Abstract
This study is to reveal the relationship between pituitary adenomas and tumor texture by comparing MRI and pathologic results. Preoperative imaging data of 38 cases of pituitary adenoma patients and collagen content of tumor specimens measured by histopathological were analyzed and compared. T2WI and diffusion coefficient assessment were used to reveal the relationship between tumor texture and collagen content. There were 13 cases of soft texture, 17 cases of medium texture and 8 cases of tough texture tumors. Signal intensity of different texture Pituitary adenomas had significant difference on T2WI and ADC map (P < 0.05). The signal intensity ratio of tumor and pons on T2WI had high consistency with tumor texture. Mean collagen contents of soft, medium and tough texture group were 1.51% ± 0.91%, 7.35% ± 2.99% and 18.10% ± 8.24%, respectively. There were significant differences in collagen content of different texture tumors (P < 0.01). The signal intensity of T2WI and ADC images have prediction value for pituitary adenomas texture and T2WI is more reliable.
Keywords: Pituitary adenoma, texture, MRI, T2WI, ADC, collagen content
Introduction
Pituitary adenoma (PA) is a common benign tumor of neurosurgery and it ranks the third in brain tumors [1]. PA can bring a variety of hazards to human body. Excessive secretion of pituitary hormone can cause a series of metabolic disorders and organ damages. PA can press normal pituitary tissue thus lead to low function of corresponding target glands. Compression and invasion of PA to its surrounding structures such as the sella, optic chiasm, optic nerve, cavernous sinus, skull base and brain-stem can result in serious obstacles to the corresponding organ functions. PA can hamper human growth, development, labor ability and reproductive function, which, causes a series of social psychological impacts. With the rapid development of modern medical technology, progress has been made in the diagnosis and treatment of PA, thereby effectively reduces the harm of PA.
To date, operation resection is the most effective therapeutic method for curing PA. Tumor texture has been considered to be an important factor that affecting the rate of operation resection [2,3]. Thus, accurately prediction of tumor texture preoperative will play an important role in guiding operative risk evaluation and surgical planning, which, can greatly ensure the operation safety. With the development of medical imaging technology, scholars begin to study the value of preoperative imaging in evaluation of PA texture and the the most watched are T2WI [4-6] and DWI [4,7-9]. However, as the methods used are different, there is no unanimous conclusion about the use of PA texture evaluation by T2WI and DWI.
In this study, T2WI and ADC images were used for the first time for comparing PA signal intensity with the intensity on the level of pons through bridge arm. Combined with intraoperative tumor texture judgment and collagen content measurement after postoperative, the predicting value of the two parameters had been investigated and the relationship between them also was discussed in the hope of providing reference for preoperative evaluation of PA texture.
Materials and methods
Patient’ data
A total of 104 cases of patients who were diagnosed as PA and were admitted to our hospital from November 2011 to April 2013 and 38 cases were enrolled in this study. Among the 38 cases, 17 were male and 21 were female. They aged from 21-73 years old, with an average age of (45.3 ± 14.6) years old. The duration was from 14 days to 21 years. The inclusion criteria were defined as follows: patients those taken 3.0-T MRI T2WI and DWI examination and were found space-occupying lesions in saddle area; patients those were confirmed as PA by postoperative pathology. The exclusion criteria were described as follows: patients those with inhomogeneous MRI signal intensity in tumor area and patients those with tumor diameter less than 1 cm.
Prior written and informed consent were obtained from every patient and the study was approved by the ethics review board of Fuzhou General Hospital, Fujian Medical University, China.
MRI scanning
Plain and enhanced MRI scanning was performed with 3.0T magnetic resonance scanner (Tim Trio; Siemens Medical Solutions, Erlangen, Germany). The parameters used for conventional scanning were as follows: conventional T1WI (TR/TE 700/8 ms) and T2WI (TR/TE 3000/98 ms) in axial, coronal and sagittal; diffusion sensitivity coefficient (b value) = 100 s/mm2; TR/TE 5100/90 ms. The parameters used for enhanced scanning were as follows: gradin-echo (GRE); TR/TE, 1900/3 ms; Fov, 240 mm × 240 mm; matrix, 256 × 256; layer thickness, 1.0 mm. GD-DTPA was used as contrast agent with the dose of 0.2 ml/kg.
