54 | 4 | 5 | 29 | 20 |
| Liu ZW [17] | 2015 | China | Chinese | Asian | 53 | 73 | 42/84 | 41.6 | Siemens | SonoVue | 47 | 7 | 6 | 66 | 22 |
| Zhao Y [6] | 2010 | China | Chinese | Asian | 17 | 22 | 6/31 | 41.1 | GE | SonoVue | 16 | 3 | 1 | 19 | 20 |
| Pan JS [18] | 2013 | China | Chinese | Asian | 17 | 72 | 10/71 | 42.7 | - | SonoVue | 13 | 2 | 4 | 70 | 22 |
| Cantisani V [19] | 2013 | Italy | Chinese | European | 19 | 34 | 13/35 | 49.4 | Aplio XG | SonoVue | 15 | 3 | 4 | 31 | 25 |
| Nemec U [20] | 2012 | Australia | Chinese | Oceania | 11 | 31 | 10/36 | 52.1 | Philips | SonoVue | 8 | 5 | 3 | 26 | 26 |
| Deng J [21] | 2014 | China | Chinese | Asian | 56 | 119 | 42/104 | 46.3 | Siemens | SonoVue | 46 | 18 | 10 | 101 | 23 |
Figure 1.
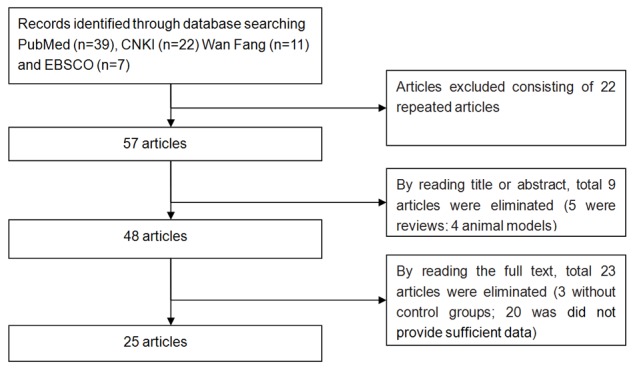
Flow chart for literature screening.
A total of 424 in 1154 nodules is malignant thyroid tumors. We preformed the sonographic contrast agent SonoVue by Intravenous administration in including studies. As a whole, seven studies were used Siemens ultrasound, two studies were used Phillips ultrasound and GE ultrasound, respectively, and three studies were used others brands.
Quantitative data synthesis
Due to the significant heterogeneity among the studies, so we choose the random effects model. Diagnostic accuracy of CEUS was measured as pooled Sen (Spe, LR+ and LR- (Figure 2). Our meta-analysis disclosed that the pooled Sen was 0.88 (95% CI 0.85-0.91); the pooled Spe was 0.90 (95% CI 0.88-0.92). There is no threshold effect that there was no significant correlation (r = -0.067, P = 0.837) between sensitivity and specificity. Furthermore, we observed that the pooled positive LR+ and negative LR- was 8.69 (95% CI 5.78-13.09) and 0.15 (95% CI 0.12-0.19), respectively. The pooled DOR of CEUS in the diagnosis of thyroid nodules was 63.18 (95% CI37.82-105.53) (Figure 3). The area under the SROC curve was 0.946 (standard error [SE] = 0.010) (Figure 4).
Figure 2.
Forest plots for the accuracy of CEUS for the diagnosis of thyroid nodules (A. Sensitivity. B. Specificity. C. Positive likelihood ratio. D. Negative likelihood ratio).
Figure 3.
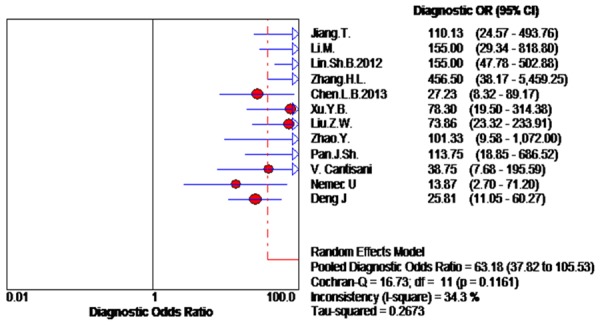
Forest plot of DOR of CEUS for the diagnosis of thyroid nodules.
Figure 4.

SROC curve for the accuracy of CEUS in the diagnosis of thyroid nodules.
