Abstract
Bile duct hamartomas (BHs), also called von Meyenburg complex (VMC), are benign biliary malformations that originate from disorganization of the small intrahepatic bile ducts. This disorganization is often associated with the abnormal involution of embryonic ductal end plates in the liver. This is clinically significant, as the development of BHs can cause diagnostic confusion with liver metastases and small hepatocellular carcinoma (SHCC). Currently, we report a specific case of BHs and review the literature to better define and diagnose BHs. In the following case, a 37 year-old male bearing a lesion in his liver is presented and undergoes both radiological and pathological diagnosis. The lesion is preliminarily suspected to be a hepatic hemangioma by examination of conventional ultrasound (US), contrast enhanced ultrasound (CEUS), computerized tomographic scanning (CT) and magnetic resonance imaging (MRI). However, SHCC is suspected by follow-up analysis of US and CEUS, due to the patient’s background history of hepatitis B and growth of the lesion and a tumor-feeding vessel in BHs via CEUS. However, BHs are finally diagnosed by biopsy pathology under the guidance of ultrasound. Therefore, we believe pathology is imperative for correct diagnosis of BHs over other similar diseases when the imaging findings are atypical. Here we report the novel and unique detection of a tumor-feeding vessel, which mimicked SHCC strongly, during the course of CEUS. We also present a comprehensive review of the previous reported radiological examination related to BHs.
Keywords: Bile duct hamartomas (BHs), pathology, ultrasound (US), contrast enhanced ultrasound (CEUS), computerized tomographic (CT), magnetic resonance imaging (MRI)
Introduction
Bile duct hamartomas (BHs), also known as von Meyenburg complex (VMC), were initially described by the Swiss pathologist Hanns von Meyenburg [1] in 1918. BHs have been previously described as benign liver malformations resulting from maldevelopment of the ductal end plates, which remain in a persistent double-layered cylindrical structure similar to the fetal ductal plate [2,3]. Therefore, pathologically, BHs can be defined by examining dilated intrahepatic bile ductules and the surrounding fibrous stroma [4,5].
BHs are typically detected during laparotomy or autopsy, and the prevalence of BH formation is age-dependent, varying from 1% in children to 5.6% in adults in a large series of autopsy samples [4,6,7]. Macroscopically, BHs can be characterized by the visualization of many grayish-whitish or yellow small nodules scattered throughout the liver, either intraparenchymally or subcapsularly [6,8-10]. BHs may be detected as single or multiple hamartomas, with a diameter ranging from 1 to 15 mm [11]. During diagnosis, its characteristics can mimic that of liver metastases, especially when the patient has previously suffered a primary malignancy [9,11].
Patients with BHs are usually symptomatic. Jaundice, epigastralgia, and fever can occur. Jaundice, a major symptom, is caused by obstruction of the biliary tract owing to the mucus secreted by biliary papillomas [4,6,12]. However, these symptoms are nonspecific. A close relationship has been reported to exist between BHs and the following congenital disorders: Caroli’s disease, polycystic liver disease, congenital hepatic fibrosis, mesenchymal hamartomas, bile duct atresia and autosomal recessive polycystic kidney disease [5,8,13,14]. While VMC is a benign condition, there is an ongoing debate as to whether BHs have the potential to induce malignant degeneration to cholangiocarcinoma and hepatocellular carcinoma [6,8,11,13-15]. Here, we report a case of VMC diagnosed through several radiological examinations, including ultrasound (US), contrast-enhance ultrasound (CEUS), computerized tomographic scanning (CT) and magnetic resonance imaging (MRI). We present novel results via CEUS that suggest the occurrence of a tumor-feeding vessel. The imaging is enhanced in the arterial phase without early disappearance in the delay phase in CEUS. Additionally, we believe imaging strategies are not sufficient to achieve a most definitive diagnosis of BHs in the absence of typical imaging findings.