MRI signal data processing
T2WI and ADC image data of each patient was evaluated. The following adenoma and pons tissue related data were collected (by scanning on the level of brachium pontis) and used for data processing: T2PA (average tumor signal intensity on T2WI); T2ME (mean pons signal intensity on T2WI); ADCPA (average tumor signal intensity on ADC diagram); ADCME (mean pons signal intensity on ADC diagram).
The ratios of tumor and pons signal intensity on T2WI and ADC were calculated and defined as RT2 (T2PA/T2ME) and RADC (ADCPA/ADCME).
Tumor texture judgment
In this study, a total of 34 cases of PA patients underwent transsphenoidal operation while the other 4 cases underwent craniotomy operation. According to Mahmoud [9] classification method, the tumors were divided into three groups. Soft texture group: tumor that is easily suctioned with aspirator. Medium texture group: the tumor that is hard to be removed with aspirator. Tough texture group: the tumor cannot be suctioned with aspirator, and bipolar electrocoagulation or sharp segmentation resection is needed. On the basis of the original grouping method, we classified tumors of soft texture mixed with medium texture into medium texture group and medium texture combined with tough texture into tough texture.
Calculation of collagen content
The operation specimen slices were performed with conventional picric acid- collagen staining method followed by taking pictures under the microscope. The images collected were analyzed with Image-Pro Plus 6.0 software and the percentage of each component was calculated with the tools the software provided. The four corners and the central 200 × vision were taken and photographed for collagen content calculation. Collagen content = (total collagen content area under microscope/total slice area under microscope) × 100%.
Statistical analysis
All the statistical analyses were performed using SPSS version 19.0 (SPSS Inc, Chicago, IL, USA) for Windows and P value less than 0.05 was considered as statistically significant. The data was expressed as mean ± standard deviation (SD).
The receiver operating characteristic curve (ROC) was used to assess the detecting value of each index for adenoma texture. Area under the ROC curve (AUC) greater than 0.7 was considered to be with certain detecting value. Kappa coefficient was calculated and the consistency of prejudgment results and tumor texture was assessed. Kappa ≥ 0.75 was considered as good consistency, 0.75 > kappa > 0.4 was regarded as general consistency and kappa < 0.4 was thought to be poor consistency.
Results
Comparisons of MRI indexes of different texture tumors
To identify whether MRI indexes vary among different texture tumors, MRI indexes of different texture tumors were compared. MRI images of soft texture, medium texture and tough texture were shown in Figures 1, 2 and 3. There were 13 cases of soft texture, 17 cases of medium texture and 8 cases of tough texture tumors. Comparisons of each MRI index of different texture tumors all had significant differences (Kruskal-Wallis H test, P < 0.05) (Table 1). To sum up, this result indicated that the harder the tumor texture, the lower the MRI index.
Figure 1.
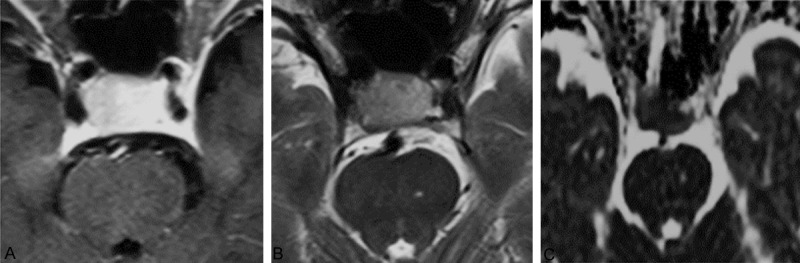
MRI images of 1 case of soft texture PA. A. Enhanced axial MRI image. B. Axial T2WI image. T2PA = 0.656 × 10-3 mm2/sec, T2ME = 0.360 × 10-3 mm2/sec, RT2 = 1.82. C. Axial ADC image. ADCPA = 0.975 × 10-3 mm2/sec, ADCME = 0.774 × 10-3 mm2/sec, RADC = 1.26.
