Abstract
Background: Neural stem cells (NSCs) are promising for ischemia stroke because they can replace damaged or lost cells. However, the adult central nervous system (CNS) does not provide an optimal microenvironment for exogenous NSCs to survive, proliferation and differentiation. We established a co-transplantation system with NSCs and astrocyte and brain microvascular endothelial cells (BMECs) to explore whether it can improve the memory ability in ischemic stroke rat. Methods: After building the ischemic stroke in 50 rats by middle cerebral artery occlusion and reperfusion (MCAO/R), transplantation of NSCs and astrocyte and BMECs were performed with different combination. Results: Laser doppler flowmetry and MRI were used to detect the ischemia of the model and 42 rats survived for the Morris water-maze test. The test shows that co-transplantation with the three different cells together can improve memory deficits in MCAO/R rat and it is the most effect group. Grafting with two cells have more effect in memory improving than one cell while transplanting NSC alone has no obvious effect on memory improving. Conclusions: In NSC niche, astrocytes and BMECs are the most important cells to regulate and interaction with NSCs. Co-transplantation NSCs with astrocyte and BMECs can improve the memory ability in ischemia rat, which maybe the result of microenvironment improve by the astrocyte and BMECs.
Keywords: Neural stem cells, middle cerebral artery occlusion and reperfusion, transplantation, hippocampus
Introduction
Stroke is an acute and progressive neurodegenerative disorder of brain that has already become one of the main reasons of various disabilities and mortality [1]. However, treatment options to date are very limited especially for ischemic patients. Tissue plasminogen activator (t-PA) is the only Food and Drug Administration (FDA) approved agent for ischemic stroke, but even so, only 1-2% patients can benefit from it for their risk of hemorrhage [2].
Neural stem cells (NSCs) have the potential of neurogenesis, synaptic plasticity and even angiogenesis that can repair the brain function after stroke [3]. Evidence has suggested that the enormous potential of NSCs transplantation treatment for ischemic stroke. NSCs can survive and differentiate to neurons and glia after intravenous administration or transplanted into the parenchyma around the damaged tissue [4-6]. With the induced of chemokines, cells were targeted migration to the site of the lesion and can enhance functional recovery [7,8].
However, a major obstacle for NSCs grafting in ischemic stroke is the vast loss of cells after transplantation. These death cells can reach even to 90% of the total transplantation cells in stroke and Parkinson’s disease [9]. The further research shows that the local microenvironment or niche is the most important and decisive factor for the survival of these NSCs. So preparing a proper niche for NSCs is the key point in brain repair recovery strategy after ischemic stroke [10].
Co-transplantation NSCs with other main cells in the niche maybe a better strategy to improve microenvironment and make the grafting more efficient. In our work, we choose astrocyte and brain microvascular endothelial cells (BMECs) as the co-transplant cells and compare the different among the various combination to study the benefit of co-transplantation to the ischemia stroke in rat.
Materials and methods
Animal and groups
Adult and healthy male Sprague-Dawley (SD) rats (around 250 g) were prepared by the Experimental Animal Center of Wuhan University. The rats were randomly divided into 5 groups and every group was 10 rats. These were Group A: Middle cerebral artery occlusion and reperfusion + NSCs transplantation (MCAO/R + NSCs); Group B: MCAO/R + NSCs + astrocytes transplantation (MCAO/R + NSCs + Astrocyte); Group C: MCAO/R + NSCs + BMECs transplantation (MCAO/R + NSCs + BMECs); Group D: MCAO/R + NSCs + BMECs + astrocytes transplantation (MCAO/R + NSCs + BMECs + Astrocyte); Group E: MCAO/R + PBS transplantation (MCAO/R + PBS). The conduction of operation procedures and postsurgical animal care were accordance with China’s Guidelines for Care and Use of Laboratory Animals. Other 10 newborn rats (less than 24 h) were provided from the same center and prepared for the cells isolation and culture.
