Abstract
To investigate the protective effect of adenovirus mediated Survivin protein overexpression on acute myocardial infarction in rats. 45 acute myocardial infarction rat models were constructed by suture method and were randomly divided into sham group, model group and treatment group. The treatment group was injected with Survivin gene packed virus via ventricle. The model group was injected with equal titer of adenovirus packed empty vector. The sham group was not ligated. These rats were killed in 96 h after treatment. The levels of Survivin, Caspase-3, caspase-7 mRNA and protein in myocardial tissues were detected by real-time fluorescence quantitative PCR and Western blot. Myocardium tissue cell apoptosis were analyzed by TUNEL staining, the immunology of myocardial infarction tissue was analyzed by TTC staining. Compared with model group and sham group, the level of survivin protein in myocardium tissue of rats in treatment group was significantly increased (P<0.05). Compared with sham group, the levels of caspase-3 and caspase-7 mRNA and protein in myocardial tissue of rats in model group and treatment group were significantly increased, but the treatment group were significantly lower than those of model group (P<0.05). The myocardium cell apoptosis index and myocardial infarction areas of rats in model group and treatment group were significantly higher than those of sham group, but the treatment group were significantly lower than those of model group (P<0.05). In conclusion, Survivin protein overexpression in myocardial tissue can significantly inhibit the expression of apoptosis promoting factor in myocardial tissue of acute myocardial infarction rats, reduce the apoptosis index of myocardial cells and the myocardial infarct size, which has great significance for protecting myocardial function.
Keywords: Acute myocardial infarction, apoptosis, Survivin, Caspase-3
Introduction
Acute myocardial infarction is one of the most common cardiovascular and cerebrovascular diseases. Its incidence is high, the progression of disease is rapid and has become the major disease to threaten human health. After myocardial infarction, the myocardial tissue is in anoxic and ischemia state. Hypoxia and ischemia for a long time can lead to apoptosis and necrosis of myocardial cells, which eventually leads to the loss of function [1,2]. The current study showed that myocardial cells mainly occurred to apoptosis in ischemic region in the early stage of infarction. So to inhibit apoptosis is of great significance to improve the prognosis of the patients. That is also a hot spot for clinical research [3,4]. Cell apoptosis is an initiative and ordered self-digestion process controlled by genes. Under normal physiological regulation, cell proliferation and apoptosis are in a state of dynamic equilibrium. Once the pathological changes occur, such as myocardial infarction, the balance will be broken, resulting in rapid apoptosis of myocardial cells [5,6]. Survivin protein is also known as survival protein, which is the most powerful inhibitor of apoptosis, and its expression level can significantly inhibit P53 and Caspase mediated apoptosis [7,8]. Survivin protein expression has tissue selectivity. Recent studies showed that it was highly expressed in most malignant tumors and normal embryonic tissue, but was not expressed or lowly expressed in normal adult tissues [9,10]. So we supposed that Survivin protein overexpression might decrease myocardial cell apoptosis in acute myocardial infarction, and then protect the myocardium. Therefore, Survivin protein was expressed by adenovirus mediated myocardial cells so as to investigate the myocardial protective mechanism of acute myocardial infarction in rats.
Materials and methods
Construction and treatment of acute myocardial infarction model
SD rats (180-200 g) were purchased from Silaike Experimental Animal Biotechnology Co., Ltd., Shanghai, China. This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (Bethesda, MD, USA). Eighth Edition, 2010. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of The Third Affiliated Hospital of Xinxiang Medical University. The experimental rats were injected with 1 ml 100 g/L chloral hydrate solution via intraperitoneal injection. They were fixed in supine position after were anesthetized. The ventilator was used for assisted respiration; The left of chest median incision was made. The tissue was separated layer by layer. The chest was opened along the fourth intercostal space. The pericardium, was separated to expose the heart. The left coronary artery was ligated with 7-0 thread at the lower edge of left atrial appendage. The symptoms of acute myocardial infarction appear immediately [11]. The chest was only opened and the left coronary artery was not ligated in rats in sham group. The rats were randomly divided into three groups: sham group, model group and treatment group. The rats in treatment group were injected with Survivin expression plasmid packed adenovirus (Kaiji Biotechnology Co., Shanghai, China) via left ventricle. The virus titer was 1×107. The rats in model group were injected with empty vector packed adenovirus (Kaiji Biotechnology Co., Shanghai, China) via the left ventricle. The titer was 1×107. The rats in sham group were injected with isometric PBS solution.
