Abstract
This report evaluates the efficiency and safety of catheter-directed thrombolysis (CDT) using tissue plasminogen activator (tPA) and argatroban for the treatment of IVC filter thrombosis complicated by heparin-induced thrombocytopenia (HIT). From October 2012 to December 2014, 19 patients with unilateral lower extremity deep venous thrombosis were treated with standard anticoagulation, filter placement and urokinase-based CDT, all of whom developed IVC filter thrombosis and HIT. A revised protocol (tPA-based CDT and argatroban-based anticoagulation) was performed to treat IVC thrombosis. The extent of lysis was graded from I to III. Technical and clinical outcomes and complications were monitored. A total of 22 filters were implanted, 20 of which were retrieved later. The technical success rate of revised CDT for IVC thrombosis was 100%. On evaluating IVC thrombus, thirteen cases (68.4%, 13/19) were identified as grade III (complete resolution of thrombus) and six (31.6%, 6/19) as grade II (50-99% resolution of thrombus). No major bleeding related to CDT occurred. HIT in all patients was successfully treated with argatroban. Two patients with malignant tumor died during the follow-up. For patients with IVC filter thrombosis complicated by HIT, it seems tPA-based CDT and argatroban is an alternative regimen.
Keywords: Inferior vena cava filter, venous thrombosis, catheter-directed thrombolysis, anticoagulation therapy
Introduction
Implantation of a filter into inferior vena cava (IVC) has become an acceptable and reliable method to prevent or reduce fatal pulmonary embolism (PE) for patients with lower-extremity deep venous thrombosis (LEDVT). However, a filter dwelled in IVC may be associated with a higher incidence of thrombotic complications such as IVC occlusion, thrombosis, and the recurrence of deep venous thrombosis [1-5]. According to the literature [6,7], IVC filter thrombosis is not a rare complication; its incidence ranges from 2% to 10%, even rising to 30% in some series, depending on the type of filters used [8].
Extensive IVC filter thrombosis may develop just from the captured clots in the filter, or extend from the ilio-femoral vein thrombosis. Type of filters used, hypercoagulable conditions, and strategies of anticoagulation therapy have been reported as major factors contributory to IVC filter thrombosis [9-12].
The clinical significance is largely dependent upon the patency loss of IVC and the extent of compensatory collateral network. The management of IVC filter thrombosis includes anticoagulation therapy and thrombus removal strategy, basically similar to LEDVT does [13]. The use of heparin, or low molecular weight heparin (LMWH) is routinely accepted as a standard coagulation therapy [14]. Most of patients with venous thrombosis will respond to such treatments. However, a rare but severe complication, heparin-induced thrombocytopenia (HIT), may occur due to exposure to heparin or LMWH [15,16] and in turn complicates the treatments for previously existed LEDVT, usually making it refractory to the standard anticoagulation therapy and catheter-directed thrombolysis (CDT), and sometimes going to extreme to induce extensive IVC filter thrombosis. Although some guidelines for managing HIT have been available for referring to, the treatments for a patient with LEDVT and HIT are still a challenge, and to an extent, contingent upon the clinician’s experiences. In the present study, we reported our experience in dealing with such cases of acute IVC filter thrombosis complicated by HIT.
Methods
Patients
Institutional Review Board approval was obtained from the ethics committee of our institution for this study. Between October 2012 and December 2014, 19 patients (fourteen of whom were male) were referred to our department with symptomatic IVC thrombosis following filter implantation. The mean age of the patients was 38.64 years (range, 23-72 years). The onset time ranged from 2 to 14 days.
All patients were diagnosed as acute LEDVT initially, with sixteen on the left versus three on the right. At this time, no involvement of the IVC was found by venography. They were then treated with LMWH-based standard anticoagulation plus urokinase-based CDT therapy. Prior to undergoing CDT, a filter was placed into the IVC. Three to 5 days after such treatments, all patients complained of no relief but aggravation of the present symptoms in comparison with onset, including bilateral lower limb swelling (n=15), pain (n=8), cyanosis (n=6), pallescence (n=7), and rising skin temperature (n=3). Therefore, further examinations were performed to evaluate if the thrombosis propagated. Risk factors were analyzed as well to evaluate patients’ coagulable status, co-existence of HIT and the like.