Main culture medium and reagents
DMEM, DMEM/F12 and B27 were purchased from Gibco (Invitrogen Corporation, USA). EGF and bFGF were obtained from Peprotech Company (USA). FBS (Fetal bovine serum) and BSA (bovine serum albumin) were purchased from Hyclone Company (Thermo scientific, USA). Collagenase/dispase gelatin and percoll were prepared by Pharmacia Company (New Jersey). Antibody such as rabbit anti-nestin antibody, rabbit anti-NF antibody, and mouse anti-GFAP antibody were obtained from Sigma Company (USA). Rabbit VIII factor antibody was purchased from Dako Cytomation (Denmark). Secondary antibodies were as list below: goat anti-mouse fluorescein isothiocyanate (Boster Company, Wuhan, China); goat anti-rabbit CY3 (Sigma).
Isolation and purification and culture of three kinds of rat cerebral cells
All animal experiments were approved ethically by the Administration committee of experimental animals, Hubei Province, China.
NSCs, astrocytes and BMECs were isolated and cultured from neonatal SD rats. Briefly, for NSCs, after the hippocampus of rat were isolated and cut into small fragments, tissues were digested by 0.25% trypsin. Then cells centrifuged and re-suspended in a serum-free medium consisting of DMEM/F12, B27, EGF and bFGF, and then seeded into culture flask. After neural stem cells had formed spheres, the neurospheres were passaged every 3-4 days.
Astrocytes were isolated and cultured as described in previous reports [11,12]. The grey matter of brain was dissected and digested by trypsin. Centrifuging and re-suspended precipitate in the DMEM with 10% FBS. Then filtered with 70-μm mesh and centrifugation and re-suspended again with complete medium contain FBS. Cultured the cells for 4-5th days and shaken at 220 rpm for 18 h at 37°C to purify the astrocytes.
For the BMECs, after removing meninges from the cortices, tissues were digested with 0.1% collagenase (type II) at 37°C for 1.5 hour. And then added 20% BSA to centrifuge and washed it with PBS. The purified microvessel pellet was digested further in 0.1% collagenase/dispase in DMEM for 1 hour at 37°C. Finally, the suspension was collected with percoll gradient solutions and centrifuged. The cell suspension added with supplemented DMEM and seeded into culture dishes. After incubated for 24-hours, remove the suspended non-BMECs matter and add fresh medium.
The modified MCAO/R model
A modified right MCAO/R procedure [13] was used in our ischemic stroke model. Briefly, the right common carotid artery (CCA), external carotid arteries (ECA), internal carotid arteries (ICA) and vagus nerve were exposed and isolated carefully. Temporal blocking the CCA and ICA by a slipknot, electrocauterization the distal end of ECA and cut it off. Then cut a small hole on the proximal end of the ECA and inserted a 4-0 silicon rubber-coated monofilament from the ECA into the lumen of the ICA. Loosen the slipknot on ICA and kept the filament going 18-20 mm beyond the carotid bifurcation to the middle cerebral artery (MCA). After 1 hour of occlusion, the filament was withdrawn and then sealed the hole on ECA by electrocauterization, then loosen the slipknot on CCA for reperfusion. Kept the rectal temperature at 37±0.5°C by using a temperature-controlled blanket. The model was assessed by the monitoring of cerebral blood flow (CBF) with a laser-doppler flowmetry (PeriFlux System 5000, Perimed, Sweden) during the operation and the magnetic resonance imaging (MRI) 24-hours after surgery.
Transplantation
Grafting was performed 12 days after the MCAO/R, using methods described in the supplemental online data [14]. The animals were fixed on stereotaxic apparatus after anesthetization. The coordinates for CA1 was chose from the atlas of Paxinos and Watson [15] as 3.2 mm posterior to the bregma, 1.9 mm lateral to the midline, and 2.9 mm ventral of the dorsal surface of the skull. Each graft contained 1×105 live cells in 1 μl of the culture medium. Group A graft contained 1 μl NSCs and 4 μl PBS, group B graft contained 1 μl NSCs and 1 μl Astrocyte and 3 μl PBS, group C graft contained 1 μl NSCs and 1 μl BMECs and 3 μl PBS, group D graft contained 1 μl NSCs and 1 μl BMECs and 1 μl Astrocyte and 2 μl PBS, group E graft contained 5 μl PBS. The injection rate is 1 μl/min by Hamilton syringe, and the needle is held in place for an additional 5 min before retraction.