Figure 2.
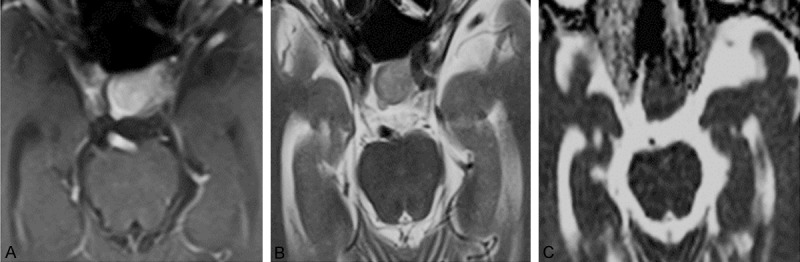
MRI images of 1 case of medium texture PA. A. Enhanced axial MRI image. B. Axial T2WI image. T2PA = 0.657 × 10-3 mm2/sec, T2ME = 0.350 × 10-3 mm2/sec, RT2 = 1.88. C. Axial ADC image. ADCPA = 0.721 × 10-3 mm2/sec, ADCME = 0.784 × 10-3 mm2/sec, RADC = 0.92.
Figure 3.
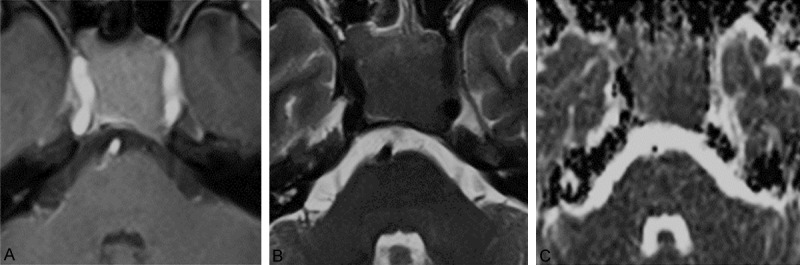
MRI images of 1 case of tough texture PA. A. Enhanced axial MRI image. B. Axial T2WI image. T2PA = 0.260 × 10-3 mm2/sec, T2ME = 0.215 × 10-3 mm2/sec, RT2 = 1.21. C. Axial ADC image. ADCPA = 0.732 × 10-3 mm2/sec, ADCME = 0.697 × 10-3 mm2/sec, RADC = 1.05.
Table 1.
Comparison of MRI indexes of different texture of tumors (n = 38)
| Texture | T2PA (mm2/sec) | RT2 | ADCPA (mm2/sec) | RADC |
|---|---|---|---|---|
| Soft | 0.649 ± 0.130 × 10-3 | 1.92 ± 0.36 | 0.987 ± 0.252 × 10-3 | 1.48 ± 0.43 |
| Medium | 0.570 ± 0.156 × 10-3 | 1.60 ± 0.45 | 0.768 ± 0.248 × 10-3 | 1.12 ± 0.34 |
| Tough | 0.483 ± 0.111 × 10-3 | 1.26 ± 0.32 | 0.742 ± 0.190 × 10-3 | 1.09 ± 0.35 |
| P | 0.04 | 0.03 | 0.02 | 0.01 |
Detecting value of MRI indexes for different texture tumors
To investigate the detecting value of MRI indexes for different texture tumors, MRI indexes including T2PA, RT2, ADCPA and RADC were compared with intraoperative texture judgment by ROC curve. It was found that for soft and tough texture tumors, AUC all were greater than 0.7 while each AUC of medium texture tumor was less than 0.7 (Table 2). According to ROC curve analysis, the critical value of MRI indexes for soft texture tumor detecting was calculated and compared with intraoperative judgment (Table 3). The kappa coefficients of T2PA, RT2, ADCPA and RADC were 0.389, 0.501, 0.391 and 0.436, all with P < 0.05 while compared with tumor texture. Meanwhile, the same comparison of tough texture tumor was carried out (Table 4). The kappa coefficients of T2PA, RT2, ADCPA and RADC were 0.379, 0.401, 0.245 and 0.118 and the P value were 0.013, 0.005, 0.307 and 0.146. In conclusion, it was notable that compared to other indexes, RT2 had high consistency with intraoperative judgment.