The management at the initial stage
Once the diagnosis of LEDVT was established, all patients received the standard anticoagulation therapy plus urokinase-based CDT after careful consideration about the risk of bleeding and benefits of clot removal. Unlike developed western countries, urokinase is a widely-used thrombolytic agent for venous thrombosis in China just because of economic concern. However, in the present study, the treatments for such patients were split into two stages: the initial stage, treated with LMWH-based anticoagulation using LMWH and urokinase-based CDT, and the second stage, treated with a revised protocol, i.e., argatroban-based anticoagulation and tissue plasminogen activator (tPA)-based CDT.
Anticoagulation therapy
Anticoagulation therapy was performed as early as possible once the diagnosis of LEDVT was made. LMWH (Fragmin, Pfizer Inc, New York, USA) was used twice a day via subcutaneous injection (5000 IU per injection).
Implanting IVC filters
Prior to CDT, a filter was placed into the infrarenal IVC against potential PE. The indications and procedures of filter implantation in the study complied strictly with the Guidelines for the Performance of Inferior Vena Cava Filter Placement for the Prevention of Pulmonary Embolism from Society of Interventional Radiology [6,17]. Before inserting the filter, a cavogram was obtained using a 4F pigtail catheter via the right jugular vein or the contralateral femoral vein approach. After confirming that IVC was free of thrombus, an OptEase (Cordis Corp., Miami Lakes, FL, USA) filter was placed into the segment of IVC 1-2 cm below the level of the renal vein confluence. No permanent filter was placed in the study.
CDT for LEDVT
After inserting filter, a thrombolytic catheter (UniFuse) with a 20- or 30-cm-length side-hole (AngioDynamics, Queensbury, NY, USA), or Cook catheter, with a 16-cm-length side hole (Cook Medical, Bloomington, IN, USA), was placed into the thrombus in lower extremity deep veins via the right jugular vein, the ipsilateral popliteal vein or the contralateral femoral vein approach. The end of the catheter was connected with a self-controllable infusion pump, allowing a total of 500,000-750,000 IU urokinase per day to be infused in continuously and evenly.
During the period, daily blood test and coagulation function examination was performed. The physical examinations were also performed twice a day to evaluate the patient’s status. Daily repeated venography via the thrombolytic catheter was routinely performed to inspect the venous response to lytic therapy.
The management at the second stage
The diagnosis of HITT
The diagnosis of HIT relied on clinical assessment as well as laboratory tests. The clinical assessment should at least include the followings: recent exposure to heparin products, presence or absence of unexpected new or progressive thrombosis and skin necrosis at heparin injection sites. The repeated laboratory tests should be performed to ascertain whether an absolute thrombocytopenia (platelet count <150,000/µL) or relative thrombocytopenia (drop in platelet count of at least 50% from baseline value) was developed. Presence of HIT antibodies was also detected by ELISA immunoassay. We also adopted a pretest clinical score called the “4Ts” [(Thrombocytopenia, Timing, Thrombosis) and the absence of other explanation(s)] to assist in diagnosis [18].
Revised anticoagulation therapy
The previously used LMWH was discontinued immediately. The revised anticoagulation of argatroban (TIPR Pharmaceutical Responsible Co., Ltd, Tianjing, China) was administrated intravenously twice a day (40 mg per administration).
CDT for IVC filter thrombosis
After confirmation of IVC filter thrombosis, the previous CDT specifically for LEDVT should be modified. If the previous CDT was via the right jugular vein or the popliteal vein approach, the thrombolytic catheter was then exchanged into a new one with a longer porous segment, allowing it to cover the thrombus as much as possible. If the previous CDT is through the contralateral femoral vein, additional coverage of caval thrombosis with a new CDT was created, usually through the right jugular vein.
To expedite the lytic process, the thrombolytic agent was also changed. Instead of urokinase, tissue plasminogen activator (tPA) (Boehringer Ingelheim China, Shanghai, China), 20 mg per day in total, was infused continuously for 2-3 days through the thrombolytic catheter(s). A cavogram and lower-extremity venogram was repeated every day during thrombolysis therapy. The location of the thrombolytic catheter was adjusted according to the conditions of the residual thrombus. If second filter was needed, it commonly was placed into the supra-renal portion of IVC.
Assisted thrombus-removal techniques
To expect a rapid reduction of clot burden, we also performed prior to CDT mechanical thrombectomy by means of manual aspiration using an 8F guiding catheter (Envoy, Cordis) or AngioJet device (Boston Scientific, Natick, MA, USA). A total of thirteen patients underwent the assisted thrombus-removal treatments, 7 of them with catheter aspiration versus 6 with AngioJet devices. The AngioJet Rheolytic Thrombectomy System was primed for use as described in the Instructions. For each patient, 2 runs of rheolytic thrombectomy were performed over the thrombotic segment.
