Abstract
Keloids are scars characterized by pathologically excessive dermal fibrosis and aberrant wound healing. Hypernomic growth of fibroblast cells and excessive accumulation of collagens, especially collagen I, made important contribution to keloid formation. Transforming growth factor-beta 1 (TGF-β1) was a key ruler for the dermal fibrosis. Here, we found that BMP and activin membrane-bound inhibitor (BAMBI), a pseudo-receptor of TGF-β1, was being decreased during the human keloid development and in vitro keloid cell growth. To investigate the effect of BAMBI on keloid development, pcDNA-BAMBI expression vector were transfected into the human primary keloid cells. Then the cell proliferation and viability were detected with EdU and MTT methods and expression of TGF-β1 and collagen I/III were examined by Western blotting analysis. The results showed that, compared to the control, BAMBI overexpression suppressed the cell proliferation and expression of TGF-β1 and collagen I (P < 0.05), whereas, TGFβ1 overexpression rescued the suppression. Finally, pcDNA-BAMBI expression vector was subcutaneously injected into transplanted human keloid in nude mice. During the 6 weeks of in vivo experiment, pcDNA-BAMBI injection significantly suppressed the growth of the implanted keloids and the ratio of collagen I in the keloids. Therefore, BAMBI had an effect on inhibition of keloid growth through suppressing TGF-β1-induced fibroblast cell proliferation and excessive accumulation of collagen I.
Keywords: Keloid, BMP and activin membrane-bound inhibitor, fibroblast cell proliferation, collagens, transforming growth factor-beta 1
Introduction
Keloid is a type of aesthetically inappreciative scar formed after the skin repairing from injuries [1]. High recurrence rates following excision make it frustrating to the patients [2]. Keloid formation is so complicated processes that up to date the principle mechanism underlying its propagation still remains unidentified. In spite of this, hypernomic growth of fibroblast cells and excessive accumulation of collagens (type III in early stage and type I in late stage) were reported to be largely responsible for keloid formation [3,4]. Several signaling pathways were highly focused as potential pathways involving keloid formation, including mitogen-activated protein kinase (MAPK), insulin-like growth factor-I (IGF-I), integrin pathways, and transforming growth factor-beta 1 (TGF-β1) [5].
TGF-β1, which was first identified in human platelets with a potential role in wound healing, is a member of the transforming growth factor beta superfamily of cytokines [6]. TGF-β1 was well known to make the normal fibroblast phenotype transformation. In addition, TGF-β1 perform multiple functions in regulation of tissue regeneration, cell differentiation, embryonic development and immune system [7,8]. Studies have showed that TGF-β1 was a key promoter of excessive proliferation of fibroblast cell and the extracellular matrix accumulation during tissue regeneration and fibrosis [9-11]. Therapeutic strategies through TGF-β1 have been proposed to alleviate the excessive fibrosis of the wounded skin [12-14].
BMP and activin membrane-bound inhibitor (BAMBI) is a protein which in humans is encoded by the BAMBI gene. BAMBI was regarded as a pseudo-receptor of TGF-β, for they shared a same transmembrane glycoprotein responsible for signal receiving but BAMBI lacked intracellular serine/threonine kinase domain required for signal transduction [9]. Such a special structure conferred BAMBI a completely distinct function from TGF-β. Recently, evidence have indicated that BAMBI deletion led to a gain of myocardial TGF-β signaling and protected the pressure-overloaded myocardium from uncontrolled extracellular matrix deposition in humans and mice [10]. Besides, studies also revealed BAMBI restrained TGF-β signaling and played a negative role in fibrosis of liver and several other tissues [15,17,18]. However, researches on the role of BAMBI in the skin fibrosis and related diseases, especially keloid formation, were rarely reported.
In this study, we found that expression of BAMBI mRNA was being decreased during the human keloid development and in vitro keloid cell growth. BAMBI overexpression restrained TGF-β1 and caused inhibition of keloid fibroblast cell proliferation and accumulation of collagen I.
