Abstract
Objective: Nuclear factor E2-related factor 2 (Nrf2) plays a part in antioxidant and phase II detoxification enzymes in cells by the up regulation of many antioxidant response elements (ARE) related gene transcription. Nrf2 not only protect the normal cells, but also can protect cancer cells from the effect of cell stress, which is helpful to the survival of cancer cell. Some studies show that the expression of Nrf2 has important clinical significance in cancer patients, but the analysis of gastrointestinal tumor Nrf2 comprehensive expression has not been reported. The aim of this study is to evaluate the expression of Nrf2 in gastric cancer by immunohistochemistry and analyze its related clinical significance. Methods: 180 cases of gastric cancer patients receive the gastrectomy and lymphadenectomy, and the resection of tissue is expressesd in paraffin embedded sections by immunohistochemical analysis of Nrf2. And the difference between groups use χ2 (chi-square criterion) test, and will be analyzed by Fisher’s exact test and Mann-Whitney U test. Use univariate and multivariate analysis, Kaplan-Meier curve and log-rank to test and evaluate the correlation between the expression of Nrf2 and the clinical pathological features. Results: The immune reaction of Nrf2 is mainly found in gastric cancer cell nucleus, which positive expression is closely related to the tumor size, depth of invasion, lymph node metastasis, lymphatic invasion and histological analysis (all P<0.05). The log-rank test shows that the survival rate of Nrf2 positive expression group is significantly lower than that of the negative expression group (P<0.01). The Nrf2 positive expression is closely related to the drug resistance of adjuvant chemotherapy on the basis of 5FU (P=0.022). Conclusion: There is a positive correlation between the expression of Nrf2 and the invasion of gastric cancer, which can be used as a potential indicator of patients’ poor prognosis.
Keywords: Nuclear factor E2-related factor 2 (Nrf2), gastric cancer, antioxidant
Introduction
Gastric cancer is the fourth most common gastrointestinal cancer, but its mortality is the second in all gastrointestinal tumors. Asian countries are the high incidence area of gastric cancer. Early diagnosis, radical operation and endoscopic treatment are the key to improve the prognosis of patients [1,2]. However, some patients are even treated with radical resection, the late recovery is still not ideal, which has closely relationship with TNM factors [3,4]. Besides clinical pathological factors, the prognosis of gastric cancer is also influenced by the biological variables, so it’s necessary to search for the new cancer related marker factors to the usage of the diagnosis and treatment of gastric cancer. The widely accepted understanding is that oxidative stress (OS) participates in the physiological and pathological process of degenerative disease. The production and increase of OS and the damage of DNA are all related to the damage and malignant transformation of gastric mucosa. Therefore, OS plays an important role in the cancerous process of gastric cancer [5,6]. Nuclear factor E2-related factor 2 (Nrf2), is the basic of the redox sensitive bZIP transcription factor, which plays a part in antioxidant and phase II detoxification enzymes in cells by the up regulation of many antioxidant response elements (ARE) related gene transcription. Nrf2 binds to Kelch-like ECH-associated protein 1 (KEAP1). And the type cul3 ubiquitin ligase E3 regulates Nrf2 ubiquitin and protease dependent degradation [7-10]. When cells exist in oxidative stress or the environment of chemical substances, Nrf2 transfer to the nucleus, form heterologous dimerization of two and Maf partner, bind to ARE sequence position of DNA and activate the transcription of downstream gene such as antioxidant and phase II detoxifying enzyme [11-13]. The significance of Nrf2 is that it can play a protective role in many diseases and pathological changes in human, including cancer, neurodegenerative diseases, cardiovascular disease, inflammation, pulmonary fibrosis, acute non-injury and etc, and it is also associated with delaying senility [14-18]. So that Nrf2 is regarded as the “useful” transcription factors which can protect the body from oxidative stress injury [19]. But some studies also report that there exist abnormal activation of Nrf2 pathway in cancer cells and tumor tissues [20]. Nrf2 not only prevent normal cells turn into cancer cells, but also protect cancer cells from the effect of cell stress at the same time which can promote the survival of cancer cells, so it’s related to the growth and invasiveness of tumor. At present, there is no overall analysis on the expression of Nrf2 in gastric cancer cell nucleus, which has a correlation with the clinical prognosis in patients. Therefore, the aim of this study is to develop the preliminary exploration on it, and further elaborate the important significance of oxidative stress related Nrf2 expression on clinical practice.