The Morris water maze test
The Morris water maze test was carried out at 8 weeks after transplantation. Briefly, the Morris water maze test consists of 1 day of pre-training trial, 4 days of hidden platform tests and a spatial probe trial 24 h after the last hidden platform test. The movement of rats was tracked and recorded by an auto-tracking system (Smart Track). There were 4 trials every day in hidden platform tests and a 3-5 min interval between each trial. Animals allowed to swim for 120 s to find the hidden platform at each trial, and after that, they allowed to take a rest on platform for 15 s. For those animals failed to the mission, the score was marked as 120 s. The time to find the platform (escape latency) and the distance to the platform were recorded and measured as a learning score in each trial. One day after the last hidden platform test, a spatial probe test was performed to measure the retention of spatial memory without the platform.
Statistical analysis
All the data were plotted as means ± SD (standard error) and analyzed with the Student’s t-test and one-way analysis of variance (ANOVA). Unpaired Student’s t-test was used to compare the difference between 2 groups and multiple groups was evaluated by analysis of variance (ANOVA). P values of less than 0.05 were considered significant.
Results
Morphology of three kinds of rat cerebral cells
NSCs incubate in serum free medium and form many small floating neurospheres in 2-3 days (Figure 1A). These cells show nestin-positive (Figure 1B) by immunostaining, suggesting that they are neural stem cells or neural progenitor cells that remain multipotential. When NSCs cultured with 1% FBS, the differentiated cells can be noticed obvious and proved to be neurons (NSE positive) (Figure 1C), astrocytes (GFAP positive) (Figure 1D) by using immunocytochemistry.
Figure 1.
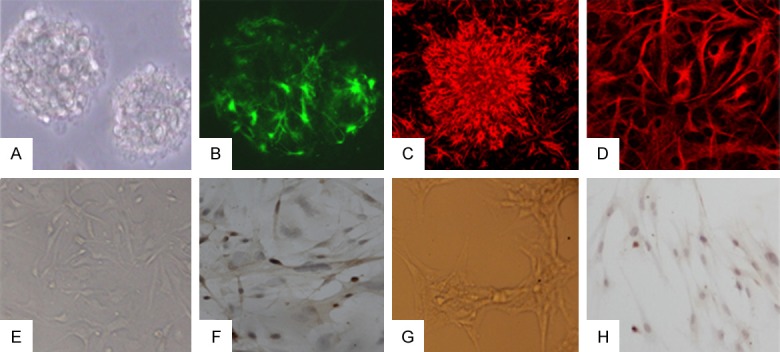
Culture and identification of three kinds of cells. A. Neural stem cells proliferate and form neurospheres. B. Neural stem cells show nestin-positive. C. Neural stem cells differentiate into neuron and show NSE positive. D. Neural stem cells differentiate into neuron and show GFAP positive. E. BMECs presented a long spindle-shape morphology. F. VIII factor related antigen immunohistochemistry stain that cytosolic and perinuclear regions were positive. G. Astrocyte culture show the long processes. H. Immunocytochemical stain show GFAP positive in cytoplasmic of astrocyte.
The BMECs could migrate out from brain microvessel fragments and presented a typical cobblestone-like cluster in 2 days and could grow up to a long spindle-shape morphology (Figure 1E) in the third day. Within 5 to 6 days, the cells grew to confluency and presented a swirl monolayer. The cytosolic and perinuclear regions were positively stained after by immunohistochemical staining with factor VIII related antigen and the region of nuclear showed a bubble-like substructure (Figure 1F).
After 3-4 days after plating, astrocytes increase significantly and astrocyte layer is forming and neurons are almost absent. And the cytoplasmic of astrocyte become larger and rich, the shape is irregular and the processes are more long (Figure 1G). Immunocytochemical analysis demonstrated cytoplasmic immunolabeling of the cytoskeletal protein GFAP, attesting to the astrocytic phenotype of the cultured cells (Figure 1H).