Case report
Here, we report the occurrence of a hypoechonical solid nodule in the right liver lobe detected by B-mode ultrasonography in a 37 year-old male. Our study had been approved by the ethics committee of Shanghai First People’s Hospital and the informed consent had been waived. Considering that the patient had a history of Hepatitis B, development of a malignant tumor could not be ruled out. At the initial screening, CT scanning missed the nodule because it was imaged in only one frame, which was reluctantly discovered in a later retrospective analysis of the CT imaging. Additionally, the patient refused to undergo a MRI examination. CEUS was further implemented, with the primary consideration being liver benign lesion. Six months later, follow-up with US, CEUS and MRI showed that the lesion had slightly increased in size (on US and CEUS) and indicated the presence of a tumor-feeding vessel. The MRI findings suggested that the lesion was benign. Because SHCC could not be ruled out on US and CEUS, considering the patient’s history of hepatitis B, a biopsy was indispensable in order to identify the final diagnosis. The patient had suffered right abdominal discomfort, but did not present with other clinical symptoms such as fever, nausea or vomiting, cough and expectoration, gastrointestinal dysfunction, jaundice, hematemesis or melena. Laboratory examination found no abnormal results, including α-FP and other tumor-related index. Next, we present the strategies used for diagnosis:
Conventional ultrasound (US)
The first ultrasound examination was conducted at initial screening. A solid non-homogeneous hypoechoic lesion was detected in the liver, measuring 8.9×13.6 mm (Figure 1A). This lesion was located in the upper segment of the right anterior hepatic lobe, and was considered a benign lesion due to its clear boundary, regular shape and the missing internal blood flow (Figure 1B). The ultrasound findings at the six-month follow-up were roughly consistent with that of the first time, with a slightly increased lesion size of 13.5×13.8 mm (Figure 1C). However, we additionally detected an internally sporadic blood flow (Figure 1D), which was initially suggestive of a malignant transformation.
Figure 1.
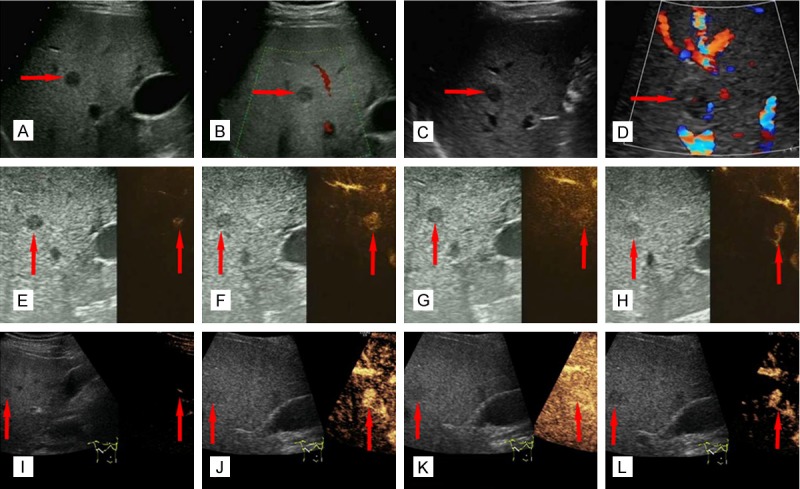
Imaging of patient findings via US and CEUS. (A) At initial examination, a heterogeneous hypoechoic nodule was located in the liver parenchyma via US (red arrow). (B) At initial examination, there was no blood flow in the interior of the nodule (red arrow). (C) The result of a second examination shows a similar heterogeneous nodule located in the liver parenchyma (red arrow). (D) The results of a second examination show sporadic internal blood flow in the interior of the nodule (red arrow). (E-H) At first CEUS examination, enhancement of the hypervascular nodule is earlier than the surrounding liver parenchyma. The arrival time, peak time and regression time of Sonovue® are (E) 12 seconds, (F) 19 seconds and (G) 24 seconds, respectively (red arrows). (H) A tumor-feeding vessel connecting the branch of hepatic artery and the lesion (red arrow). (I-L) At second CEUS examination, enhancement of the hypervascular nodule is also earlier than the surrounding liver parenchyma. The arrival time, peak time and regression time of Sonovue® are (I) 10 seconds, (J) 17 seconds, and (K) 24 seconds, respectively. (L) The tumor-feeding vessel is again detected (red arrows).