Table 2.
The AUC of different MRI indexes for tumor texture detection
| Index | AUC of different MRI indexes for tumor texture detection | ||
|---|---|---|---|
|
|
|||
| Soft | Medium | Tough | |
| T2PA | 0.723 | 0.524 | 0.767 |
| RT2 | 0.790 | 0.555 | 0.815 |
| ADCPA | 0.763 | 0.681 | 0.588 |
| RADC | 0.774 | 0.654 | 0.642 |
Table 3.
Comparison between MRI indexes and soft texture tumor intraoperative judgment (n = 38)
| MRI index | Judgment criteria | Tumor texture | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Youden index | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Soft | Medium or tough | |||||||
| T2PA | ≥ 0.562 | 11 | 10 | 84.6 | 60.0 | 52.4 | 88.2 | 0.446 |
| < 0.562 | 2 | 15 | ||||||
| RT2 | ≥ 1.77 | 10 | 6 | 76.9 | 76.0 | 62.5 | 86.4 | 0.529 |
| < 1.77 | 3 | 19 | ||||||
| ADCPA | ≥ 0.856 | 9 | 7 | 69.2 | 72.0 | 56.3 | 81.8 | 0.452 |
| < 0.856 | 4 | 18 | ||||||
| RADC | ≥ 1.23 | 9 | 6 | 69.2 | 76.0 | 60.0 | 82.6 | 0.452 |
| < 1.23 | 4 | 19 | ||||||
Table 4.
Comparison of judge MRI index and tough texture tumor intraoperative judgment (n = 38)
| MRI index | Judgment criteria | Tumor texture | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Youden index | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Soft | Medium or tough | |||||||
| T2PA | < 0.650 | 8 | 15 | 100 | 50.0 | 34.8 | 100 | 0.500 |
| ≥ 0.650 | 0 | 15 | ||||||
| RT2 | < 1.72 | 8 | 13 | 100 | 56.7 | 38.1 | 100 | 0.567 |
| ≥ 1.72 | 0 | 17 | ||||||
| ADCPA | < 1.031 | 8 | 23 | 100 | 23.3 | 25.8 | 100 | 0.233 |
| ≥ 1.031 | 0 | 7 | ||||||
| RADC | < 0.85 | 3 | 4 | 37.5 | 93.3 | 42.9 | 78.8 | 0.308 |
| ≥ 0.85 | 5 | 26 | ||||||
Relationship between tumor texture and collagen content
To identify the relationship between tumor texture and collagen content, collagen contents in different texture tumors were tested. Mean collagen contents of soft, medium and tough texture group were 1.51% ± 0.91%, 7.35% ± 2.99% and 18.10% ± 8.24%, respectively. The schematic tissue slices of soft, medium and tough texture tumor were shown in Figure 4. There were significant differences in collagen content of different texture tumors (Kruskal-Wallis H test, P < 0.01). According to the ROC curve analysis, AUC of collagen content detection for soft, medium and tough texture tumors were 0.972, 0.655 and 0.908. Hence, one can see that collagen content had higher detection value for soft and tough texture tumors. Further calculation showed that the critical values of collagen content in soft and tough texture tumors were 3.74% and 8.93%, this result was compared with intraoperative judgment (Table 5). The kappa coefficients of detecting values of collagen content for soft and tough texture tumors were 0.887 and 0.599, all had statistical correlation (P < 0.01). Given all this, it is possible to assume that collagen content is positively correlated to tumor texture and the harder the texture, the more collagen content the tumor contains.
Figure 4.