Assessment of stroke
Laser doppler flowmetry was used to record and monitor the cerebral blood flow (CBF) of rat throughout the stroke model to control the incomplete occlusion or poor insertion. The rat which the percentage of CBF drops less than 80% was excluded from the experiment (Figure 2A, 2B). MRI was used to detect the ischemia of the model 24 hours after the operation and which one had been expired from the group with no signal of the infarction (Figure 2C, 2D).
Figure 2.
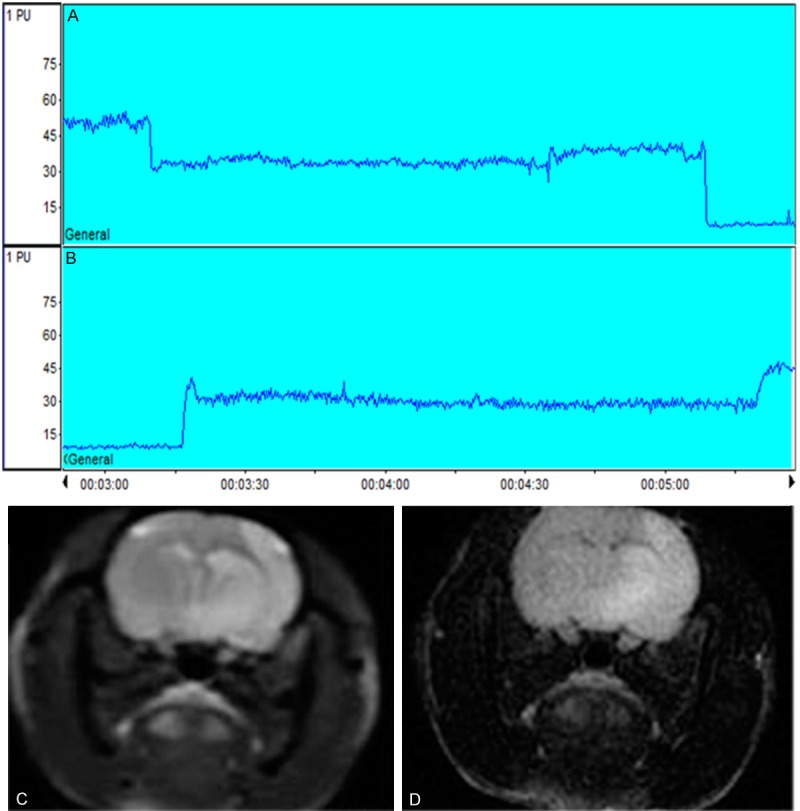
Representative cerebral blood flow and magnetic resonance imaging of MCAO/R model of rat. A, B. Laser Doppler Flowmetry showed that the CBF was two drops from baseline during occlusion procedure and two jumps during reperfusion procedure, the percentage drop from baseline ≥80%. C, D. Brain edema accompanying focal ischemia is visualized in T2-weighted and T2-Flair MRI images.
Morris water-maze test
42 rats survive for the Morris water-maze test and other 8 rats dead during the complication of model of MCAO/R and the grafting. During the 4-day training period of hidden platform test, the swimming time and path length to reach the platform declined day by day in all groups. Mean escape latency times have significant difference among groups by one-way ANOVA (F=29.63, P<0.01). For more information, we have made t-test between the groups and we find that there is significant difference between Group A and B (P<0.05), Group A and C (P<0.05), Group A and D (P<0.01), Group B and D (P<0.01), Group C and D (P<0.01), Group C and E (P<0.05), Group D and E (P<0.01). While there is no significant difference between Group A and E (P>0.05), Group B and C (P>0.05), Group B and E (P>0.05) (Table 1) (Figure 3A, 3B).
Table 1.