Filter retrieval
When no or minimal residual thrombus was remained in the IVC and iliofemoral veins, the thrombolytic therapy was terminated and the IVC filter was retrieved.
The retrieval techniques for OptEase have been described everywhere. In brief, a 10F guiding catheter (Cordis Corp., Miami Lakes, FL, USA) was inserted into the IVC near the filter. Due to its specific structure, the OptEase filter should be retrieved via the femoral vein approach. The snare for retrieving filter was passed through the catheter. The retrieval hook of the filter was engaged with the snare, and the filter was then trapped into the guiding catheter. After doing so, the filter was retracted and removed. A repeated cavogram was obtained following retrieval to inspect for complications. All patients accepted long-term anticoagulation treatment with oral warfarin following the retrieval procedure.
Outcome evaluation
The technical and clinical outcome, complications, and postoperative PE were monitored. All patients were asked a follow up at 6 months for angiographic and clinical evaluation and annually thereafter.
Based on the changes in venography, the extent of lysis was graded from I to III. Grade III lysis was defined as complete resolution of thrombus on visual assessment of venograms. Grade II and grade I lysis were deemed present when the extent of thrombus resolution was 50-99% and <50%, respectively.
Results
The demographic and clinical data of all patients were listed in Tables 1 and 2.
Table 1.
Demographic data of patients (n=19)
| Factors | No. of patients (% total) |
|---|---|
| Male gender | 14 (73.7%) |
| Age ≤40 yrs | 12 (63.2%) |
| Initial left-sided DVT | 16 (84.2%) |
| Risk factors for DVT | |
| Malignant tumor | 2 (10.5%) |
| Post trauma | 4 (21.1%) |
| Calf soft tissue infection | 3 (15.8%) |
| Post operation | 2 (10.5%) |
| Postpartum | 2 (10.5%) |
| APS | 1 (5.3%) |
| Unknown | 5 (26.3%) |
| Symptoms of caval thrombosis | |
| Unilateral limb swelling | 4 (21.1%) |
| Bilateral limb swelling | 15 (88.9%) |
| Pain | 8 (42.1%) |
| Limb cyanosis | 6 (31.6%) |
| Limb pallescence | 7 (36.8%) |
| Rising skin temperature | 3 (15.8%) |
DVT, deep venous thrombosis; APS, antiphospholipid syndrome.
Table 2.
Patients treated with the revised protocol (n=19)
| Factors | value |
|---|---|
| Filters in total | 22 |
| No. of filters initially inserted | 19 |
| No. of filters secondly inserted | 3 |
| Duration of tPA-based CDT | 3.08 ± 0.45 days |
| The total dose of tPA used | 59.71 ± 15.02 mg |
| No. of patients with assisted techniques (% total) | 19 |
| Guiding catheter aspiration | 7 (36.8%) |
| AnjioJet rheolytic thrombectomy | 6 (31.6%) |
| None | 6 (31.6%) |
| No. of lysis grade (% total) achieved in caval thrombosis* | 19 |
| Grade I | 0 |
| Grade II | 6 (31.6%) |
| Grade III | 13 (68.4%) |
| No. of complications (% total)** | 19 |
| Major | 0 |
| Minor | 4 (21.1%) |
tPA, tissue plasminogen activator; CDT, catheter-directed thrombolysis.
Lysis grade: grade III, complete resolution of thrombus on visual assessment of venograms; Grade II, 50-99% resolution; grade I, <50%.
Major complications including massive bleeding, severe allergy, renal dysfunction and the like, usually requiring prompt treatments.
On initial venogram, all LEDVTs basically involved the iliac veins. Additional involvement of femoral veins was found in 12 patients. Sixteen of 19 patients were with left-sided LEDVT versus 3 patients right-sided. No caval thrombosis was found initially.
The outcome of the first stage
A total of 19 filters were successfully placed in the infrarenal segment of IVC. The CDT was through the right jugular vein in 11 patients, the popliteal vein in 5 patients and the contralateral femoral vein in 3 patients. The average duration of CDT at the first stage was 3.6 ± 0.7 days (range, 3-5 days). The total dose of urokinase used was 2,236,800 ± 348,350 IU (range 1,500,000-3,000,000 IU). During this process, no severe complications were found and all patients showed a good compliance to the CDT.
The diagnosis of HIT
All nineteen patients were diagnosed with HIT. They all got a high score (≥6) estimated with the “4Ts” system. They all experienced a dramatic drop of platelet count to -116,000/µL and -84,500/µL 3 and 4 days after exposure to LMWH respectively. The platelet count began to return 3-4 days after instead use of argatroban, getting to normal about twelve days. The longitudinal changes of the platelet count were showed in Figure 1.