Discussion
Previous studies reported that a separate nodule noticed on ultrasound examination was missed on palpatory examination [22]. Common clinical practice chose the US to screen patients with suspected thyroid nodules firstly, because it’s relatively inexpensive, worldwide available, non-invasive, lack of radiation and so on. But US is not a high degree of confidence in diagnosing or excluding malignancy thyroid nodules [23], With the development of science and technology, microbubble contrast agents were employed for US, then CEUS has emerged and gradually matured for diagnosing thyroid nodules [24]. Several early studies have implied that assessing nodules vascularity is essential in diagnosing nodules features [25]. Finding a valid modality to characterize the nodule lesion is necessary to differentiate malignant form benign nodules. Several recent studies indicated that CEUS is more accurate than other traditional inspections in detection and characterization nodules [4,20]. But CEUS is less accurate than pathological diagnosis relatively.In this meta-analysis pathological diagnosis is used as golden criterion that means absolutely exact accuracy. Therefore, CEUS just as an auxiliary diagnosis, before the pathological diagnosis is employed in differential diagnosis malignant form benign thyroid nodules. CEUS is still widely used in clinical practice but the accuracy of CEUS has controversy. So we made meta-analysis to systematically evaluate the accuracy of CEUS for differential diagnosis between benign and malignant thyroid nodules in our study.
A comprehensive and reliable conclusion on the accuracy of CEUS in the diagnosis of thyroid nodules was provided by our study. This Meta-analysis absorbed 12 independent studies including 1003 thyroid nodules patients. The results showed that the pooled Sen, Spe, and DOR of CEUS in the diagnosis of thyroid nodules were 88, 90 and 63.18%, respectively. In summary, our results demonstrate that CEUS might have high accuracy for diagnosing thyroid nodules. So CEUS may be a good tool for differential diagnosis between benign and malignant thyroid nodules and plays an important role in predicting prognosis of thyroid nodules patients. Our results demonstrated that there was no significant correlation between Sen and Spe in the meta-analysis. Heterogeneity existed in early individual studies, but we were not carried out subgroup analyses. As a whole, our finding strongly suggests that CEUS is a high accuracy in qualitative diagnosis of thyroid nodules, which is consistent with early studies.
Limitations and strengths
There are some limitations in this meta-analysis. Firstly, our results indicated to be representative of statistically significant heterogeneity due to relatively low-quality included studies. Secondly, it exists some subjects’ selection bias in retrospective studies. In additions, the reliability and validity are adversely affected in our results due to the majority of included studies originated from China.
Conclusions
The meta-analysis implies that CEUS may have high accuracy in differential diagnosis between benign and malignant thyroid nodules. Therefore, CEUS may be a good tool for the diagnosis of thyroid nodules. However, further detailed studies are still required to confirm our finding due to the limitation mentioned above.
Acknowledgements
All authors have made substantial contributions to this article: Buyun Sun Xiangming Zhu contributed to the conception and design. Feng Jiang and Cheng Wang contributed to the analysis and interpretation of data, and drafting of the article. Lin Lan and Lianping He contributed to the acquisition of data and revision of the article. All authors read and approved the final manuscript.
Disclosure of conflict of interest
None.
References
- 1.Guth S, Theune U, Aberle J, Galach A, Bamberger CM. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest. 2009;39:699–706. doi: 10.1111/j.1365-2362.2009.02162.x. [DOI] [PubMed] [Google Scholar]
- 2.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Sherman SI, Tuttle RM American Thyroid Association Guidelines Taskforce. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109–42. doi: 10.1089/thy.2006.16.109. [DOI] [PubMed] [Google Scholar]
- 3.Jakobsen JA, Oyen R, Thomsen HS, Morcos SK Members of Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR) Safety of ultrasound contrast agents. Eur Radiol. 2005;15:941–5. doi: 10.1007/s00330-004-2601-0. [DOI] [PubMed] [Google Scholar]
- 4.Zhang B, Jiang YX, Liu JB, Yang M, Dai Q, Zhu QL, Gao P. Utility of contrast-enhanced ultrasound for evaluation of thyroid nodules. Thyroid. 2010;20:51–7. doi: 10.1089/thy.2009.0045. [DOI] [PubMed] [Google Scholar]