Changes in escape latency of acquisition tests in the water maze task
| Group N | 1st day | 2nd day | 3rd day | 4th day |
|---|---|---|---|---|
| A 8 | 68.3±18.4 | 52.7±18.0 | 41.5±16.7 | 35.6±12.8 |
| B 8 | 65.9±15.7 | 48.1±16.6 | 39.7±13.5 | 30.4±11.3 |
| C 10 | 65.5±16.3 | 42.9±19.3 | 35.4±14.4 | 31.6±11.9 |
| D 9 | 58.2±14.9 | 38.6±13.1 | 25.7±9.8 | 15.5±6.3 |
| E 7 | 73.4±20.6 | 57.2±15.2 | 43.8±12.1 | 33.5±13.6 |
Figure 3.
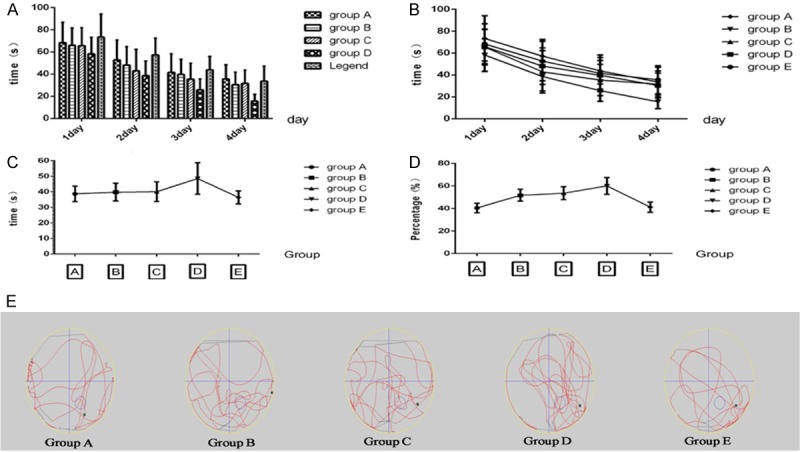
Morris water-maze test show the difference of improving memory deficits in MCAO/R rat among the grafting groups. A. Interleaved bar analysis of escape latency among grafting groups show the difference between group D and other groups in acquisition tests. B. Superimposed symbols with connecting line show that the swimming time are shorten gradually with the passage of days in acquisition tests. C. Mean connected line display the platform quadrant swimming time changes in spatial probe tests. D. Mean connected line of platform quadrant swimming distance/total swimming distance in spatial probe tests. E. The swimming locus of animal movement tracked and recorded by an auto-tracking system in spatial probe tests. Black point is the start position, the circle third quadrant represent the platform position placed in the acquisition tests.
Comparison between groups show that there is significant difference between Group A and B (t=4.234, P=0.0241), Group A and C (t=3.698, P=0.0343), Group A and D (t=7.255, P=0.0054), Group B and D (t=6.631, P=0.007), Group C and D (t=3.730, P=0.0336), Group C and E (t=3.207, P=0.0491), Group D and E (t=22.71, P=0.0002). While there is no significant difference between Group A and E (t=1.501, P=0.2302), Group B and C (t=1.419, P=0.2511), Group B and E (t=1.336, P=0.2737).
In the spatial probe trial on the fifth day, rat which co-transplantation with the three different cells together (Group D) traveled into the third quadrant, where the hidden platform was previously placed, significantly more times than other groups (P<0.05) (Table 2). The percentage of the platform quadrant swimming distance/total swimming distance among the groups is also significant difference (F=18.48, P<0.01), which are 40.5%, 51.8%, 53.6%, 60.1% and 41.2% respectively (Figure 3C-E) (Table 2).
Table 2.
Changes in spatial probe tests in the water maze task
| Group | Platform quadrant swimming time (s) | Platform quadrant swimming distance/total swimming distance (%) |
|---|---|---|
| A | 38.7±5.0 | 40.5±4.2 |
| B | 39.8±5.7 | 51.8±5.2 |
| C | 40.1±6.3 | 53.6±5.7 |
| D | 48.6±10.1 | 60.1±7.4 |
| E | 36.4±4.2 | 41.2±4.6 |
In platform quadrant swimming time, t-test shows that there is significant difference between Group A and D (t=2.507, P=0.0242), Group B and D (t=2.171, P=0.0464), Group C and D (t=2.227, P=0.0398), Group E and D (t=2.983, P=0.0099). The percentage of the platform quadrant swimming distance/total swimming distance among the groups is also significant difference (F=18.48, P<0.01) by one-way ANOVA.