Figure 1.
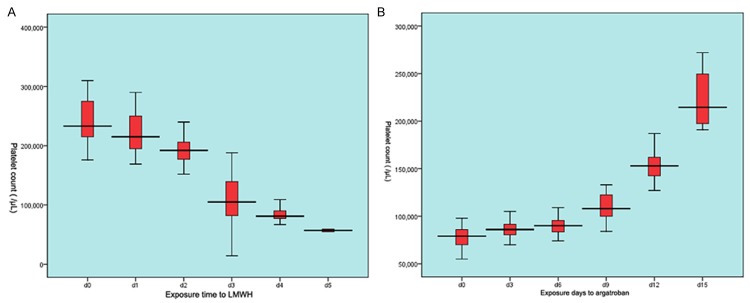
The longitudinal response of platelet count to LMWH and argatroban. (A) showed a dramatical drop of platelet count after exposure to LMWH (lower molecular weight heparin). The platelet count returned steadily to normal level twelve days after administration of argatroban (B).
On repeated venography two to 5 days after urokinase-based CDT, the infrarenal IVC and filter was filled with newly developed thrombosis, causing complete caval occlusion in 12 patients but partial occlusion with seemingly unaffected caval flow in the others. The previous DVT proved not to be cleared but progressed in all patients, and newly formed thrombus in the contralateral iliofemoral veins was found in 3 patients.
The outcomes of the second stage
The revised CDT
The technical success rate of the revised CDT was 100%. The duration time of the revised CDT was 3.08 ± 0.45 days (range, 2-4 days) and the total dose of tPA was 59.71 ± 15.02 mg (range, 40-80 mg). Grade III of lysis was achieved in 13 (68.4%) and 12 patients (63.2%) in the setting of IVC filter thrombosis and iliofemoral thrombosis respectively, versus grade II in 6 (31.6%) and 7 (36.8%) patients respectively (Figures 2, 3). No major bleeding occurred, but minor ecchymosis was found in four patients (21.1%, 4/19).
Figure 2.
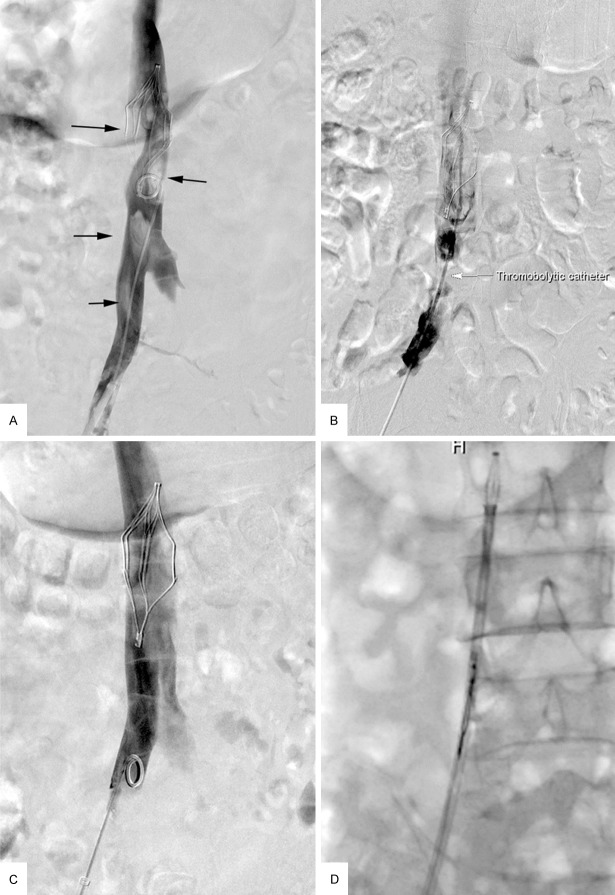
A-D. A 60-year-old man with left-extremity deep venous thrombosis (DVT) developed filter-related inferior vena cava (IVC) thrombosis. A. Venogram revealed a sign of “filling defect” within the IVC, indicating thrombus (black arrow). B. A thrombolytic catheter was placed within the thrombus via the right femoral vein (white arrow). C. Follow-up venogram revealed complete resolution of thrombus (grade III). D. The filter was retrieved successfully.
Figure 3.