Contrast enhanced ultrasound (CEUS)
During the first examination, a 2.5 ml solution of the microbubble contrast agent Sonovue® was injected into the median cubital vein. Twelve seconds later, the dye began to delineate the lesion outline (Figure 1E), and reached peak contrast at 19 seconds (Figure 1F). The contrast lasted 24 seconds and then began to subside (Figure 1G). In the retrospective analysis six months later, a tumor-feeding vessel was observed during the contrast enhancement course, which was not detected at first examination of the CEUS data (Figure 1H).
At the six-month follow-up, both the arrival and peak contrast times were two seconds earlier than that of the first examination, 10 seconds (Figure 1I) and 17 seconds (Figure 1J), respectively. Similar to the initial screening, imaging started to subside at 24 seconds (Figure 1K). Again, the tumor-feeding vessel was also detected (Figure 1L). By CEUS examination, the lesion demonstrated a slight increase in size, which was an 11×15 mm in measurement, as compared to the initial measurement of 8.9×13.6 mm. CEUS of the nodule displayed an enhancement in the arterial phase, similar to the majority of liver cancers. However, the malignant liver lesions on CEUS typically disappear earlier than the surrounding liver parenchyma in the delay phase, but that trait did not emerge in our patient. Thus, valid conclusions could not be drawn to differentiate BHs and liver metastases via US and CEUS alone. Because of the changes in size and hemodynamics of lesion, a malignant lesion (SHCC or liver metastasis) was initially suspected.
Contrast enhanced computed tomographic (CECT)
To perform CECT, the arterial phase scanning and the porto-venous phase scanning were respectively started at 8 s and 35 s after a bolus of Ultravist 370 was injected. At the initial screening, CT imaging did not recognize the liver nodule as it was detected in only one cross-section. However, the nodule was detected in the following retrospective study of CT imaging by improving the brightness and contrast. Compared with the surrounding liver parenchyma, the density of the lesion was slightly lower and non-homogeneous. There was no enhancement in either hepatic arterial phase or in portal venous phase of iohexol-enhanced imaging (Figure 2A, 2B).
Figure 2.

Imaging of patient findings via CT and MRI. (A, B) CECT examination displayed no enhancement in both of hepatic arterial phase and portal venous phase (red arrows). (C-E) Conventinal MRI examination. (C) T1WI, the lesion is hypointense (red arrow). (D) T2WI, the lesion is hyperintense (red arrow). (E) DWI, the lesion shows free diffusion (red arrow). (F-H) Gadolinium-enhanced T1WI. (F) The lesion displays rim enhancement in the hepatic arterial phase, and marginal tuberculiform enhancement in both (G) portal venous phase and (H) hepatic vein (red arrows).
Magnetic resonance imaging (MRI)
A MRI examination was performed at the six-month follow-up. A round lesion measuring 15 mm in diameter was found in the upper segment of the right anterior hepatic lobe, with hypo-intense signals on T1-weighted images (T1WI) (Figure 2C), hyper-intense signals on T2-weighted images (T2WI) (Figure 2D), and high diffusion on diffusion-weighted imaging (DWI) (Figure 2E), respectively. After Magnevist was injected intravenously, the lesion was clearly imaged with rim enhancement in the hepatic arterial phase (Figure 2F) of enhanced T1WI, and marginally nodular enhancement was detected in portal venous phase (Figure 2G) and delayed phase (Figure 2H). Therefore, a liver hemangioma was suggested via the MRI results.
Biopsy under ultrasound guidance
Due to inconclusive diagnosis by the imaging studies, a biopsy was performed with a disposable core tissue biopsy needle, MN1820, Bard® Magnium®, USA, under the guidance of ultrasound instrument equipped with a puncture probe, Hitachi, Japan (Figure 3A, 3B). Three strips of tissue samples measuring 1 cm in diameter and 21 mm in length were obtained. The sample strips were placed on sterilized glass and turned over to prepare smear glass. Next, the strips were dipped in absolute alcohol to fix the specimens for pathological examination.
Figure 3.