Materials and methods
Western blot test the expression of Nrf2 in gastric cancer cell lines
Gastric cancer cell lines which include MKN74, MNK45, KATOIII and NUGC4 are purchased by the Chinese academy of sciences cell institute. Cell incubation liquid which contains RPMI 1640, 10% fetal bovine serum (FBS), 100 units/ml penicillin and 100 µg/ml streptomycin, which is incubated in 37°C environment, and then cells obtained by centrifugation. The total proteins are extracted by lysates after the phosphate buffer solution (PBS) has been washed. Prepare the nuclear extracts and cytoplasmic groups of Nrf2 Western blotting analysis by using the nuclear/cytoplasmic component kit (K266-25, BioVison, California, USA). Denaturation of the protein from the nucleus or cytoplasm transfers to the hybrid membrane after being separated on SDS polyacrylamide gel, and then close overnight in 5% skim milk Tris buffer solution (TBS). The membrane is incubated overnight (sc-365949, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) by anti Nrf2 mouse monoclonal antibody which is in the concentration of 1:500. TBS-Tween 20 (TBST) conjugates horseradish peroxidase (HAF007, RD Syestems, Minneapolis, USA) by anti mouse IgG when the membrane has been rinsed, and then incubate for 15 minutes. Use ECL-Plus detection reagent to show the immunoreactive bands in X ray film, and distinguish nuclear and cytoplasmic components at the same time. When the membranes has been washed by WB solution for 15 minutes, the cell nucleus and cytoplasmic component will be marked and detected again by anti Lamin B1 antibody (ab16048, Abcam, Cambridge, USA, 1:1000) and anti α tubulin antibody (CP06, Calbiochem, MA, USA, 1:1000).
Patient and specimen
The study includes 180 cases of gastric adenocarcinoma patients in total, and the tumors of them have invaded the submucosa tissues over. All of patients have received the subtotal gastrectomy and lymphadenectomy from January 2006 to December 2012. There are 59 cases of patients have received the distal gastrectomy, 17 cases of patients have received proximal gastrectomy, 99 cases of patients have received total gastrectomy and 5 cases of patients have received partial gastrectomy in 180 cases of patients. The male to female ratio is 121:59, and the average age is 65 years old (33-82 years old). The cases of I-III staging of gastric cancer are 50 cases, 36 cases and 94 cases. Histopathologic classification is based on tumor lymph node metastasis classification of seventh edition that 75 cases are differentiated type (papillary, high differentiation and moderately differentiated tubular adenocarcinoma) and other 105 cases are undifferentiated type (low differentiated adenocarcinoma, mucinous adenocarcinoma and signet ring cell carcinoma). All of patients have not received chemotherapy before the operation, at the same time the related contents of the research have been informed consent by the patients and their family and approved by our hospital ethics committee.