Materials and methods
Ethics statement
The study population was consisted of 37 patients (mean age 22 ± 5 years). The study was approved by the Ethical Committee of Yuhuangding Hospital, and all subjects gave written informed consent to participate in the study. All animal procedures described herein were approved by the Experimental Animal Center of Shandong Province. The mice were monitored in their home cage in a stress-free environment where they were given food and water ad libitum in a humidity- and temperature-controlled room under a 12-hour light-dark cycle. After experimentation, to minimize pain without drugs, the mice were rapidly euthanized by cervical dislocation and decapitation by an experienced animal handler.
Cell isolation and culture
Tissues were isolated from keloid and adjacent normal dermal of 37 patients with about 4-month chest keloid scar. The tissue mass was cut with scissors into approximately 1 mm3 sections under sterile-free condition and digested with 0.25% trypsin for about 60 min at 37°C in a shaking water bath. Then, DMEM/F12 medium containing 10% FBS was added to stop digestion. The solution was passed through 100-μm nylon filters to remove undigested tissue and large cell aggregates and then centrifuged at 2,000 rpm for 5 min to pellet. The pellet was washed twice with serum-free medium. The cells were taken from liquid nitrogen and then thawed in 37°C water bath. After centrifugated at 1000 g for 5 min, the cells were suspended by DMEM/F12 complemented with 10% FBS (Invitrogen, Carlsbad, CA) and seeded into 12 well plates at the density of 105/cm2. The cells were incubated in a humidified incubator with an atmosphere of 95% air-5% CO2 at 37°C.
Real-time quantitative PCR
Total RNA was isolated using TRIzol reagent (Invitrogen) following the manufacturer’s instructions. The RNA concentration was quantified using a spectrophotometer measuring OD260/280 ratio (1.80-1.95). The integrity of RNA was checked by electrophoresis on 1.0% agarose gel with ethidium bromide staining. Real-time qPCR reactions were carried out in a final volume of 25 μl, using SYBR Premix Ex Taq (TaKaRa), 0.4 mM of each primer, and 200 ng of cDNA template. The primers of BAMBI, TGFβ1, collagen I and collagen III were designed and produced by Ribobio Company (Guangzhou, China). Each individual sample was run in triplicate wells. PCR amplification cycles were performed using iQTM 5 Multicolor Real-Time PCR Detection System (Bio-Rad) and SYBR Premix Ex Taq II kit (Invitrogen). The reactions were initially denatured at 95°C for 3 min followed by 35 cycles of 95°C for 15 s, 60°C for 1 min. The change of transcript abundance of all tested genes was calculated using 2-ΔΔCt method. All mRNA amounts were normalized to β-actin.
Western blotting
Cells were lysed in lysis buffer (Beyotime) supplemented with 1 mM PMSF. Protein concentration was determined with BCA protein assay kit (Tiangen). Twenty micrograms of protein of each sample were separated by 12% SDS-PAGE and electro-transferred to PVDF membrane (Millipore) for immunoblotting analysis. The following primary antibodies were used: Anti-BAMBI (1:300, Abcam), Anti-TGFβ1 (1:300, Abcam), Anti-collagen I (1:600, Abcam) Anti-collagen III (1:600, Abcam), and Anti-β-actin (1:500, Abcam) which was used as the internal reference. After incubation with the appropriate HRP-conjugate secondary antibody, proteins were detected using a ChemiDoc XRS imaging system and analysis software Quantity One (Bio-Rad).
Cell proliferation and DNA replication
Cell proliferation was evaluated using the MTT (Sigma) assay. The cells were seeded in 12-well culture plates at 0.5 × 105/well, transfected with the 1 μg pcDNA-BAMBI or/and 2 μg pcDNA-TGF-β1 with Lipofectamine 3000 (Invitrogen) according to the manufacture’s instruction. The cells were incubated for 0, 24, 48, and 72 h before adding the MTT reagent to each well at a final concentration of 0.5 mg/ml and incubated at 37°C for 4 h. After medium removal, 500 μl of dimethyl sulfoxide was added to each well. Viable cells were measured by absorbance at a 550 nm wavelength using a microplate reader (BioRad, Hercules, CA).