From the analysis, we can find that Group D had significant difference to all other groups, which means co-transplantation with the three different cells together can improve memory deficits in MCAO/R rat and it is the most effect group. Group B and C has also significant difference to group A, it means that grafting with two cells have more effect in memory improving than one cell. Group A has no significant difference with group E which show transplanting NSC alone has no obvious effect on memory improving. Group B also has no significant difference with Group E while group C has significant difference with Group E which suggests grafting NSC and BMECs have more effect than grafting NSC and astrocyte. This may hint that the BMECs has more effect than astrocyte in this model.
Discussion
The NSCs niche in the CNS are composed with the interactions among cells, the vasculature, the extracellular matrix and basal lamina [16]. Astrocytes may interact with NSCs and produce some growth factors and receptors to regulate their renewal and differentiation. Meanwhile, BMECs can produce a special microenvironment and influence the characteristics of NSCs [16]. Cells in germinal zones secrete vascular endothelial growth factors (VEGF) and attract microvessel growth toward them in the development of the CNS [17]. Thus, in adult rat CNS, NSCs mainly localize in the dentate gyrus of the hippocampus and subventricular zone where the brain microvascular and their cells are close to these stem cells [18].
So, optimization the NSCs niche by other helper cells and graft NSCs with them together maybe better than a single transplantation of NSCs. In 2003, Lo [19] and his colleagues have proposed a concept of Nerve Vascular Unit (NVU), which refers to an overall structure and function unit consisting of brain neurons, BMECs, glial cells, even with pericytes, basement membrane, microglia cells and extracellular matrix [19-21]. This concept emphasizes the dynamic interactions among all of the components and their important influence on the pathophysiology of stroke [22]. NVU is acknowledgedly composed by neurons as the core component together with BMECs, astrocytes, pericyte, basal membrane, microglia and extracellular matrix. Previous investigations have demonstrated that astrocytes play a crucial role between the communications of neurons and BMECs [23,24]. Therefore, neurons, BMECs, and astrocytes are the key components of NVU.
In the adult mammal CNS, astrocytes can provide structural, metabolic and tropic support for neurons. Actually, astrocytes play a key role in the brain involved in the maintenance of the extracellular environment and in the stabilization of cell-cell communications under physiological and pathological conditions [25-28]. At the same time, in the stem cell niche, astrocytes act uniquely as sensors and regulators for their meandering processes contact all cells and their end feet terminate on the brain microvessels. So, astrocytes are prepared to detect changes in neurons and translate signals from the microvasculature and other cells in germinal regions. And these cells are extensively connected each other with gap junctions and they may provide rapid propagation of signaling in the NSC niche. Furthermore, astrocytes themselves can produce grow factors which support neurogenesis in vitro [29].
It is also proved that BMECs, a main component of microvessels and the blood-brain barrier, not only have the function of barrier protection and nutrition transport as semipermeable membrane, but also play a key role in the proliferation and differentiation of NSCs. In brain, NSCs is close to BMECs, and in most cases nerve regeneration is followed by the proliferation of BMECs, especially in some pathological stimulation such as ischemic injury [30,31]. BMECs can excrete diverse bioactive molecules, which help stimulate the proliferation of NSCs and their differentiation to terminal nerve cells, and also help neuron and its precursor migrate to the injured region of brain [32-35]. Ying et al used transwell co-culture with BMECs and NSCs to compare the effects of BMECs and VEGF on the behaviors of NSCs. They found the nestin-positive cell ratio for the BMECs group is higher than that for VEGF-A group after 7 days’ treatment with VEGF-A or BMECs. After removing VEGF-A or BMECs, the cells were cultured in differentiated conditioned medium for another 4 days. The NF-positive cell ratio for the BMEC group is also higher than that for the VEGF-A group, which indicates that BMECs take an advantage over VEGF-A in both promoting the self-renewal, proliferation, or survival of NSCs and enhancing the potency of differentiation to neuron [36]. Shen et al, also found BMECs have a strong ability to promote the growth of NSCs. They established a coculture system of endothelial cell line from mouse brain with NSCs separated from mouse embryo cerebral cortex (10th to 11th day), and effects of the endothelial cells on the self-renewal and differentiation of NSCs were observed [37].