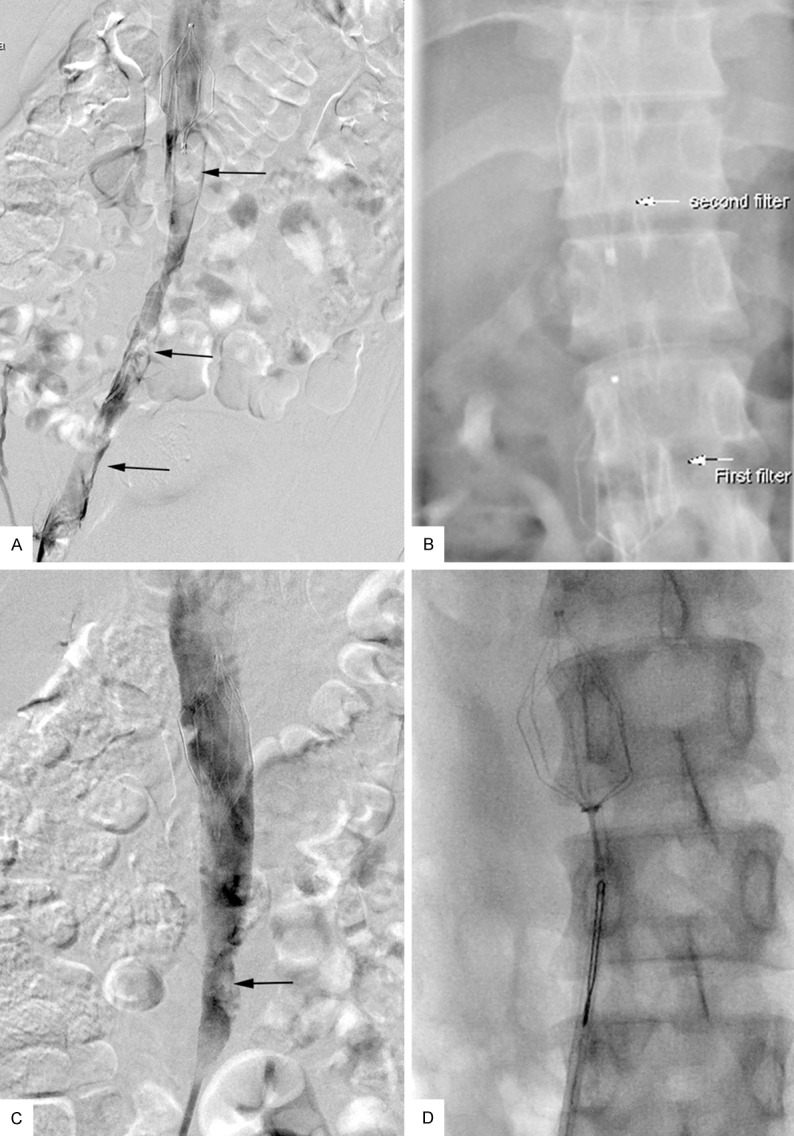
A-D. A 24-year-old man with right-extremity DVT developed extensive IVC thrombosis after insertion of a filter. A. Venogram revealed extensive thrombosis within the IVC and iliac vein (black arrow). B. A second filter was placed above the first filter (white arrow). C. Follow-up venogram revealed grade II resolution of thrombus. Minor residual thrombus was noted (black arrow). D. The filters were retrieved successfully.
Clinical outcomes
All patients reported a relief of clinical symptoms at discharge. The median follow-up time was 12.11 ± 6.15 months (range, 2-25 months). During the follow-up, no clinically detectable PE or lower extremity swelling were observed except one with lung carcinoma reported a recurrent LEDVT. No further endovascular intervention was performed for the recurrence. During the follow-up, two patients with malignant tumors died of the complications directed to the progression of tumors.
Filter retrieval
Three patients underwent insertion of a second filter (Figure 2), making a total of 22 filters inserted into the IVC. Two patients who had malignant tumor declined the attempt of retrieval. Twenty filters were ultimately retrieved; a success rate of 100%. No procedure-related complications occurred.
Discussion
In the present study, we reported that some cases with LEDVT, even treated with LMWH-based anticoagulation and urokinase-based CDT, soon got a HIT and new or progressive IVC filter thrombosis, which was finally removed with a revised protocol. In our opinion, the revised protocol (argatroban-based anticoagulation and tPA-based CDT) was over the initial protocol, the advantages including: a rapid removal of thrombus, effectiveness in salvaging HIT, significant improvement on clinical presentations, and a short catheter indwelling time. Also, the revised protocol seemed to be highly safe, as proved in our study that no severe complications developed.