DNA replication was determined after transfection for 48 h using Apollo® 567 EdU Staining Kit (Roche, Basel, Switzerland) according to the manufacture’s instruction, and the positive cells were analyzed with flow cytometry
In vivo study
Keloid tissue donor: male, 22 years old, 4 years ago after the chest skin burn. The scar progressive increased, and was dark red and confirmed to be keloid.
Nude mice model of keloid scar: Twenty-four BALB/C healthy male nude mice (about 8-week old, weighing about 16 g). After intraperitoneal injection of 10% chloral hydrate at a dose of 0.3 mg/g weight, pieces of keloids (6 mm × 4 mm × 3 mm) from the patient were implanted into subcutaneous pockets of the 24 mice. Cut an incision of 5 mm on the right side after undermining the separation of skin with cavity to form a suitably sized bag. The incisions were sutured and disinfected.
Statistical analysis
All data were obtained from at least three independent experiments. Values were expressed as means ± SEM. Statistics were calculated with SPSS 18.0. Multiple comparisons were assessed by one-way ANOVA followed by Dunnett’s tests. Difference between groups was considered statistically significant if P < 0.05.
Results
Expression of BAMBI mRNA was being decreased during the human keloid development and in vitro primary keloid cell growth
The mRNA levels of BAMBI, TGFβ1, collagen I and collagen III were detected during the development of human keloid development. The real-time qPCR analysis showed that, at the developmental time point of 1 month, 3 month, 6 month, 1 year, 2 year and 3 year, TGFβ1 mRNA level was being elevated. Whereas, the mRNA level of the pseudo-receptor of TGFβ exhibited a completely reverse pattern of expression. As a main contributor of keloid formation, mRNA level of collagen I exhibited an excessive increasing pattern all during the keloid growth. While as for collagen III, which was identified to an early contributor of keloid formation, its mRNA was being upregulated until to 3 months but downregulated to quite a low level after 6 months (Figure 1A).
Figure 1.
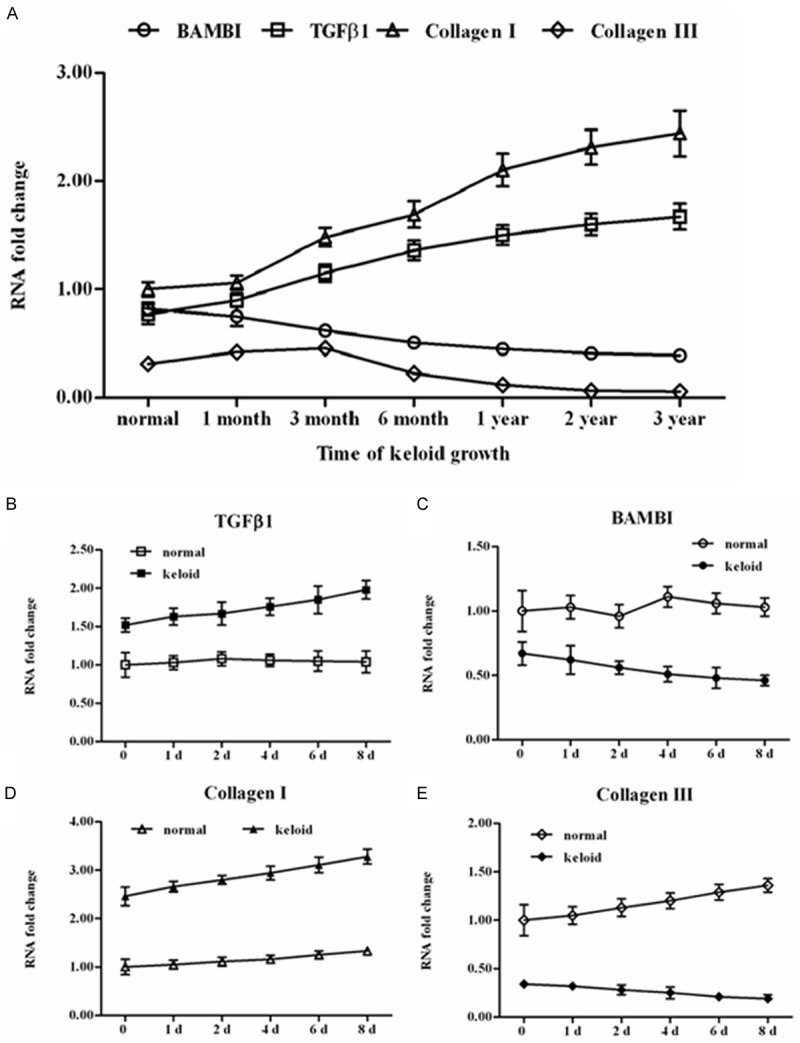
BAMBI was being decreased during the growth of human keloid tissue and primary keloid cells. Tissue samples were collected from 37 patients with keloid at growth time points of 1 month, 3 month, 6 month, 1 year, 2 year, and 3 year. Primary fibroblast cells (in keloids and their adjacent normal skin) were isolated with trypsin digestion method. The cells were incubated in a humidified incubator with an atmosphere of 95% air-5% CO2 at 37°C for 8 days. The cells