RT-PCR
The rats in three groups were executed in 96 h after treatment. 0.1 g myocardial tissue was removed and placed in 1 ml Trizol solution (Invitrogen, Carlsbad, CA, USA). 200 μl chloroform was added after homogenized. The mixture was vibrated, placed on ice for 15 min to stratify the solution and centrifuged for 15 minutes at 12000 rpm/min. The supernatant was transferred to 500 μl isopropanol, placed on ice for 15 min after mixing, centrifuged for 10 min at 15, 000 g to precipitate RNA and washed twice with pre-cooling 75% ethanol. The precipitate was dissolved in DEPC treated double distilled water. The sample concentration was determined. RNA was transcribed into cDNA by reverse transcription kit (TaKaRa, Dalian, China) as the template of PCR.
The following primers were designed according to Survivin, Caspase-3 and Caspase-7 sequences provided by GenBank: Survivin-3-F: 5’-ATGGGTGCCCCGACGTT-3’, Survivin-3-R: 5’-TCAATCCATGGCAGCCAG-3’. Caspase-3-F: 5’-GGTATCCATGGAGAACACT-3’, Caspase-3-R: 5’-AAAAA TAGAGTTCTTTTGTGAG-3’. Caspase-7-F: 5’-CTACCGCCGTGGGAACGATG-3’, Caspase-7-R: 5’-CGAAGGCCCATACCTGTCAC-3’. GAPDH-F: 5’-ACCACAGTCCATGCCATCAC-3’, GAPDH-R: 5’-TCCACCACCCTGTTGCTGTA-3’. The primers were diluted to 10 µmmol•L-1. The conditions were optimized according to primer specificity and annealing temperature at first. Then the following reaction systems were prepared: 10 µl 2× SYBR Green universal type qPCR Master Mix (Roche, Basel, Switzerland), 0.6 µl upstream/downstream primers (10 µmmol•L-1), 8.8 µl 1:100 diluted cDNA. The total reaction volume was 20 µl. The reaction mixture was centrifuged at 1500 rpm/min to thrown to the tube bottom. PCR was conducted according to the following reaction conditions: pre-degeneration at 95°C for 30 s; degeneration at 95°C for 3; annealing extension at 60°C for 30 s; The solubility curve was constructed. Finally, the data was read directly from the real-time PCR instrument (Applied Biosystems, Foster City, CA, USA).
Western bolt
The rat myocardial tissue after different treatments was placed in tissue lysates for sonication, then on ice for 30 min to make the tissue being fully cracked, then centrifuged for 15 min at 15, 000 g. The supernatant was removed to determine protein concentration. 4× sample buffer was added. The boiling water was used to boil for 30 min. The sample was added after centrifuged. SDS-PAGE was conducted and the protein was transferred to PVDF membrane. 5% defatted milk powder was used to close down. Survivin, Caspase-3 and Caspase-7 primary antibodies (Santa-Cruz, CA, USA) were coated overnight, washed with PBST for 3 times in the next day. Then HRP conjugated Sheep anti mouse secondary antibody (ZSGB-BIO, Beijing, China) was used to coat for 1 h at room temperature. PBST was used to wash for 3 times. The luminous liquid (Wako, Osaka, Japan) was added, colored and photographed. Meanwhile, GAPDH was taken as the internal reference. The protein band gray was analyzed with gray calculation software. The relative expression of target protein was calculated.
TUNEL staining
The rats in three groups were executed after treated for 96 h. The myocardial tissue was removed to prepare paraffin sections. The sections were immersed in xylene for 2 times, 5 min each time; successively rinsed once with gradient ethanol (100, 95, 90, 80 and 70%), 3 min every time; washed with PBS twice; The tissue was treated with Proteinase K working solution for 15-30 min at 21-37°C, washed with PBS twice; The TUNEL reaction mixture was prepared. 50 μl TUNEL reaction mixture was added in the specimens. The slide or sealing film was stamped at 37°C for 60 min in the dark wet box. PBS was washed for 3 times. One drop of PBS was added to count apoptotic cells under the microscope (Olympus AX80, Olympus, Tokyo, Japan) (The excitation wavelength was 450~500 nm).