Lesion biopsy under ultrasound guidance and pathological examination. (A) The lesion is clearly presented by two-dimensional sonogram. (B) The biopsy needle is inserted into the lesion through the liver parenchyma. (C-F) Pathological examination. (C, D) The lesion displays hyperplasia of the fibrocollagenous stroma and bile ducts ((H) and (E) staining, (C) ×100, (D) ×400). (E, F) The bile duct epithelia is positive for CK (Immunohistochemistry of CK, (E) ×100, (F) ×400).
The resulting pathological sections were reviewed by specialists in hepatobiliary pathology. It was determined that the morphology and structure of the hepatic cells had no obvious abnormalities. However, the small bile duct cells were in an irregular arrangement surrounded by plenty of fibrocollagenous stroma. In addition, the bile duct epithelia were significantly hyperplastic without cellular heteromorphism (Figure 3C, 3D). The results of the immunohistochemistry demonstrated that cytokeratins (CK) CK8, CK18 and CK19 staining were all positive, while hepatocyte staining was negative (Figure 3E, 3F). Due to this staining, the histological origin of the lesion was considered to be bile duct epithelium. Therefore, bile duct hamartoma was finally defined by pathology.
The patients were additionally followed-up both four months and nine months after the biopsy. At these times, the size of the lesion was 13.6×14.9 mm and 13.7×15.7 mm, respectively. The nodule increased one to two mm in nine months. Due to the benign results of biopsy before, BHs were considered the correct pathologic results.
Discussion
As mentioned above, BHs are considered benign liver malformations resulting from derangement of brephic bile duct cells, which is proposed to be triggered by a disruptive or ischemic factor during bile duct lamina remodeling [8,13]. These nodules typically measure less than 1 cm on gross observation, strongly simulating liver metastases on macroscopical inspection and in imaging studies [9,10]. Microscopically, BHs consist of a variable numbers of dilated, tortuous or branching intraheatic bile ducts surrounded by monoptychial columnar or cubical bile duct epithelium. The background is composed of abundant fibrocollagenous stroma with little vascular proliferation. Furthermore, the dilated bile ducts may have some bile in their lumina [6,10].
Two kinds of classification methods for BHs have been mentioned in the literature. Based on the degree of biliary tract dilatation within the lesions, BHs can be divided into three classes: (i) predominantly solid, (ii) predominantly cystic and (iii) intermediate lesions [4,8,16,17]. The connections between the abnormal dilated cysts and the draining bile ducts BHs are also divided in two types: (i) draining or (ii) non-draining [8,18]. However, it is also strongly believed that there is no communication either among the deformed bile ducts or between them and the normal biliary system [19].
Sonographic findings of BHs detect hypoechoic, hyperechoic, or mixed non-homogeneous echoic lesions [10,11,13,20,21]. A specific ultrasonic characteristic of BHs is the so-called “multiple comet-tail echoes” that have been inferred to be strong diagnostic evidence towards BHs [10,11,21]. However, this finding was not significant in our case study. Instead, CEUS was used to evaluate the microcirculation inside the lesion. Recently, Murphy-Lavallee et al. [11,22] have suggested a unique phenomenon wherein all metastases display degrees of enhancement in the arterial phase on CEUS, no matter the appearances on contrast enhanced CT and contrast enhanced MRI [11]. There has been no contrast enhancement reported in most cases of CEUS examination for BHs, but this finding is currently being disputed [10,13]. For example, Berry et al. [11,23] and Phillip et al. [11] reported cases of multiple BHs that did not demonstrate any enhancement in all three phases of the process (arterial, portal venous, and delayed phase) via CEUS. On the other hand, Hohmann et al. [11,24] highlighted the presence of contrast enhancement on CEUS in a BH measuring 5 mm in size. Imaging of the lesion was synchronized and consistent with that of the normal liver parenchyma in arterial phase and portal venous phase, and ‘washout’ in the delayed phase [11]. Our results of CEUS roughly agree with the findings of Hohmann et al. The imaging of the lesion displayed enhancement in arterial phase, similar to imaging in liver malignancies. These differences may vary based on lesion size. Typically, liver malignancies on CEUS show that the lesion disappears earlier than the surrounding liver parenchyma in the delay phase. In our case, they are synchronous in portal venous phase and delay phase. Innovatively, we have also found a tumor-feeding vessel during the course of CEUS, which has never been reported. Thus, valid conclusions cannot be drawn between imaging differences in BH and liver malignancies.