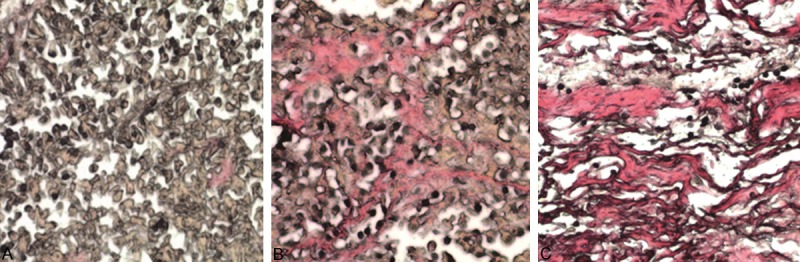
Picric acid-collagen staining of three PA cases of different texture. A. Soft texture PA under microscope. A large number of tumor cells (black), a small amount of fresh red collagen tissue (arrows) and rich cytoplasm (brown yellow) were seen. B. Medium texture PA under microscope. Moderate strip or bundle collagen fiber (red), tumor cells (black), and rich cytoplasm (brown yellow) was seen. C. Tough texture PA under microscope. A large number of collagen fibers (red), a small amount of tumor cells (black) and little cytoplasm (brown yellow) was seen.
Table 5.
Comparison of collagen content and tumor intraoperative judgment (n = 38)
| Pre judgment results | Collagen judgment standard | Intraoperative findings and cases | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Youden index | |
|---|---|---|---|---|---|---|---|---|
| Soft | Medium/tough | |||||||
| Soft | ≤ 3.74% | 13 | 2 | 100.0 | 92.0 | 86.7 | 100.0 | 0.920 |
| > 3.74% | 0 | 23 | ||||||
| Tough | Soft/medium | |||||||
| Tough | ≥ 8.93% | 7 | 5 | 87.5 | 83.3 | 58.3 | 96.2 | 0.708 |
| < 8.93% | 1 | 25 | ||||||
Relationship between tumor type and tumor texture
To identify the relationship between tumor type and tumor texture, texture of different PA types was compared. PA clinical endocrine diagnosis was performed according to the 2004 WHO classification criteria [10]. There were 25 cases of non functional adenoma, 8 cases of growth hormone adenoma, 3 cases of lactotroph adenoma, 1 case of thyrotroph adenoma and 1 case of adrenocorticotropic hormone adenoma. As shown in Table 6, 75.0% (6/8) of patients in tough texture group were non functional adenoma. All the samples were subjected to immunohistochemical examination and there were 11 cases of gonadotropin adenomas, 9 cases of null cell adenoma, 7 cases of growth hormone adenoma, 6 cases of PRL adenoma, 2 cases of TSH adenoma, 1 case of ACTH adenoma and 2 cases of plurihormonal adenoma. It was shown by Table 6 that in tough texture group, most of the patients were hormone-active adenoma. Together, the results argued that non functional adenoma and hormone-active adenoma were common in tough texture PA.
Table 6.
Comparison of tumor type and tumor texture (n = 38)
| Grouping | Texture (cases) | ||
|---|---|---|---|
|
|
|||
| Soft | Medium | Tough | |
| Clinical endocrine type | |||
| Functional type | 4 | 7 | 2 |
| Non functional type | 9 | 10 | 6 |
| Immunohistochemical types | |||
| Hormone active adenoma | 9 | 14 | 6 |
| Null cell adenoma | 4 | 3 | 2 |
Discussion
In this study, signal intensity of tumor and pons in MRI images was firstly compared and the result showed that T2WI had quit high reliability in tumor texture detecting. In previous studies, some scholars compared adenoma signal intensity directly. Influenced by movement and tissue density, even with the same scanning parameters, there are differences of MRI signal intensity in the same parts of different patients. Hence, it is necessary to take another reference for comparative study to reduce the disturbance. Some scholars [4,7,9] have compared the MRI signal intensity in adenoma and cerebral white matter, however, there was no detailed description of which parts of the brain white matter scanned. In this study, we found that there was difference in signal intensity in the same patient because tissue density of different parts of the brain white matter tissue was not unified. Magnetic susceptibility artifacts induced by cerebrospinal fluid and brain gray matter can interfere with DWI and ADC images, as a result, the acquisition error was larger when scanning brain white matter. It was found that the signal intensity of pons on bridge arm level was relatively homogeneous and stable on axial MRI, and the result calculated with taking this part as a reference was less disturbed by magnetic susceptibility artifacts.