Immunohistochemical analysis of Nrf2 in gastric cancer
Tumor specimen will be embedded in paraffin sections when it has been fixed in PBS with 10% formalin (the thickness is 4 um), which is used for immunohistochemical analysis by fixed on the glass slides. Use xylene to make formalin chemical treatment for sections, at the same time the sections are dewatered by a series of concentration gradient ethanol. Endogenous peroxidase activity of sections is destroyed when dipping in 3% catalase-methanol solution at room temperature for 10 minutes. After being washed three times by PBS (5 minutes each time), sections are processed by 1% bovine serum at room temperature for 30 minutes to block the nonspecific reaction. The sections which are stained by anti Nrf2 antibody should be preprocessed by using citrate buffer solution in advance to get antigenicity at 121°C for 10 minutes. The sections are closed for 10 minutes at room temperature with 3% skim milk powder PBS kit after washed three times by PBS (5 minutes each time). Then sections are incubated overnight at 4°C in the PBS containing anti Nrf2 antibody (sc-365949, Santa Cruz Biotechnology, Inc., 1:200). Use anti biotin-peroxidase kit with streptavidin (Nichirei, Tokyo, Japan) to stain the sections next. The incubation of sections and diaminobenzidine tetrahydrochloride will achieve visualization of immune complexes when the sections have been washed three times by PBS. And then, after washing sections, use hematoxylin to double stain and fix the sections. Normal placental tissue regard as a positive control of Nrf2. The expression of Nrf2 is evaluated by the number of the nuclei of the tumor cells which are marked by anti Nrf2 antibody, and all sections are evaluated by two independent observers (who don’t know the clinical data and prognosis). Choose 10 representative regions in the tumor and evaluate the expressions of 100 cells by high power microscope (×400). We will choose the undifferentiated region to evaluate if a section exist differentiation and undifferentiated regions at the same time even the area is smaller than the differentiation area. The average labeling index of Nrf2 is evaluated by the number of positive cells within each region. The expression of Nrf2 is based on the ratio of positive cells and the staining intensity, and the latter distinguishes it by three grades: 0, 1+, 2+, which is evaluated by the degree of reaction according to Solis and other method in the form of a percentage. The score of immunohistochemistry is obtained by the product of the staining intensity and the degree of reaction (0-200). Because the median value of Nrf2 expression in gastric cancer is 110 in this study, the cut-off value is 100.
Correlation between the expression of Nrf2 and chemical resistance in gastric cancer
In order to evaluate whether the expression of Nrf2 has influence on chemical resistance in gastric cancer, we compare the levels of Nrf2 expression of the patients with gastric cancer who have received 5-fluorouracil (5-FU) chemotherapy. “5-FU resistance” is defined that patients’ tumor recurrence after they received adjuvant chemotherapy, at the same time only the patients who are in II and III staging of gastric cancer receive chemotherapy, and the patients who can’t receive chemotherapy because of side reaction are ruled out, so finally 72 cases of patients are included in the study analysis.
Data analysis
All data record and analysis are performed by using SPSS 16 software, and use χ2 (chi-square criterion) test to examine the differences between groups, and will be analyzed by Fisher’s exact test and Mann-Whitney U test. Use univariate and multivariate analysis, Kaplan-Meier curve and log-rank to test and evaluate the correlation between the expression of Nrf2 and the clinical and pathological features. If P<0.05, the differences have statistical significance.
Results
Expression of Nrf2 in gastric cancer cell lines
Western blotting analysis was performed in order to detect the protein level of Nrf2. The result showed that Nrf2 is mainly expressed in the nucleus, and only a small amount of expression in the cytoplasm (Figure 1).
Figure 1.
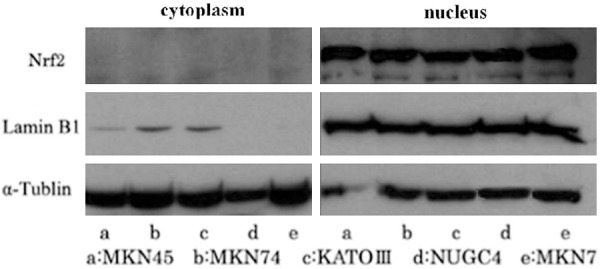
Expression of Nrf2 in gastric cancer cell lines, which is mainly expressed in the nucleus.
Correlation between the immunohistochemical expression of Nrf2 and the clinical pathological features
Use immunohistochemistry to determine the expression of Nrf2 for 180 cases of paraffin sections of gastric cancer. Table 1 shows the clinical pathological features of the patients. Nrf2 immunoreactivity is mainly found in the nucleus (Figure 2). Nrf2 immune activities were different regarding as differentiated and undifferentiated gastric cancer cells from same sample, and the positive rate of Nrf2 immunoreactivity of undifferentiated tumor cells was higher. In 180 cases of patients, we classified 111 cases (61.7%) as Nrf2 positive group which Nrf2 immunohistochemical scores are more than 100, the remaining 69 cases (38.3%) are negative group. Table 2 showed the correlation between the expression of Nrf2 and the clinical pathological features. The positive expression of Nrf2 was significantly related to the gender, volume of tumor, depth of invasion, lymph node metastasis, lymphatic invasion and analysis of tissue clinical stages.