- 5.Bartolotta TV, Midiri M, Galia M, Runza G, Attard M, Savoia G, Lagalla R, Cardinale AE. Qualitative and quantitative evaluation of solitary thyroid nodules with contrast-enhanced ultrasound: initial results. Eur Radiol. 2006;16:2234–41. doi: 10.1007/s00330-006-0229-y. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Y, Huang DZ, Huang XL. Value of contrast-enhanced ultrasonography in differential diagnosis of benign or malignant thyroid nodules. Chinese Journal of Medical Imaging Technology. 2010;26:1441–43. [Google Scholar]
- 7.Whiting PF, Weswood ME, Rutjes AW, Reitsma JB, Bossuyt PN, Kleijnen J. Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Med Res Methodol. 2006;6:9. doi: 10.1186/1471-2288-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamza TH, Arends LR, van Houwelingen HC, Stijnen T. Multivariate random effects meta-analysis of diagnostic tests with multiple thresholds. BMC Med Res Methodol. 2009;9:73. doi: 10.1186/1471-2288-9-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zintzaras E, Ioannidis JP. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics. 2005;21:3672–3. doi: 10.1093/bioinformatics/bti536. [DOI] [PubMed] [Google Scholar]
- 10.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 11.Jiang T. Value of ultrasonic elastography and contrast-enhanced ultrasound in diagnosing hashimoto’s thyroiditis combining with malignant nodule. Chinese Modern Doctor. 2013 [Google Scholar]
- 12.Li M. Value of contrast -enhanced ultrasound and ultrasonic elastography in differential diagnosis of benign and malignant thyroid nodules. Journal of Clinical Ultrasound in Medicine. 2011 [Google Scholar]
- 13.Lin SB. Value of Ultrasound Imaging and Ultrasound Elastograph in Diagnosing Thyroid Malignant and Benign Nodules. China Cancer. 2012:39. [Google Scholar]
- 14.Zhang HL. Diagnosis of thyroid “warm” nodule by contrast enhanced ultrasound. Journal of Modern Oncology. 2013 [Google Scholar]
- 15.Chen LB. Two Dimensional Ultrasonograhy (2D -US) Combined with Contrast Enhanced Ultrasonogrphy (CEUS) in Differentiation of Thyroid Malignant and Benign Masses. Journal of Medical Research. 2013:42. [Google Scholar]
- 16.Xu YB. Value of contrast-enhanced ultrasonography in the diagnosis of microalcified thyroid nodules. Journal of Clinical Ultrasound in Medicine. 2013:15. [Google Scholar]
- 17.Liu WZ, Li QS, Gong XH. Study on significance of contrast-enhanced ultrasound in diagnosis of calcified thyroid nodules. Journal of Clinical and Experimental Medicine. 2015:14. [Google Scholar]
- 18.Pan JS. Diagnosis of thyroid nodulesby clinical value of combined contrast-enhanced ultrasonography with core-needle biopsy. Lingnan modern clinical in surgery. 2013 [Google Scholar]
- 19.Cantisani V, Consorti F, Guerrisi A, Guerrisi I, Ricci P, Di Segni M, Mancuso E, Scardella L, Milazzo F, D’Ambrosio F, Antonaci A. Prospective comparative evaluation of quantitative-elastosonography (Q-elastography) and contrast-enhanced ultrasound for the evaluation of thyroid nodules: preliminary experience. Eur J Radiol. 2013;82:1892–8. doi: 10.1016/j.ejrad.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Nemec U, Consorti F, Guerrisi A, Guerrisi I, Ricci P, Di Segni M, Mancuso E, Scardella L, Milazzo F, D’Ambrosio F, Antonaci A. Quantitative evaluation of contrast-enhanced ultrasound after intravenous administration of a microbubble contrast agent for differentiation of benign and malignant thyroid nodules: assessment of diagnostic accuracy. Eur Radiol. 2012;22:1357–65. doi: 10.1007/s00330-012-2385-6. [DOI] [PubMed] [Google Scholar]
- 21.Deng J, Zhou P, Tian SM, Zhang L, Li JL, Qian Y. Comparison of diagnostic efficacy of contrast-enhanced ultrasound, acoustic radiation force impulse imaging, and their combined use in differentiating focal solid thyroid nodules. PLoS One. 2014;9:e90674. doi: 10.1371/journal.pone.0090674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brander A, Viikinkoski P, Tuuhea J, Voutilainen L, Kivisaari L. Clinical versus ultrasound examination of the thyroid gland in common clinical practice. J Clin Ultrasound. 1992;20:37–42. doi: 10.1002/jcu.1870200107. [DOI] [PubMed] [Google Scholar]
- 23.Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, Cronan JJ, Doubilet PM, Evans DB, Goellner JR, Hay ID, Hertzberg BS, Intenzo CM, Jeffrey RB, Langer JE, Larsen PR, Mandel SJ, Middleton WD, Reading CC, Sherman SI, Tessler FN Society of Radiologists in Ultrasound. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005;237:794–800. doi: 10.1148/radiol.2373050220. [DOI] [PubMed] [Google Scholar]
- 24.Postema M, Gilja OH. Contrast-enhanced and targeted ultrasound. World J Gastroenterol. 2011;17:28–41. doi: 10.3748/wjg.v17.i1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spira D, Neumeister H, Spira SM, Hetzel J, Spengler W, von Weyhern CH, Horger M. Assessment of tumor vascularity in lung cancer using volume perfusion CT (VPCT) with histopathologic comparison: a further step toward an individualized tumor characterization. J Comput Assist Tomogr. 2013;37:15–21. doi: 10.1097/RCT.0b013e318277c84f. [DOI] [PubMed] [Google Scholar]