were collected at day 0, 1, 2, 4, 6, and 8. Total RNA was extracted from the above tissue samples and cells and mRNA levels of BAMBI, TGFβ1, collagen I and collagen III were detected with real-time qPCR. A. Expression of the mRNAs of BAMBI, TGFβ1, collagen I and collagen III during human keloid growth. B-E. Expression of the mRNAs of BAMBI, TGFβ1, collagen I and collagen III in primary keloid cells and their adjacent normal skin fibroblast cells in vitro.
Then keloid cells and adjacent normal dermal cells were isolated from 37 patients with about 4-month chest keloid scar. The two types of primary cells were cultured in vitro and the mRNA levels of the above 4 genes in the cells were examined. The results showed that mRNA levels of TGFβ1 and collagen I in the primary keloid cells were much higher than those in the normal cells (Figure 1B and 1D), while mRNA levels of BAMBI and collagen III were just opposite (Figure 1C and 1E). Moreover, in the keloid cells expression of TGFβ1 and collagen I exhibited an increasing pattern during their growth (Figure 1B and 1D), while BAMBI and collagen III was being decreased (Figure 1C and 1E). These results suggested that BAMBI was negatively associated with TGFβ1 and collagen I.
Overexpression of BAMBI suppressed keloid cell proliferation and expression of collagen I gene through restraining TGFβ1
To explore the effect of BAMBI on keloid development, pcDNA-BAMBI or pcDNA-TGFβ1 expression vector were transfected alone or co-transfected into the human primary keloid cells. After transfection for 48 h, the cell proliferation was detected with EdU staining and analyzed by flow cytometry. The results showed that, compared to the control, BAMBI overexpression suppressed the cell proliferation (71.2% vs 42.3%, P < 0.05), whereas, pcDNA-TGFβ1 transfection rescued the suppression (65.0%) (Figure 2A). Then the cell viability during the transfection was examined with MTT method. The results indicated that at time point of 24, 48 and 72 h, the cell viability was markedly restrained by the pcDNA-BAMBI transfection (Figure 2B). Similarly, pcDNA-TGFβ1 transfection rescued the cell viability (Figure 2B). Then the protein levels of TGFβ1, collagen I and collagen III were tested after transfection for 48 h. The Western blotting analysis displayed that BAMBI overexpression decreased the protein levels of TGFβ1 and collagen I, but had no confluence on that of collagen III. Consistent with the results of cell proliferation detection, pcDNA-BAMBI transfection also rescued the protein levels of TGFβ1 and collagen I (Figure 2C).
Figure 2.