Table 1.
Basic information of the patients
| Case number | |
|---|---|
| Operation method | |
| Total gastrectomy | 99 |
| Distal resection | 59 |
| Proximal resection | 17 |
| Partial resection | 5 |
| Staging | |
| IA/IB | 50 |
| IIA/IIB | 36 |
| IIIA/IIIB/IIIC | 94 |
| Differentiation | |
| Differentiated type | 75 |
| Undifferentiated type | 105 |
Figure 2.
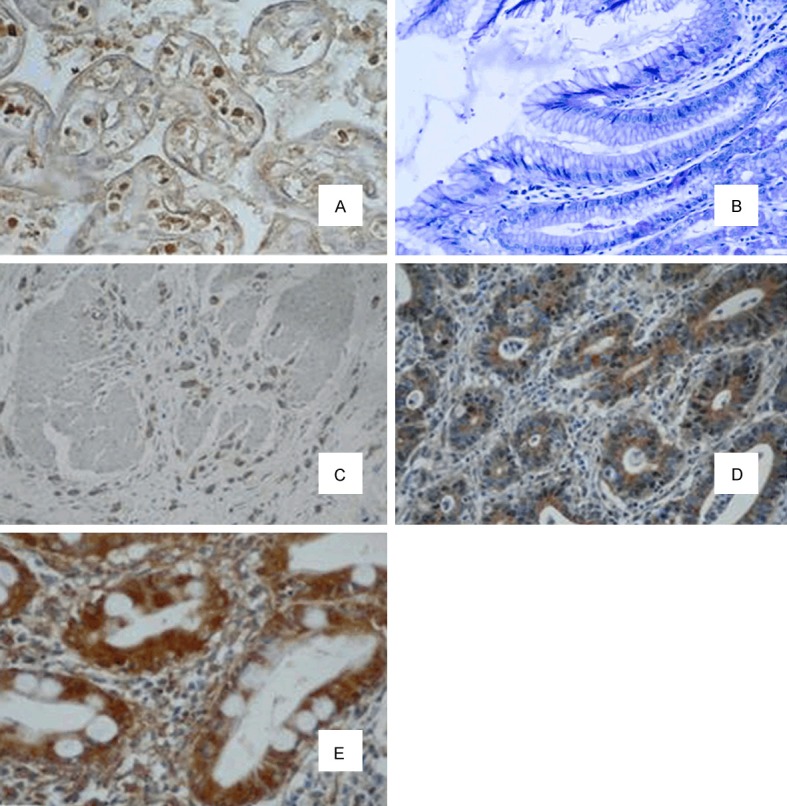
Immunoblot analysis of Nrf2 in the specimen. A. Positive control; B. Normal gastric tissue. C-E. Gastric tissue; C. Nfr2 of negative of coloring; D. Nrf2 of weak positive staining (+1); E. Nrf2 of positive (+2, ×400 amplification).
Table 2.