BH lesions are always low-density on CT scanning images. In the published literature, no enhancement of BHs has been captured in all phases of CECT after intravenous injection of iodine contrast medium [13], as in our case. However, exceptions exist in two cases [13,25] in which the imaging of lesions was homogeneously enhanced on post-contrast imaging of CECT.
The typical imaging features of BHs on MRI are hypo signal on T1WI and hyper signal on T2WI [11,19]. However, controversy exists when considering enhancement of gadolinium-enhanced T1WI [11]. Both enhancement [13,18,21,26,27] and non-enhancement [11,13] have been reported. In our case, rim enhancement was detected in the hepatic arterial phase, and marginal tuberculiform enhancement was detected in both the portal venous phase and the delayed phase of enhanced T1WI. This was speculated to be resulting from the compressed liver parenchyma surrounding the lesion and inflammatory cell infiltration [21,27]. As a promising part of MRI, diffusion-weighted imaging (DWI) has attracted a lot of attention in differentiating benign lesions from malignant lesions, due to diffusion rate. It is thought that benign lesions display free diffusion on DWI, while malignant lesions show restricted diffusion [11]. According to this theory, the lesion in our case could be inferred to be benign. Additionally, it has been suggested that the false negative rate of CT and MRI remains high for liver metastasis less than 1.5 cm in size, which may also explain the discrepancies in the T2W1 enhancement. This size coincides with the vast majority of BHs, so the differential diagnosis would be difficult and may vary with lesion size [28].
Magnetic resonance cholangiopancreatography (MRCP) imaging has the ability to identify the communication between BHs and the normal biliary system, also known as the ‘starry sky’ phenomenon. The dilated bile ducts of BHs are hyper intense isolated nodules scattered in both of the liver lobes, and may or may not communicate with the draining bile ducts [6,10,13,21,27,28]. MRCP can facilitatefacilitate the differential diagnosis of BHs and other bile duct anomalies such as Caroli disease by analyzing the association with the draining bile ducts [13]. However, based on whether there are connections between the abnormal dilated cysts and the draining bile ducts, some believe that BHs can be divided in two types, existence or absence of the communication [8,13].
The most important differential diagnosis of BHs would be to rule out liver metastases, which would interfere with different treatment strategies [29], especially when the patient has a history of malignancy [2,28]. Other similar diseases include primary hepatocellular carcinoma, intrahepatic cholangiocarcinoma and circumscribed inhomogeneous fatty liver [2,28], simple hepatic cyst, small liver abscess, and lymphoma. To our knowledge, CEUS of BHs has been reported in few papers, and the accuracy of CEUS in the diagnosis of BHs has not been reported. CT helps slightly in differentiating BHs from liver metastasis but has poor tissue contrast resolution [21]. Comparatively, if accompanied with typical imaging, MRI may be of the greatest value in the differential diagnosis of BHs and liver metastasis, although it remains controversial, as described above. In addition, the dilated bile ducts of BHs can display the characteristic “starry sky” appearance in some cases on MRCP [6,10,13,21]. We believe that pathological examination is required in those lesions with atypical radiological performance, such as solitary lesions or enhanced post-gadolinium during MRI [21], as in our case.
BHs may also be confused with peribiliary gland hamartoma (PGH), also known as intrahepatic bile duct adenoma, originating from peribiliary glands. Microscopic examination of PGH reveals disordered peribiliary gland acini embedded in fibrotic tissue which causes chronic inflammation [8,30]. Microscopical examination of BHs shows small irregular and dilated bile ducts embedded in fibrocollagenous stroma without inflammation [8,10,31,32]. Part of the dilated bile ducts turn into small cysts and maybe contain inspissated bile in them [8,30,33]. However, it is difficult to distinguish BHs from PGH by macroscopical examination or imaging methods. The only difference is that PGH has been considered to have delayed or prolonged enhancement on contrast enhanced CT and MRI [8]. The most precise identifying method to distinguish BH and PGH should be immunohistochemistry. The serous and mucous cells comprised in acini and tubules of PGH express D10 and 1F6, which are also secreted by the intrahepatic peribiliary glands [30,34,35]. Another related investigation demonstrates that PGH can be marked specifically by five foregut antigens (D10, 1F6, MUC6, MUC5AC, and TFF2) as well as the exocrinosity of acid mucin [35]. Therefore, imaging examination may help somewhat in indicating the most likely differential diagnosis of BHs and PGH, with the final diagnosis depending on pathology.