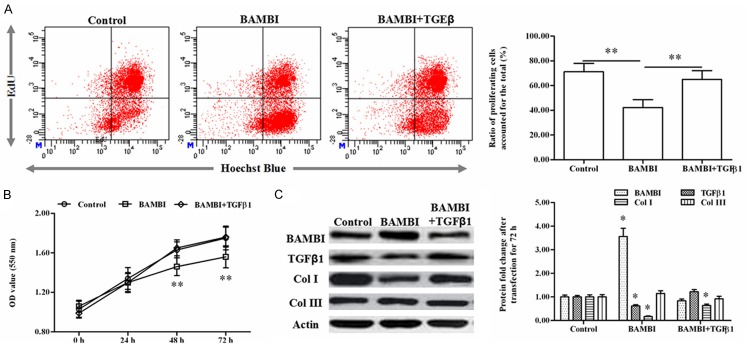
Overexpression of BAMBI suppressed keloid cell proliferation and expression of collagen I gene through restraining TGFβ1 in vitro. The pcDNA-BAMBI or pcDNA-TGFβ1 expression vector was transfected alone or co-transfected into the keloid cells in vitro. A. The cell proliferation was detected with EdU staining and analyzed by flow cytometry after transfection for 48 h. B. Cell viability was detected with MTT approach during the 72 h of transfection. C. The proteins levels of BAMBI, TGFβ1, collagen I and collagen III after transfection for 48 h. * indicates P < 0.05, ** indicates P < 0.01.
BAMBI inhibited progression of the transplanted human keloid in nude mice
Pieces of keloids (6 mm × 4 mm × 3 mm) from a male patient with chest keloid scar were implanted into subcutaneous pockets of 24 nude mice which were divided into 2 groups. Every week the volume of the keloids and their collagen I contents in the BAMBI-injection group and the control were measured. The results indicated that, from the second week to the end of the experimental period, the mean volume of the keloids in the BAMBI-injection group was notably smaller than that of the control (Figure 3A). Moreover, from the week 2 to week 6, the collagen I mass ratio of the keloids in the BAMBI-injection was also much lower than that of the control (Figure 3B). Finally, HE staining was applied to examine the morphological changes of the implanted keloids and its adjacent normal dermal. The observation depicted that, the size of the fiber in the keloids with no injection was much thicker than that of the normal dermal and the BAMBI-injection group (Figure 3C). Simultaneously, the color of nucleus in the keloids with no injection was also shallower than the other groups (Figure 3C). From the histological observation, we could realize that keloids treated with BAMBI-injection were closer to the normal skin tissues. These above data revealed that BAMBI overexpression inhibited progression of the transplanted human keloid in vivo.
Figure 3.
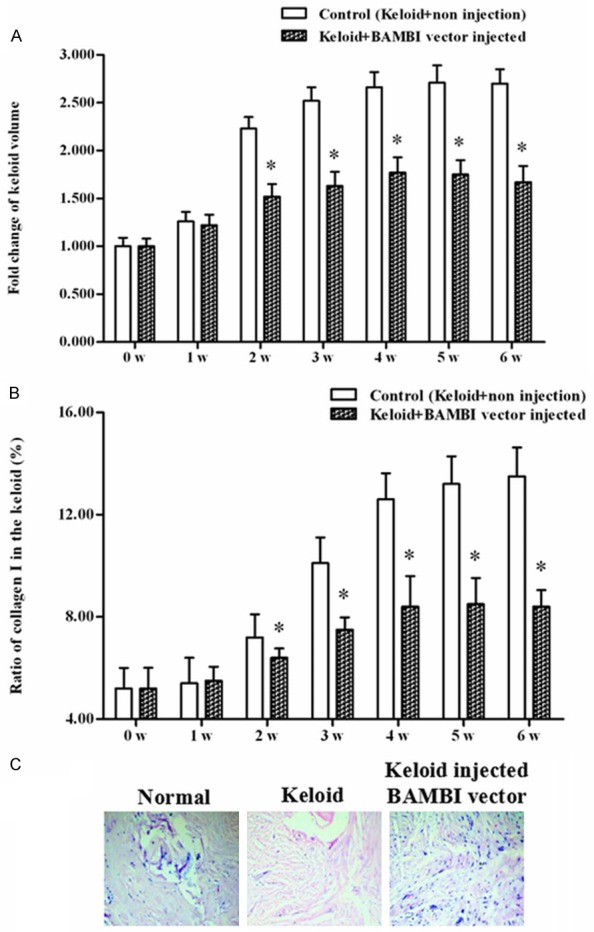
Overexpression of BAMBI suppressed keloid cell proliferation and excessive accumulation of collagen I in vivo. Pieces of keloids (6 mm × 4 mm × 3 mm) from a male patient with chest keloid scar were implanted into subcutaneous pockets of 24 nude mice which were divided into 2 groups: non-injected control and pcDNA-BABMI injected group. Every week the volume of the keloids and their collagen I contents in the BAMBI-injection group and the control were measured. A. The volume of the keloids during the 6-week transplant experiment. B. The proportion of collagen I occupied in the total mass of the keloid tissue. C. HE staining for normal skin, control keloid, and keloid injected with BAMBI expression vector. * indicates P < 0.05, ** indicates P < 0.01.