That is to say, in NSC niche, astrocytes and BMECs are the most important cells to regulate and interaction with NSCs. So, co-transplantation NSCs with astrocytes and BMECs maybe a promising strategy for the treatment of ischemic stroke. In our work, we have established the triple cells co-transplantation system with rat NSCs, astrocytes and BMECs. After graft these cells into the brain of MACO/R model rat with different combination, we found that the learning and memory ability of these rat improved to some extend respectively. The rats which transplantation of NSCs with astrocyte and BMECs together have got the most achievement and they are better than those who grafted only two cells. The rats grafted only one cell have got the worst improvement. From our experiment, we get the conclusion that co-transplantation NSCs with astrocyte and BMECs can improve the memory ability in ischemia rat, which maybe the result of microenvironment improve by the astrocyte and BMECs. The exact mechanism maybe some synergistic effects happened among NSCs and astrocytes and BMECs after co-transplantation and need further research.
Acknowledgements
This work is supported by Nature Science Foundation of Hubei Province of China (2009CDB422), Hubei Province health and family planning scientific research project (WJ2015MB092) and the foundation of China Scholarship Council.
Disclosure of conflict of interest
None.
References
- 1.Kim G, Kim E. The Effects of Antecedent Exercise on Motor Function Recovery and Brain-derived Neurotrophic Factor Expression after Focal Cerebral Ischemia in Rats. J Phys Ther Sci. 2013;25:553–6. doi: 10.1589/jpts.25.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaffer H, Morris VB, Stewart D, Labhasetwar V. Advances in Stroke Therapy. Drug Deliv Transl Res. 2011;1:409–19. doi: 10.1007/s13346-011-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindvall O, Kokaia Z. Stem cell research in stroke: how far from the clinic? Stroke. 2011;42:2369–75. doi: 10.1161/STROKEAHA.110.599654. [DOI] [PubMed] [Google Scholar]
- 4.Chu K, Kim M, Park KI, Jeong SW, Park HK, Jung KH, Lee ST, Kang L, Lee K, Park DK, Kim SU, Roh JK. Human neural stem cells improve sensorimotor deficits in the adult rat brain with experimental focal ischemia. Brain Res. 2004;1016:145–53. doi: 10.1016/j.brainres.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 5.Jeong SW, Chu K, Jung KH, Kim SU, Kim M, Roh JK. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke. 2003;34:2258–63. doi: 10.1161/01.STR.0000083698.20199.1F. [DOI] [PubMed] [Google Scholar]
- 6.Pollock K, Stroemer P, Patel S, Stevanato L, Hope A, Miljan E, Dong Z, Hodges H, Price J, Sinden JD. A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp Neurol. 2006;199:143–55. doi: 10.1016/j.expneurol.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel J, Yenari MA, Weissman IL, Uchida N, Palmer T, Steinberg GK. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci U S A. 2004;101:11839–44. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belmadani A, Tran PB, Ren D, Miller RJ. Chemokines regulate the migration of neural progenitors to sites of neuroinflammation. J Neurosci. 2006;26:3182–91. doi: 10.1523/JNEUROSCI.0156-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang Y, Agren L, Lyczek A, Walczak P, Bulte JW. Neural progenitor cell survival in mouse brain can be improved by co-transplantation of helper cells expressing bFGF under doxycycline control. Exp Neurol. 2013;247:73–9. doi: 10.1016/j.expneurol.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim H, Cooke MJ, Shoichet MS. Creating permissive microenvironments for stem cell transplantation into the central nervous system. Trends Biotechnol. 2012;30:55–63. doi: 10.1016/j.tibtech.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa S, Deli MA, Kawaguchi H, Shimizudani T, Shimono T, Kittel A, Tanaka K, Niwa M. A new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem Int. 2009;54:253–63. doi: 10.1016/j.neuint.