Widespread use of IVC filters for LEDVT patients has been closely associated with increasing number of complications. Owing to the draining of lateral veins, not all cases of IVC filter thrombosis are clinically meaningful. However, thrombus propagation into the IVC filter, even above the level of renal vein, is potentially a catastrophic complication, leading to a spectrum of sequelae ranging from mild ambulatory lower-extremity swelling to incapacitating edema at rest, venous claudication and/or venous ulcers, and even recurrence of PE and renal failure [9,19].
The mechanism of filter thrombosis covers several potential factors, including hemodynamic changes after IVC filter implantation, vein wall injuries resulting from the stimulus of the filter’s radial force, and captured thrombus in filters [20]. In addition, the failure of anticoagulant therapy may increase the risk of thrombosis [9,12]. In the present study, the patients suffered from a progressive thrombosis having been refractory to a standard anticoagulation and urokinase-based CDT, which might be attributed to the followings: filter induced caval wall injuries and hemodynamic insult; and HIT. The both then might produce a synergistic effect expediting the caval thrombosis.
Treatment with anticoagulants has been historically directed toward prevention of recurrent venous thromboembolism. Anticoagulants are encouragingly put in use as early as possible for the candidates, especially who has a confirmed DVT. Nowadays, LMWH is the standard agent for anticoagulation. It is very common that thrombocytopenia occurs in patients receiving heparin or LMWH, but only a minority can be called HIT [18]. The underestimation of HIT may compromise the outcomes in patients with a previous thrombosis. It might be the case when a previous thrombosis is refractory to LMWH and lytic agents. Once HIT is suspected or confirmed, Heparin or LMWH should be replaced with appropriate non-heparin anticoagulant. Argatroban, a direct thrombin inhibitor, is approved for treating thrombosis complicating HIT. It is also recommended that warfarin not be started until substantial resolution of thrombocytopenia has occurred (preferably, platelet count >150,000/µL) [18].
Nowadays, urokinase and tPA are the most common thrombolysis agents [13]. In China, urokinase is greeted due to its wide availability and affordable cost. Xiao et al. [20] reported that after infusion of urokinase through a thrombolytic catheter, the residual thrombus in the IVC was <10% in four of five patients with a thrombo-occlusive IVC filter, the blood flow in the IVC was recovered in all five patients, and swelling of the lower extremity disappeared or was relieved in all 10 limbs. Angle et al. [21] reported a similar thrombolytic success rate of 88% in seven of eight patients using local catheter-directed infusion of urokinase. However, urokinase-based CDT needs a long dwelling time of catheter in the veins that might discomfort the patients and increases the risks for developing complications.
In the present study, urokinase was ineffective to LEDVT in all patients. Presumably, the following factors were responsible. First, two of 19 patients in our study suffered from concurrent malignant tumor and four females were postpartum. These patients perhaps were in a state of hypercoagulation, instigating an insensitive response to urokinase and being prone to recurrence of DVT. Second is the existence of HIT resulting in propagation of DVT.
We then used a revised protocol to expect a rapid removal of thrombus. In such complicated cases with caval thrombosis and HIT, the revised protocol consisting of argatroban and tPA proved to be highly effective and safe. The merits of such a protocol include: a steady resolution of thrombocytopenia over an average of two weeks; a rapid removal of clot burden, a favorable outcomes in terms of thrombus resolution and clinical improvements; a minority of minor complications. We surmise that these merits are most likely to be associated with a synergistic effect between argatroban and tPA, as proved in some animals studies [22,23].
Our results are in line with that of Janivier et al. [24], who reported a case of bilateral renal vein thrombosis caused by IVC filter migration, which was successfully treated using catheter-directed infusion of tPA. Sharifi et al. [25] reported that 33 patients with massive symptomatic iliac and femoropopliteal DVT underwent CDT with tPA and argatroban. Complete resolution of thrombus on venography was achieved in 30 patients (91%).
However, massive bleeding is still a main concern over the combination of argatroban and tPA. In their small phase II study, Barreto et al. [26] reported that four of 65 patients (6.2%) with acute ischemic stroke using tPA and argatroban simultaneously suffered a significant intracerebral hemorrhage. Sharifi et al. [25] suggested that the following measures might reduce the risk of bleeding: using a lower dose of argatroban; injecting both drugs within the thrombus rather than using bolus injection; and using imaging guidance to reduce the probability of inadvertent arterial puncture. We are in agreement with this opinion. In addition, close monitoring of the serum fibrinogen level is very important. A rapidly decreased fibrinogen level indicates that reducing the dose of tPA is necessary.