Discussion
In this study, we found expression of BAMBI mRNA was being decreased during the clinical human keloid development and growth of primary keloid cell. BAMBI mRNA exhibited a reverse expression pattern with TGF-β1 and collagen I mRNAs, suggesting an inhibitive role in the expression role of these two genes. BAMBI overexpression and injection experiments suppressed the growth of human keloid and excessive accumulation of collagen I in vitro and in vivo. These data testified that BAMBI had a negative impact on the growth of human keloid cells.
Primarily identified as a pseudo-receptor of TGF-β, BAMBI has previously been shown to negatively regulate the signaling activity of TGF-β, activin, BMP and Wnt/β-catenin pathway [11]. Numerous research focused on its role in anti-tumorigenesis through inhibiting the signaling activity of TGF-β and Wnt/β-catenin pathways [12,13]. Recent years, TGF-β was highlighted to a key regulator in tumor metastasis and fibrosis of multiple tissues, including liver, kidney, lung, breasts, and skin. TGF-β induced NF-κB activity was proven to be essential for tumor epithelial-mesenchymal transition and metastasis [14]. TGF-β1 was also identified a reliable target of hepatic fibrosis and liver injury and the most potent fibrogenic cytokine [15]. Moreover, in keloid formation, TGF-β1 was also believed to play a leading role in the three members of the TGF-β members. In this current study, the role of BAMBI overexpression in the regulation of TGF-β1 expression and skin fibrosis was investigated. Our data showed that BAMBI was an effective inhibitor of TGF-β1 in skin fibroblast cell growth and excessive accumulation of collagen I.
Tissue fibrosis has been considered to be a sign of tissue lesions. Fibrosis is an inherent response to chronic damage upon immense apoptosis or necrosis [16]. Continuous fibrosis led to increase of fibrous connective tissue, reduce of parenchymal cells, continued progress in structural damage, and eventually caused organ dysfunction and failure [17,18]. Therefore, it is meaningful to explore the mechanism underlying tissue fibrosis, as well as to seek for a reliable target for inhibiting the fibrosis. BAMBI might be such a reliable target and could be applied in fibrosis inhibition in other tissues.
Collectively, BAMBI had an effect on inhibition of keloid growth through suppressing TGF-β1-induced fibroblast cell proliferation and excessive accumulation of collagen I. To our knowledge, it is the first report that BAMBI was a reliable target for fibrosis inhibition in skin.
Disclosure of conflict of interest
None.