Conclusion
BHs are benign liver malformations, with an extremely low probability of malignant transformation. Recently, they have attracted a lot of attention because they simulate the performance of liver metastasis on gross observation and imaging examination. As mentioned, it is difficult with imaging studies alone to differentiate BH from similar disease. However, when imaging, we believe MRI is most important for differential diagnosis. Pathological examination is necessary when a history of primary malignancy exists or the patient has atypical appearance on radiological images. In addition, we present diagnosis of BHs from a small lesion in the liver of a young patient with hepatitis. We also present a novel association between BHs and tumor-feeding vessels that has never been reported, potentially creating a new link between benign lesions and tumor vasculature. Of course, we cannot rule out that our case may be an individual phenomenon.
Acknowledgements
This project was carried out with financial support from the National Natural Science Foundation of China (Grant Nos. 30332369/H1205 and 81171352/H1805) and the Science and Technology Support Projects of the Science and Technology Commission of Shanghai (Grant No. 124119a5300).
Disclosure of conflict of interest
None.
Abbreviations
- BHs
Bile duct hamartomas
- VMC
Von Meyenburg complex
- US
Ultrasound
- CEUS
Contrast enhanced ultrasound
- CT
Computerized tomographic scanning
- MRI
Magnetic resonance imaging
- T1WI
T1-weighted images
- 2WI
T2-weighted images
- DWI
Diffusion-weighted imaging
- CK
Cytokeratin
- MRCP
Magnetic resonance cholangiopancreatography
- PGH
Peribiliary gland hamartoma
References
- 1.H vM. Über die Zyztenleber. Beitr Pathol Anatom. 1918;64:47–532. [Google Scholar]
- 2.Michalakis K, Coppack SW, Koak Y. Von Meyenburg complex in a bariatric patient. J Gastrointestin Liver Dis. 2011;20:120. [PubMed] [Google Scholar]
- 3.Terada T, Moriki T. Monolobar ductal plate malformation disease of the liver. Pathol Int. 2010;60:407–412. doi: 10.1111/j.1440-1827.2010.02535.x. [DOI] [PubMed] [Google Scholar]
- 4.Karahan OI, Kahriman G, Soyuer I, Ok E. Hepatic von Meyenburg complex simulating biliary cystadenocarcinoma. Clin Imaging. 2007;31:50–53. doi: 10.1016/j.clinimag.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 5.Hettinger M, Klotz M, Bolay L, Weber P. [Multiple bile duct hamartomas of the liver--a rare differential diagnosis to liver metastasis] . Ultraschall Med. 2005;26:150–153. doi: 10.1055/s-2004-813339. [DOI] [PubMed] [Google Scholar]
- 6.van Vlerken LG, van Leeuwen MS, Schipper ME, van Erpecum KJ. The “Von Meyenburg complex”: an unusual cause of cholangitis? Clin Res Hepatol Gastroenterol. 2011;35:762–764. doi: 10.1016/j.clinre.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Redston MS, Wanless IR. The hepatic von Meyenburg complex: prevalence and association with hepatic and renal cysts among 2843 autopsies [corrected] . Mod Pathol. 1996;9:233–237. [PubMed] [Google Scholar]
- 8.Ioannidis O, Iordanidis F, Paraskevas G, Ntoumpara M, Tsigkriki L, Chatzopoulos S, Kotronis A, Papadimitriou N, Konstantara A, Makrantonakis A, Sakkas A, Kakoutis E. Incidentally discovered white subcupsular liver nodules during laparoscopic surgery: biliary hamartoma and peribiliary gland hamartoma. Klin Onkol. 2012;25:468–470. [PubMed] [Google Scholar]