TTC staining
After the mouse in each group was sacrificed, the heart was quickly removed and washed with PBS. The atrium and right ventricle were removed. The left ventricle was left, quickly frozen at -20°C for 20 min, then cut into slices with the freezing microtome. The thickness of each piece was 2 mm. The slices were placed in preheated 37°C TTC staining solution, incubated for 15 min at 37°C away from light. The myocardial tissue was turned over for homogeneous staining. PBS was used to wash for three times. At this moment, the infarction area showed white and non-infarct area showed deep red. The different staining areas were measured with image processing software. The area of myocardial infarction = (infarct size/myocardial area × myocardial weight)/left ventricular weight × 100.
Statistical analysis
All data were analyzed using SPSS 17.0 statistical software (SPSS Inc, Chicago, IL, USA). The measurement data were shown using X±S. The analysis of variance was used to compare he measurement data among multiple groups. LSD method was used to compare between two-two comparison. P<0.05 showed that the difference had statistical significance.
Results
Changes of survivin mRNA and protein levels in the myocardium tissue of rats in three groups
Survivin mRNA expressions in acute myocardial infarction tissue in rat after different treatments were analyzed by real-time fluorescence quantitative PCR. The results were as shown in Figure 1A. Compared with model group and sham group, Survivin mRNA expression in myocardial tissue of rats in Survivin group was significantly increased (P<0.05). Western blot results showed that the expression of Survivin protein in myocardium tissue in Survivin group was significantly higher than those of model group and sham group (P<0.05), indicating that adenovirus mediated survivin expression plasmid can express survivin protein in myocardial tissues.
Figure 1.
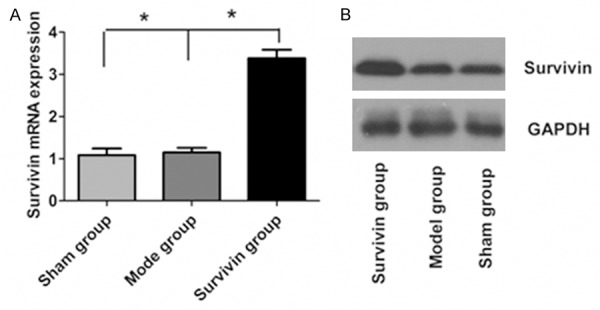
Changes of Survivin mRNA and protein levels in myocardial tissues of rats in three groups. A. Survivin mRNA in three groups; B. Survivin protein in three groups.
Changes of Caspase-3, Caspase-7 mRNA and protein levels in the myocardium tissue of rats in three groups
Real-time fluorescence quantitative PCR results were as shown in Figure 2A, 2B. Compared with sham group, Caspase-3 and Caspase-7 mRNA expressions in myocardial tissue of rats in model group and Survivin group were significantly increased, the difference was statistically significant (P<0.05). Compared with model group, Caspase-3 and Caspase-7 mRNA levels in myocardial tissue of rats in Survivin group were significantly down-regulated (P<0.05). Western Blot results were shown in Figure 1C. Compared with control group, Caspase-3 and Caspase-7 protein levels in model group and Survivin group were up-regulated, but the amplitude was significantly lower in Survivin group (P<0.05).
Figure 2.
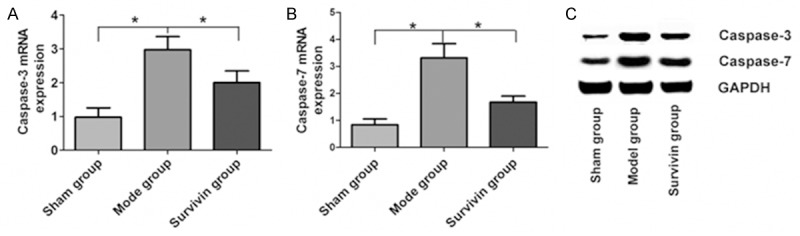
Changes of Caspase-7 and Caspase-3 mRNA and protein levels in myocardial tissues of rats in three groups. A. Caspase-3 mRNA levels in myocardial tissues of rats in three groups; B. Caspase-7 mRNA levels in myocardial tissues of rats in three groups; C. Caspase-3 and Caspase-7 protein levels in myocardial tissues of rats in three groups.