References
- 1.Ogawa R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids. Plast Reconstr Surg. 2010;125:557–568. doi: 10.1097/PRS.0b013e3181c82dd5. [DOI] [PubMed] [Google Scholar]
- 2.Leventhal D, Furr M, Reiter D. Treatment of keloids and hypertrophic scars: a meta-analysis and review of the literature. Arch Facial Plast Surg. 2006;8:362–368. doi: 10.1001/archfaci.8.6.362. [DOI] [PubMed] [Google Scholar]
- 3.Meenakshi J, Jayaraman V, Ramakrishnan K, Babu M. Keloids and hypertrophic scars: a review. Indian J Plast Surg. 2005;38:175. [Google Scholar]
- 4.Wolfram D, Tzankov A, Pülzl P, PIZA-KATZER H. Hypertrophic scars and keloids-a review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg. 2009;35:171–181. doi: 10.1111/j.1524-4725.2008.34406.x. [DOI] [PubMed] [Google Scholar]
- 5.Arno AI, Gauglitz GG, Barret JP, Jeschke MG. Up-to-date approach to manage keloids and hypertrophic scars: A useful guide. Burns. 2014;40:1255–1266. doi: 10.1016/j.burns.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaughn SP, Broussard S, Hall CR, Scott A, Blanton SH, Milunsky JM, Hecht JT. Confirmation of the Mapping of the Camurati-Englemann Locus to 19q13. 2 and Refinement to a 3.2-cM Region. Genomics. 2000;66:119–121. doi: 10.1006/geno.2000.6192. [DOI] [PubMed] [Google Scholar]
- 7.Hameedaldeen A, Liu J, Batres A, Graves GS, Graves DT. FOXO1, TGF-β Regulation and Wound Healing. Int J Mol Sci. 2014;15:16257–16269. doi: 10.3390/ijms150916257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arteaga CL. Inhibition of TGFβ signaling in cancer therapy. Curr Opin Genet Dev. 2006;16:30–37. doi: 10.1016/j.gde.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Liu C, Chen X, Yang L, Kisseleva T, Brenner DA, Seki E. Transcriptional Repression of the TGF-β Pseudoreceptor BAMBI by NF-κB p50 Enhances TGF-β Signaling in Hepatic Stellate Cells. J Biol Chem. 2014;289:7082–7091. doi: 10.1074/jbc.M113.543769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villar AV, García R, Llano M, Cobo M, Merino D, Lantero A, Tramullas M, Hurlé JM, Hurlé MA, Nistal J. BAMBI (BMP and activin membrane-bound inhibitor) protects the murine heart from pressure-overload biomechanical stress by restraining TGF-β signaling. BBA-Mol Basis Dis. 2013;1832:323–335. doi: 10.1016/j.bbadis.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Liu K, Song X, Ma H, Liu L, Wen X, Yu J, Wang L, Hu S. Knockdown of BAMBI inhibits β-catenin and transforming growth factor β to suppress metastasis of gastric cancer cells. Mol Med Rep. 2014;10:874–880. doi: 10.3892/mmr.2014.2305. [DOI] [PubMed] [Google Scholar]
- 12.Zhou L, Park J, Jang KY, Park HS, Wagle S, Yang KH, Lee KB, Park BH, Kim JR. The overexpression of BAMBI and its involvement in the growth and invasion of human osteosarcoma cells. Oncol Rep. 2013;30:1315–1322. doi: 10.3892/or.2013.2569. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Yu Z, Xiao Q, Sun X, Zhu Z, Zhang J, Xu H, Wei M, Sun M. Expression of BAMBI and its combination with Smad7 correlates with tumor invasion and poor prognosis in gastric cancer. Tumour Biol. 2014;35:7047–7056. doi: 10.1007/s13277-014-1962-5. [DOI] [PubMed] [Google Scholar]
- 14.Huber MA, Azoitei N, Baumann B, Grünert S, Sommer A, Pehamberger H, Kraut N, Beug H, Wirth T. NF-κB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114:569–581. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenner DA. Molecular pathogenesis of liver fibrosis. Trans Am Clin Climatol Assoc. 2009;120:361–368. [PMC free article] [PubMed] [Google Scholar]
- 16.Park SA, Kim MJ, Park SY, Kim JS, Lee SJ, Woo HA, Kim DK, Nam JS, Sheen YY. EW-7197 inhibits hepatic, renal, and pulmonary fibrosis by blocking TGF-β/Smad and ROS signaling. Cell Mol Life Sci. 2015;72:2023–2039. doi: 10.1007/s00018-014-1798-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.FitzSimmons SC. The changing epidemiology of cystic fibrosis. J Pediatr. 1993;122:1–9. doi: 10.1016/s0022-3476(05)83478-x. [DOI] [PubMed] [Google Scholar]
- 18.Birbrair A, Zhang T, Wang Z-M, Messi ML, Mintz A, Delbono O. Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. Am J Physiol Cell Physiol. 2013;305:C1098–113. doi: 10.1152/ajpcell.00171.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