- 9.van Baardewijk LJ, Idenburg FJ, Clahsen PC, Mollers MJ. [Von Meyenburg complexes in the liver: not metastases] . Ned Tijdschr Geneeskd. 2010;154:A1674. [PubMed] [Google Scholar]
- 10.Zheng RQ, Zhang B, Kudo M, Onda H, Inoue T. Imaging findings of biliary hamartomas. World J Gastroenterol. 2005;11:6354–6359. doi: 10.3748/wjg.v11.i40.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lung PF, Jaffer OS, Akbar N, Sidhu PS, Ryan SM. Appearances of von meyenburg complex on cross sectional imaging. J Clin Imaging Sci. 2013;3:22. doi: 10.4103/2156-7514.112804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aoki S, Okayama Y, Kitajima Y, Hayashi K, Imai H, Okamoto T, Akita S, Gotoh K, Ohara H, Nomura T, Joh T, Yokoyama Y, Itoh M. Intrahepatic biliary papilloma morphologically similar to biliary cystadenoma. J Gastroenterol Hepatol. 2005;20:321–324. doi: 10.1111/j.1440-1746.2005.03242.x. [DOI] [PubMed] [Google Scholar]
- 13.Mortele B, Mortele K, Seynaeve P, Vandevelde D, Kunnen M, Ros PR. Hepatic bile duct hamartomas (von Meyenburg Complexes): MR and MR cholangiography findings. J Comput Assist Tomogr. 2002;26:438–443. doi: 10.1097/00004728-200205000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Nasr D, Bidot ML, Roche M, Paveliu S, Morel P, Naouri A, Odet E, Esquis P, Pilleul F, Bernard P. [Von Meyenburg disease found during laparoscopic surgery: report of two cases] . Ann Chir. 2006;131:468–470. doi: 10.1016/j.anchir.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Kanematsu M, Kondo H, Semelka RC, Matsuo M, Goshima S, Hoshi H, Moriyama N, Itai Y. Early-enhancing non-neoplastic lesions on gadolinium-enhanced MRI of the liver. Clin Radiol. 2003;58:778–786. doi: 10.1016/s0009-9260(03)00217-4. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez Posadilla M, Linares Torres P, Jorquera Plaza F, Rueda Castanon R, Gonzalez de Francisco T, Olcoz Goni JL. [Importance of magnetic resonance imaging in the diagnosis of multiple small biliary hamartomas (von Meyenburg complexes)] . Gastroenterol Hepatol. 2006;29:378–379. doi: 10.1157/13089723. [DOI] [PubMed] [Google Scholar]
- 17.Federle MP, VS A. In: Hepatobiliary and pancreas. Abdomen. Diagnostic imaging. Federle MP, editor. Amirsys Inc; 2004. pp. 1–114. [Google Scholar]
- 18.Wohlgemuth WA, Bottger J, Bohndorf K. MRI, CT, US and ERCP in the evaluation of bile duct hamartomas (von Meyenburg complex): a case report. Eur Radiol. 1998;8:1623–1626. doi: 10.1007/s003300050599. [DOI] [PubMed] [Google Scholar]
- 19.Lin S, Weng Z, Xu J, Wang MF, Zhu YY, Jiang JJ. A study of multiple biliary hamartomas based on 1697 liver biopsies. Eur J Gastroenterol Hepatol. 2013;25:948–952. doi: 10.1097/MEG.0b013e32835fb9ee. [DOI] [PubMed] [Google Scholar]
- 20.Xu AM, Xian ZH, Zhang SH, Chen XF. Intrahepatic cholangiocarcinoma arising in multiple bile duct hamartomas: report of two cases and review of the literature. Eur J Gastroenterol Hepatol. 2009;21:580–584. doi: 10.1097/MEG.0b013e3282fc73b1. [DOI] [PubMed] [Google Scholar]
- 21.Shin YM. Biliary hamartoma presented as a single mass. Korean J Hepatol. 2011;17:331–334. doi: 10.3350/kjhep.2011.17.4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy-Lavallee J, Jang HJ, Kim TK, Burns PN, Wilson SR. Are metastases really hypovascular in the arterial phase? The perspective based on contrast-enhanced ultrasonography. J Ultrasound Med. 2007;26:1545–1556. doi: 10.7863/jum.2007.26.11.1545. [DOI] [PubMed] [Google Scholar]