Changes of apoptosis in the myocardial tissues of rats in three groups
The myocardial cell apoptosis of rats was analyzed by TUNEL staining after different treatments. Compared with sham group, cell apoptosis in myocardial tissue of rats in model group were significantly increased, but significantly decreased in adenovirus mediated Survivin protein overexpression in myocardial cell apoptosis, the difference was statistically significant (P<0.05) (Figure 3).
Figure 3.
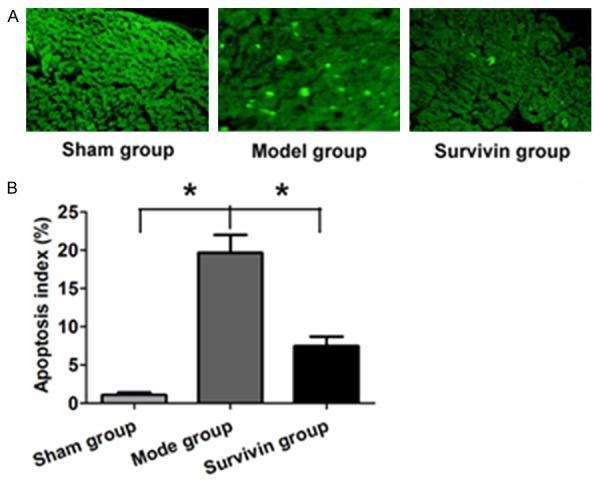
Changes of apoptotic cells in myocardial tissues of three groups. A. Myocardial cells apoptosis analysis in the three groups by TUNEL staining; B. Myocardial cells apoptosis in different treated groups by quantitative analysis.
Comparison of myocardial infarction of rats in three groups
Compared with sham group, the infarction size of myocardial tissue of rats in model group was significantly increased. Compared with model group, the myocardial infarct size in acute myocardial infarction rats was decreased significantly after treated by adenovirus mediated Survivin (P<0.05) (Figure 4).
Figure 4.
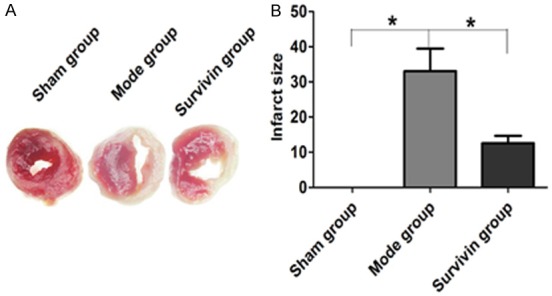
Changes of myocardial infarction of rats in three groups.
Discussion
Acute myocardial infarction is a common clinical cardiovascular and cerebrovascular disease. It can result in the formation of thrombosis in myocardial tissue, deficiency in both blood and oxygen, anaerobic phenomenon occurs on the infarct zone, the oxidative phosphorylation is blocked, finally the cell apoptosis and necrosis appear [12]. A large number of studies have shown that in the early stage of acute myocardial infarction, the number of apoptotic myocardial cells far exceeds that of necrotic myocardial cells. Therefore, apoptosis holds the dominant position in the early of acute myocardial infarction. The early acute myocardial infarction is also the golden period of its treatment. To effectively inhibit cell apoptosis induced by hypoxia and ischemia has important significance to reduce myocardial infarction size [13]. Apoptosis is programmed cell death process initiated by various stimulations in vitro and in vivo and is an initiative death process regulated by various genes. Apoptosis runs through the whole life with cell proliferation and plays a pivotal role in in the maintenance of normal body development [14]. Therefore, to effectively inhibit the early apoptosis in acute myocardial infarction plays an important protective role to myocardium.