This study was not randomized and had no control arm, and was a retrospective study with a small sample size. Future studies directly and prospectively comparing tPA and urokinase are therefore required. Nevertheless, to our knowledge this report is the first to describe the efficacy and safety of tPA and argatroban in the treatment of IVC filter thrombosis and HIT for which urokinase and LMWH are ineffective.
In conclusion, we surmise that for those patients with DVT and HIT having no positive response to urokinase and LMWH, tPA plus argatroban may be an effective regime in thrombolytic therapy.
Acknowledgements
This study was supported by grants from the Nanjing Medical Science and Technology Development Foundation (grant no. YKK13105) and from the Nanjing Science and Technology Development Foundation (grant no. 201402049).
Disclosure of conflict of interest
None.
References
- 1.Kinney TB. Update on inferior vena cava filters. J Vasc Interv Radiol. 2003;14:425–440. doi: 10.1097/01.rvi.0000064860.87207.77. [DOI] [PubMed] [Google Scholar]
- 2.Berczi V, Bottomley JR, Thomas SM, Taneja S, Gaines PA, Cleveland TJ. Long-term retrievability of IVC filters: should we abandon permanent devices? Cardiovasc Intervent Radiol. 2007;30:820–827. doi: 10.1007/s00270-007-9153-z. [DOI] [PubMed] [Google Scholar]
- 3.PREPIC Study Group. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study. Circulation. 2005;112:416–422. doi: 10.1161/CIRCULATIONAHA.104.512834. [DOI] [PubMed] [Google Scholar]
- 4.Joels CS, Sing RF, Heniford BT. Complications of inferior vena cava filters. Am Surg. 2003;69:654–659. [PubMed] [Google Scholar]
- 5.Streiff MB. Vena caval filters: a comprehensive review. Blood. 2000;95:3669–3677. [PubMed] [Google Scholar]
- 6.Grassi CJ, Swan TL, Cardella JF, Meranze SG, Oglevie SB, Omary RA, Roberts AC, Silverstein MI, Towbin RB, Lewis CA. Quality improvement guidelines for percutaneous permanent inferior vena cava filter placement for the prevention of pulmonary embolism. J Vasc Interv Radiol. 2003;14:S271–275. [PubMed] [Google Scholar]
- 7.Yan BP, Kiernan TJ, Gupta V, Ajani AE, Schainfeld RM. Combined pharmacomechanical thrombectomy for acute inferior vena cava filter thrombosis. Cardiovasc Revasc Med. 2008;9:36–40. doi: 10.1016/j.carrev.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Patel SH, Patel R. Inferior vena cava filters for recurrent thrombosis: current evidence. Tex Heart Inst J. 2007;34:187–194. [PMC free article] [PubMed] [Google Scholar]
- 9.Tardy B, Mismetti P, Page Y, Décousus H, Da Costa A, Zeni F, Barral G, Bertrand JC. Symptomatic inferior vena cava filter thrombosis: clinical study of 30 consecutive cases. Eur Respir J. 1996;9:2012–2016. doi: 10.1183/09031936.96.09102012. [DOI] [PubMed] [Google Scholar]
- 10.Mohan CR, Hoballah JJ, Sharp WJ, Kresowik TF, Lu CT, Corson JD. Comparative efficacy and complications of vena caval filters. J Vasc Surg. 1995;21:235–245. doi: 10.1016/s0741-5214(95)70265-2. [DOI] [PubMed] [Google Scholar]
- 11.Nazzal M, Chan E, Nazzal M, Abbas J, Erikson G, Sediqe S, Gohara S. Complications related to inferior vena cava filters: a single-center experience. Ann Vasc Surg. 2010;24:480–486. doi: 10.1016/j.avsg.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Crochet DP, Brunel P, Trogrlic S, Grossetëte R, Auget JL, Dary C. Long-term follow-up of Vena Tech-LGM filter: predictors and frequency of caval occlusion. J Vasc Interv Radiol. 1999;10:137–142. doi: 10.1016/s1051-0443(99)70455-0. [DOI] [PubMed] [Google Scholar]