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Noble M, Murray K. Purified astrocytes promote the in vitro division of a bipotential glial progenitor cell. EMBO J. 1984;3:2243–7. doi: 10.1002/j.1460-2075.1984.tb02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 14.Hosseini N, Nasehi M, Radahmadi M, Zarrindast MR. Effects of CA1 glutamatergic systems upon memory impairments in cholestatic rats. Behav Brain Res. 2013;256:636–45. doi: 10.1016/j.bbr.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Paxinos G, Watson CR, Emson PC. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J Neurosci Methods. 1980;3:129–49. doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- 16.Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13:543–50. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–94. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Galli R, Gritti A, Bonfanti L, Vescovi AL. Neural stem cells: an overview. Circ Res. 2003;92:598–608. doi: 10.1161/01.RES.0000065580.02404.F4. [DOI] [PubMed] [Google Scholar]
- 19.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 20.Guo S, Kim WJ, Lok J, Lee SR, Besancon E, Luo BH, Stins MF, Wang X, Dedhar S, Lo EH. Neuroprotection via matrix-trophic coupling between cerebral endothelial cells and neurons. Proc Natl Acad Sci U S A. 2008;105:7582–7. doi: 10.1073/pnas.0801105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat rev Neurosci. 2004;5:347–60. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 22.del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23:879–94. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- 23.Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 24.Moretto G, Xu RY, Monaco S, Rizzuto N, Kim SU. Expression and distribution of GAP-43 in human astrocytes in culture. Neuropathol Appl Neurobiol. 1995;21:362–7. doi: 10.1111/j.1365-2990.1995.tb01071.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang DD, Bordey A. The astrocyte odyssey. Prog Neurobiol. 2008;86:342–67. doi: 10.1016/j.pneurobio.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, Stout RF Jr, Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R, Verkhratsky A. Glial cells in (patho) physiology. J Neurochem. 2012;121:4–27. doi: 10.1111/j.1471-4159.2012.07664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schousboe A, Sickmann HM, Bak LK, Schousboe I, Jajo FS, Faek SA, Waagepetersen HS. Neuron-glia interactions in glutamatergic neurotransmission: roles of oxidative and glycolytic adenosine triphosphate as energy source. J Neurosci Res. 2011;89:1926–34. doi: 10.1002/jnr.22746. [DOI] [PubMed] [Google Scholar]
- 28.Ransom BR, Ransom CB. Astrocytes: multitalented stars of the central nervous system. Methods Mol Biol. 2012;814:3–7. doi: 10.1007/978-1-61779-452-0_1. [DOI] [PubMed] [Google Scholar]
- 29.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 30.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–16. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang R, Zhang Z, Zhang C, Zhang L, Robin A, Wang Y, Lu M, Chopp M. Stroke transiently increases subventricular zone cell division from asymmetric to symmetric and increases neuronal differentiation in the adult rat. J Neurosci. 2004;24:5810–5. doi: 10.1523/JNEUROSCI.1109-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–50. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Louissaint A Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–60. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 34.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–78. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 35.Wurmser AE, Palmer TD, Gage FH. Neuroscience. Cellular interactions in the stem cell niche. Science. 2004;304:1253–5. doi: 10.1126/science.1099344. [DOI] [PubMed] [Google Scholar]
- 36.Guo Y, Shi D, Li W, Liang C, Wang H, Ye Z, Hu L, Li Y. Effects of cerebral microvascular endothelial cells and vascular endothelial growth factor on the proliferation and differentiation of NSCs: a comparative study. Br J Neurosurg. 2010;24:62–8. doi: 10.3109/02688690903506077. [DOI] [PubMed] [Google Scholar]
- 37.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–40. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]