Survivin is a new member in anti-apoptosis protein family. Survivin has tumor specificity. It is only expressed in tumor and embryonic tissues, also closely related with tumor cell differentiation, proliferation, invasion and metastasis. It is a very strong anti-apoptosis inhibitor [15,16]. Survivin inhibits cell apoptosis mainly through two ways [17,18]: ① To directly inhibit the activities of terminal effector enzymes Caspase-3 and Caspase-7 so as to block cell apoptosis process induced by all stimulations; ② Interaction between Survivin, cyclin-dependent kinases CDK4 and CDK2 blocks apoptotic signaling pathways. Survivin overexpression can significantly inhibit tumor cells apoptosis. There was no report on the biological effect of Survivin overexpression in myocardium tissue of acute myocardial infarction rats. In this study, the effect of Survivin protein overexpression on myocardial tissue of acute myocardial infarction rats was analyzed.
The study showed that adenovirus mediated Survivin gene expression vector could significantly improve the expression of Survivin protein in myocardium. Adenovirus is a kind of particle without capsule. Among them, DNA exists in the form of linear double chains. It has many advantages, for example, host range is wide, can infect both dividing and non-dividing cells, preparation titer is high, exogenous gene loading capacity is big, so it has been widely applied in basic research and clinical trials, and adenovirus vector has become one of the commonly used gene therapy vectors in the world [19]. Our study showed that Caspase-3 and Caspase-7 mRNA and protein levels in myocardium tissue after acute myocardial infarction were significantly increased, and were significantly reduced after treated with Survivin, but still higher than those of sham group. Caspase-3 and Caspase-7 are the key effector molecules in the apoptosis pathway, and the its down-regulation may inhibit cell apoptosis [20]. TUNEL staining further verified that the number of Survivin protein overexpressed myocardium cell apoptosis was decreased significantly. The myocardial cell apoptosis in rats was significantly inhibited in the early stage, so Survivin protein overexpressed myocardial infarction range in rats was also significantly down-regulated mediated by adenovirus.
In short, adenovirus mediated Survivin protein overexpression can significantly inhibit myocardium cell apoptosis in acute myocardial infarction rat and reduce the range of myocardial infarction. This study will provide new theoretical basis for the treatment of acute myocardial infarction.
Acknowledgements
We are grateful to all the participants in this study. We thank Prof. Yin for helpful comments.
Disclosure of conflict of interest
None.
References
- 1.DiNicolantonio JJ, Fares H, Niazi AK, Chatterjee S, D’Ascenzo F, Cerrato E, Biondi-Zoccai G, Lavie CJ, Bell DS, O’Keefe JH. β-Blockers in hypertension, diabetes, heart failure and acute myocardial infarction: a review of the literature. Open Heart. 2015;2:e000230. doi: 10.1136/openhrt-2014-000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fordjour PA, Wang Y, Shi Y, Agyemang K, Akinyi M, Zhang Q, Fan G. Possible mechanisms of C-reactive protein mediated acute myocardial infarction. Eur J Pharmacol. 2015;760:72–80. doi: 10.1016/j.ejphar.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Luo KQ, Long HB, Xu BC. Reduced apoptosis after acute myocardial infarction by simvastatin. Cell Biochem Biophys. 2015;71:735–740. doi: 10.1007/s12013-014-0257-1. [DOI] [PubMed] [Google Scholar]
- 4.Malick M, Gilbert K, Barry M, Godbout R, Rousseau G. Desvenlafaxine reduces apoptosis in amygdala after myocardial infarction. Brain Res Bull. 2014;109:158–163. doi: 10.1016/j.brainresbull.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Machado NG, Alves MG, Carvalho RA, Oliveira PJ. Mitochondrial involvement in cardiac apoptosis during ischemia and reperfusion: can we close the box? Cardiovasc Toxicol. 2009;9:211–227. doi: 10.1007/s12012-009-9055-1. [DOI] [PubMed] [Google Scholar]