Previous reports compared MRI indexes of different texture tumors directly, ignoring the prediction value of each index for single texture tumor. In this study, ROC curve was used to evaluate the detecting value of each MRI index for different texture tumors. The result showed that T2PA, RT2, ADCME and RADC had certain predictive value for soft and tough texture adenomas. However, forecast results were not highly in accordance with intraoperative judgment results, only RT2 was more reliable. Studies with more samples should be taken for further confirmation.
It was pointed that collagen content is the main factor that influences the texture of PA [4,7-9,11-13]. Pierallini et al. [7] showed that the collagen content in three groups of PA were 1.34% ± 1.21%, 6.89% ± 1.91% and 7.23% ± 4.80%. Naganuma et al. [6] recommended that adenoma with collagen content more than 5% be defined as “fibrosis” adenoma. In this study, collagen content of the three kinds of texture tumors were 1.51% ± 0.91%, 7.34% ± 3.10% and 15.45% ± 8.58%. Meanwhile, collagen content of 3.74% and 8.93% were considered as critical value in the detection of soft and tough texture tumors respectively. The prediction results were highly consistent with tumor texture. There were certain differences among our results and those of the other scholars. This might be caused by uneven distribution of collagen tissue in tumor, also it might be affected by different experimental methods and sample sizes. In the present study, most of the tough texture PA were nonfunctional adenoma and this was consistent with previous reports [6,14]. However, it was reported that in prolactinoma patients, the degree of tumor fibrosis was independent of tumor shrinkage and prolactin secretion. This difference might be due to the diverse measurement standard and further research remained to be carried.
Because of tough texture adenoma only accounts for 10% of all PA cases [15], and DWI imaging is prone to be affected by movement and tissue density, in this study, cases those with inconsistent MRI signal intensity were excluded, which, making the small sample size further reduced. There is certain subjectivity in intraoperative judgment of PA texture and different operator may have different judgment results. At the same time, collagen distribution was not uniform in most tumors although the imaging signal was uniform and most of the tumors were removed by suction method. This made it difficult to guarantee the integrity of the operation samples, further affected collagen region measurement under the microscope. As a result of the forementioned reasons, there were some errors in collagen content calculation. More accurate detection methods remain further investigation.
In conclusion, the signal intensity of T2WI and ADC images had prediction value for PA texture and T2WI was more reliable. Collagen content of most soft texture adenoma was less than 3.74% and more than 8.93% in tough texture adenoma.
Acknowledgements
This work was supported by by the Twelfth Five Year Plan of Nanjing Military Medical Innovation Key Project (2011Z034). We greatly appreciate all the help from professor Yinghao Yu in Department of Pathology and associate professor Qun Zhong from Department of Radiology of our hospital for technical assistance in pathology and iconography diagnosis.
Disclosure of conflict of interest
None.