- 23.Berry JD, Boxer ME, Rashid HI, PS S. Case report: Microbubble contrast-enhanced ultrasound characteristics of multiple biliary hamartomas (von Meyenberg complexes) Ultrasound. 2004;12:95–97. [Google Scholar]
- 24.Hohmann J, Loddenkemper C, Albrecht T. Assessment of a biliary hamartoma with contrast-enhanced sonography using two different contrast agents. Ultraschall Med. 2009;30:185–188. doi: 10.1055/s-2008-1027324. [DOI] [PubMed] [Google Scholar]
- 25.An C, Park S, Choi YJ. Diffusion-weighted MRI in intrahepatic bile duct adenoma arising from the cirrhotic liver. Korean J Radiol. 2013;14:769–775. doi: 10.3348/kjr.2013.14.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong J, Kang W, Xu J. MR imaging and MR Cholangiopancreatography of multiple biliary hamartomas. Quant Imaging Med Surg. 2012;2:133–134. doi: 10.3978/j.issn.2223-4292.2012.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semelka RC, Hussain SM, Marcos HB, Woosley JT. Biliary hamartomas: solitary and multiple lesions shown on current MR techniques including gadolinium enhancement. J Magn Reson Imaging. 1999;10:196–201. doi: 10.1002/(sici)1522-2586(199908)10:2<196::aid-jmri14>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 28.Nagano Y, Matsuo K, Gorai K, Sugimori K, Kunisaki C, Ike H, Tanaka K, Imada T, Shimada H. Bile duct hamartomas (von Mayenburg complexes) mimicking liver metastases from bile duct cancer: MRC findings. World J Gastroenterol. 2006;12:1321–1323. doi: 10.3748/wjg.v12.i8.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mimatsu K, Oida T, Kawasaki A, Aramaki O, Kuboi Y, Katsura Y, Amano S. Preoperatively undetected solitary bile duct hamartoma (von Meyenburg complex) associated with esophageal carcinoma. Int J Clin Oncol. 2008;13:365–368. doi: 10.1007/s10147-007-0747-x. [DOI] [PubMed] [Google Scholar]
- 30.Kim YS, Rha SE, Oh SN, Jung SE, Shin YR, Choi BG, Byun JY, Jung ES, Kim DG. Imaging findings of intrahepatic bile duct adenoma (peribiliary gland hamartoma): a case report and literature review. Korean J Radiol. 2010;11:560–565. doi: 10.3348/kjr.2010.11.5.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tohme-Noun C, Cazals D, Noun R, Menassa L, Valla D, Vilgrain V. Multiple biliary hamartomas: magnetic resonance features with histopathologic correlation. Eur Radiol. 2008;18:493–499. doi: 10.1007/s00330-007-0790-z. [DOI] [PubMed] [Google Scholar]
- 32.Liu CH, Yen RF, Liu KL, Jeng YM, Pan MH, Yang PM. Biliary hamartomas with delayed 99mTc-diisopropyl iminodiacetic acid clearance. J Gastroenterol. 2005;40:540–544. doi: 10.1007/s00535-004-1581-9. [DOI] [PubMed] [Google Scholar]
- 33.Christine AL, Elizabeth M. In: Gastrointestinal and Liver Pathology. Christine AL, Elizabeth M, editors. Philadelphia: Churchill Livingstone; 2005. pp. 600–602. [Google Scholar]
- 34.Bhathal PS, Hughes NR, Goodman ZD. The so-called bile duct adenoma is a peribiliary gland hamartoma. Am J Surg Pathol. 1996;20:858–864. doi: 10.1097/00000478-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Hughes NR, Goodman ZD, Bhathal PS. An immunohistochemical profile of the so-called bile duct adenoma: clues to pathogenesis. Am J Surg Pathol. 2010;34:1312–1318. doi: 10.1097/PAS.0b013e3181ead722. [DOI] [PubMed] [Google Scholar]