- 6.Burke AP, Virmani R. Pathophysiology of acute myocardial infarction. Med Clin North Am. 2007;91:553–572. doi: 10.1016/j.mcna.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Mobahat M, Narendran A, Riabowol K. Survivin as a preferential target for cancer therapy. Int J Mol Sci. 2014;15:2494–2516. doi: 10.3390/ijms15022494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung CH, Huang CC, Tsai FY, Lee JY, Cheng SM, Chang YC, Huang YC, Chen SH, Chang JY. Survivin-biology and potential as a therapeutic target in oncology. Onco Targets Ther. 2013;6:1453–1462. doi: 10.2147/OTT.S33374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boidot R, Végran F, Lizard-Nacol S. Transcrip tional regulation of the survivin gene. Mol Biol Rep. 2014;41:233–240. doi: 10.1007/s11033-013-2856-0. [DOI] [PubMed] [Google Scholar]
- 10.Vegran F, Boidot R, Oudin C, Riedinger JM, Lizard-Nacol S. Distinct expression of Surv ivin splice variants in breast carcinomas. Int J Oncol. 2005;27:1151–1157. [PubMed] [Google Scholar]
- 11.Chen TL, Zhu GL, He XL, Wang JA, Wang Y, Qi GA. Short-term pretreatment with atorvastatin attenuates left ventricular dysfunction, reduces infarct size and apoptosis in acute myocardial infarction rats. Int J Clin Exp Med. 2014;7:4799–4808. [PMC free article] [PubMed] [Google Scholar]
- 12.Arboix A, Alio J. Acute cardioembolic cerebral infarction: answers to clinical questions. Curr Cardiol Rev. 2012;8:54–67. doi: 10.2174/157340312801215791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbate A, Salloum FN, Vecile E, Das A, Hoke NN, Straino S, Biondi-Zoccai GG, Houser JE, Qureshi IZ, Ownby ED, Gustini E, Biasucci LM, Severino A, Capogrossi MC, Vetrovec GW, Crea F, Baldi A, Kukreja RC, Dobrina A. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008;117:2670–2683. doi: 10.1161/CIRCULATIONAHA.107.740233. [DOI] [PubMed] [Google Scholar]
- 14.Rossi D, Gaidano G. Messengers of cell death: apoptotic signaling in health and disease. Haematologica. 2003;88:212–218. [PubMed] [Google Scholar]
- 15.Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, Reed JC. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58:5315–5320. [PubMed] [Google Scholar]
- 16.Baratchi S, Kanwar RK, Kanwar JR. Survivin: a target from brain cancer to neurodegenerative disease. Crit Rev Biochem Mol Biol. 2010;45:535–554. doi: 10.3109/10409238.2010.516740. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki A, Ito T, Kawano H, Hayashida M, Hayasaki Y, Tsutomi Y, Akahane K, Nakano T, Miura M, Shiraki K. Survivin initiates procaspase 3/p21 complex formation as a result of interaction with Cdk4 to resist Fas-mediated cell death. Oncogene. 2000;19:1346–1353. doi: 10.1038/sj.onc.1203429. [DOI] [PubMed] [Google Scholar]
- 18.Shankar SL, Mani S, O’Guin KN, O’Guin KN, Kandimalla ER, Agrawal S, Shafit-Zagardo B. Survivin inhibition induces human neural tumor cell death through caspase-independent and -dependent pathways. J Neurochem. 2001;79:426–436. doi: 10.1046/j.1471-4159.2001.00596.x. [DOI] [PubMed] [Google Scholar]
- 19.Puntel M, A K M GM, Farrokhi C, Vanderveen N, Paran C, Appelhans A, Kroeger KM, Salem A, Lacayo L, Pechnick RN, Kelson KR, Kaur S, Kennedy S, Palmer D, Ng P, Liu C, Krasinkiewicz J, Lowenstein PR, Castro MG. Safety profile, efficacy, and biodistribution of a bicistronic high-capacity adenovirus vector encoding a combined immunostimulation and cytotoxic gene therapy as a prelude to a phase I clinical trial for glioblastoma. Toxicol Appl Pharmacol. 2013;268:318–330. doi: 10.1016/j.taap.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan W, Dai Y, Xu H, Zhu X, Cai P, Wang L, Sun C, Hu C, Zheng P, Zhao BQ. Caspase-3 modulates regenerative response after stroke. Stem Cells. 2014;32:473–486. doi: 10.1002/stem.1503. [DOI] [PubMed] [Google Scholar]