References
- 1.Jane JA Jr, Laws ER Jr. The surgical management of pituitary adenomas in a series of 3,093 patients. J Am Coll Surg. 2001;193:651–9. doi: 10.1016/s1072-7515(01)01101-2. [DOI] [PubMed] [Google Scholar]
- 2.Zhao B, Wei YK, Li GL, Li YN, Yao Y, Kang J, Ma WB, Yang Y, Wang RZ. Extended transsphenoidal approach for pituitary adenomas invading the anterior cranial base, cavernous sinus, and clivus: a single-center experience with 126 consecutive cases. J Neurosurg. 2010;112:108–17. doi: 10.3171/2009.3.JNS0929. [DOI] [PubMed] [Google Scholar]
- 3.Han ZL, He DS, Mao ZG, Wang HJ. Cerebrospinal fluid rhinorrhea following trans-sphenoidal pituitary macroadenoma surgery: experience from 592 patients. Clin Neurol Neurosurg. 2008;110:570–9. doi: 10.1016/j.clineuro.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki C, Maeda M, Hori K, Kozuka Y, Sakuma H, Taki W, Takeda K. Apparent diffusion coefficient of pituitary macroadenoma evaluated with line-scan diffusion-weighted imaging. J Neuroradiol. 2007;34:228–35. doi: 10.1016/j.neurad.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Musleh W, Sonabend AM, Lesniak MS. Role of craniotomy in the management of pituitary adenomas and sellar/parasellar tumors. Expert Rev Anticancer Ther. 2006;6(Suppl 9):S79–S83. doi: 10.1586/14737140.6.9s.S79. [DOI] [PubMed] [Google Scholar]
- 6.Naganuma H, Satoh E, Nukui H. Technical considerations of transsphenoidal removal of fibrous pituitary adenomas and evaluation of collagen content and subtype in the adenomas. Neurol Med Chir (Tokyo) 2002;42:202–212. doi: 10.2176/nmc.42.202. [DOI] [PubMed] [Google Scholar]
- 7.Pierallini A, Caramia F, Falcone C, Tinelli E, Paonessa A, Ciddio AB, Fiorelli M, Bianco F, Natalizi S, Ferrante L, Bozzao L. Pituitary macroadenomas: preoperative evaluation of consistency with diffusion-weighted MR imaging--initial experience. Radiology. 2006;239:223–31. doi: 10.1148/radiol.2383042204. [DOI] [PubMed] [Google Scholar]
- 8.Boxerman JL, Rogg JM, Donahue JE, Machan JT, Goldman MA, Doberstein CE. Preoperative MRI evaluation of pituitary macroadenoma: imaging features predictive of successful transsphenoidal surgery. AJR Am J Roentgenol. 2010;195:720–8. doi: 10.2214/AJR.09.4128. [DOI] [PubMed] [Google Scholar]
- 9.Mahmoud OM, Tominaga A, Amatya VJ, Ohtaki M, Sugiyama K, Sakoguchi T, Kinoshita Y, Takeshima Y, Abe N, Akiyama Y, El-Ghoriany AI, Abd Alla AK, El-Sharkawy MA, Arita K, Kurisu K, Yamasaki F. Role of PROPELLER diffusion-weighted imaging and apparent diffusion coefficient in the evaluation of pituitary adenomas. Eur J Radiol. 2011;80:412–7. doi: 10.1016/j.ejrad.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 10.Delellis RA, Lloyd RV, Heitz PU, Eng C. Pathology and Genetics. Tumours of Endocrine Organs. Lyon: IARC Press; 2004. WHO Classification of Tumours; pp. 10–3. [Google Scholar]
- 11.Bahuleyan B, Raghuram L, Rajshekhar V, Chacko AG. To assess the ability of MRI to predict consistency of pituitary macroadenomas. Br J Neurosurg. 2006;20:324–6. doi: 10.1080/02688690601000717. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Li W, Shi D, Ye Z, Qin F, Guo Y, Yuan X. Expression of TGFbeta1 and pituitary adenoma fibrosis. Br J Neurosurg. 2009;23:293–6. doi: 10.1080/02688690802617046. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Li WS, Shi DJ, Ye ZP, Tai F, He HY, Liang CF, Gong J, Guo Y. Correlation of MMP (1) and TIMP (1) expression with pituitary adenoma fibrosis. J Neurooncol. 2008;90:151–6. doi: 10.1007/s11060-008-9647-9. [DOI] [PubMed] [Google Scholar]
- 14.Menucci M, Quinones-Hinojosa A, Burger P, Salvatori R. Effect of dopaminergic drug treatment on surgical findings in prolactinomas. Pituitary. 2011;14:68–74. doi: 10.1007/s11102-010-0261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iuchi T, Saeki N, Tanaka M, Sunami K, Yamaura A. MRI prediction of fibrous pituitary adenomas. Acta Neurochir (Wien) 1998;140:779–86. doi: 10.1007/s007010050179. [DOI] [PubMed] [Google Scholar]