- 13.Meissner MH, Gloviczki P, Comerota AJ, Dalsing MC, Eklof BG, Gillespie DL, Lohr JM, McLafferty RB, Murad MH, Padberg F, Pappas P, Raffetto JD, Wakefield TW Society for Vascular Surgery, American Venous Forum. Early thrombus removal strategies for acute deep venous thrombosis: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2012;55:1449–1462. doi: 10.1016/j.jvs.2011.12.081. [DOI] [PubMed] [Google Scholar]
- 14.Kearon C, Akl EA. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood. 2014;123:1794–1801. doi: 10.1182/blood-2013-12-512681. [DOI] [PubMed] [Google Scholar]
- 15.Jaax ME, Greinacher A. Management of heparin-induced thrombocytopenia. Expert Opin Pharmacother. 2012;13:987–1006. doi: 10.1517/14656566.2012.678834. [DOI] [PubMed] [Google Scholar]
- 16.Linkins LA, Dans AL, Moores LK, Bona R, Davidson BL, Schulman S, Crowther M. Treatment and prevention of heparin-induced thrombocytopenia: antithrombotic therapy and prevention of thrombosis. 9th edition. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e495S–530S. doi: 10.1378/chest.11-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caplin DM, Nikolic B, Kalva SP, Ganguli S, Saad WE, Zuckerman DA. Quality improvement guidelines for the performance of inferior vena cava filter placement for the prevention of pulmonary embolism. J Vasc Interv Radiol. 2011;22:1499–1506. doi: 10.1016/j.jvir.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Warkentin TE. Heparin-Induced Thrombocytopenia Diagnosis and Management. Circulation. 2004;110:e454–e458. doi: 10.1161/01.CIR.0000147537.72829.1B. [DOI] [PubMed] [Google Scholar]
- 19.Senitko M, Sims R, Dolmatch B, Vazquez MA, Lu CY. Inferior vena cava (IVC) filter thrombosis in a renal transplant recipient. Clin Nephrol. 2009;71:456–459. doi: 10.5414/cnp71456. [DOI] [PubMed] [Google Scholar]
- 20.Xiao L, Shen J, Tong JJ, Zhang Z, Mu XL, Yi ZJ, Bai S, Xu K. Transcatheter thrombolytic therapy for symptomatic thrombo-occlusion of inferior vena cava filter. Exp Ther Med. 2013;5:533–538. doi: 10.3892/etm.2012.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angle JF, Matsumoto AH, Al Shammari M, Hagspiel KD, Spinosa DJ, Humphries JE. Transcatheter regional urokinase therapy in the management of inferior vena cava thrombosis. J Vasc Interv Radiol. 1998;9:917–925. doi: 10.1016/s1051-0443(98)70422-1. [DOI] [PubMed] [Google Scholar]
- 22.Valji K, Arun K, Bookstein JJ. Use of a direct thrombin inhibitor (argatroban) during pulse-spray thrombolysis in experimental thrombosis. J Vasc Interv Radiol. 1995;6:91–95. doi: 10.1016/s1051-0443(95)71067-3. [DOI] [PubMed] [Google Scholar]
- 23.Boeve TJ, Reed GL, de Oliveira NC, Titus J, Janssens S, Giugliano RP, Torchiana D, Daggett W, Schwarz R, Jang IK. Comparison of argatroban and hirudin for the reperfusion of thrombotic arterial occlusion by tissue plasminogen activator. J Thromb Thrombolysis. 1998;6:103–108. doi: 10.1023/A:1008889202725. [DOI] [PubMed] [Google Scholar]
- 24.Janvier AL, Hamdan H, Malas M. Bilateral renal vein thrombosis and subsequent acute renal failure due to IVC filter migration and thrombosis. Clin Nephrol. 2010;73:408–412. doi: 10.5414/cnp73408. [DOI] [PubMed] [Google Scholar]
- 25.Sharifi M, Bay C, Nowroozi S, Bentz S, Valeros G, Memari S. Catheter-directed thrombolysis with argatroban and tPA for massive iliac and femoropopliteal vein thrombosis. Cardiovasc Intervent Radiol. 2013;36:1586–1590. doi: 10.1007/s00270-013-0569-3. [DOI] [PubMed] [Google Scholar]
- 26.Barreto AD, Alexandrov AV, Lyden P, Lee J, Martin-Schild S, Shen L, Wu TC, Sisson A, Pandurengan R, Chen Z, Rahbar MH, Balucani C, Barlinn K, Sugg RM, Garami Z, Tsivgoulis G, Gonzales NR, Savitz SI, Mikulik R, Demchuk AM, Grotta JC. The argatroban and tissue-type plasminogen activator stroke study: final results of a pilot safety study. Stroke. 2012;43:770–775. doi: 10.1161/STROKEAHA.111.625574. [DOI] [PMC free article] [PubMed] [Google Scholar]