Correlation between the expression of Nrf2 and clinical pathological factors
| Clinical factors | Expression of Nrf2 | P value | |
|---|---|---|---|
|
| |||
| Negative group (n=69, 38.3%) | Positive group (n=111, 61.7%) | ||
| Age | non | ||
| <65 ages | 24 | 47 | |
| ≥65 ages | 45 | 64 | |
| Gender | <0.05 | ||
| Male | 53 | 65 | |
| Female | 16 | 46 | |
| Tumor volume | <0.01 | ||
| <5 cm | 36 | 27 | |
| ≥5 cm | 33 | 84 | |
| Tumor invasion depth | <0.01 | ||
| T1a, T1b, T2 | 32 | 17 | |
| T3, T4a, T4b | 37 | 94 | |
| Lymphatic metastasis | <0.05 | ||
| Yes | 35 | 74 | |
| No | 34 | 37 | |
| Staging | <0.01 | ||
| IA, IB | 28 | 21 | |
| IIA, IIB | 14 | 22 | |
| IIIA, IIIB, IIIC | 27 | 68 | |
| Lymphatic invasion | <0.05 | ||
| Yes | 51 | 92 | |
| No | 18 | 15 | |
| Differentiation | <0.01 | ||
| Differentiated type | 37 | 34 | |
| Undifferentiated type | 32 | 74 | |
Correlation between the immunohistochemical expression of Nrf2 and 5FUdrug resistance
In 77 cases, 61 cases are Nrf2 positive who received 5FU adjuvant chemotherapy, 16 cases were negative. In 61 cases of positive patients, 44 cases (72.9%) showed 5FUdrug resistance. While in 16 cases of negative patients, only 5 cases (38.5%) showed drug resistance. The difference has statistical significance (P=0.022).
Analysis of survival rate
Kaplan Meier analysis shows that the overall survival rate of Nrf2 positive group was significantly lower than that of Nrf2 negative group (62% vs. 83%, P<0.01) (Figure 3). At the same time, univariate analysis showed that the tumor depth of invasion, volume of tumor, lymph node metastasis, histological type and lymphatic invasion were also significantly related the factors which significantly influenced the prognosis of patients. Multivariate analysis of the above factors showed that lymph node metastasis was the related indicator of independent prognosis (Table 3).
Figure 3.
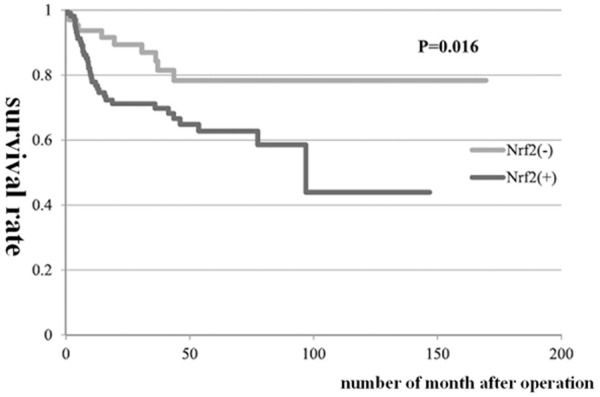
Result of survival prognosis after operation of 180 cases of patients on the basis of Nrf2 expression.
Table 3.
Univariate and multivariate analysis of prognostic factors of gastric cancer
| Clinical factors | Single argument | Multiple variables | ||
|---|---|---|---|---|
|
|
||||
| P value | P value | Risk Ratio | 95% CI | |
| Age | 0.43 | - | - | - |
| Gender | 0.52 | - | - | - |
| Depth of invasion | <0.01 | 0.26 | 2.43 | 0.55-11.47 |
| Tumor volume | <0.01 | 0.33 | 1.55 | 0.51-4.04 |
| Lymphatic metastasis | <0.01 | 0.04 | 5.00 | 1.15-21.37 |
| Lymphatic invasion | <0.05 | 0.28 | 0.31 | 0.06-2.27 |
| Tissue typing | <0.05 | 0.19 | 1.64 | 0.77-3.11 |
| Nrf2 | <0.05 | 0.44 | 1.39 | 0.45-2.67 |
Discussion
Nrf2 is regarded as antioxidant which can protect normal cells from oxidative stress damage. This is one of the protection mechanisms of the cell itself. While some studies report that there are many tumors existing in Nrf2 and theirs over expression of the downstream gene. Nrf2 can not only prevent the transformation of normal cells into cancer cells, but also assist the survival and proliferation of cancer cells at the same time [19-25]. In this study, it is the first time for us to report the correlation between the expression of Nrf2 in gastric cancer cells and the clinical pathological features.
This study shows that Nrf2 immunoreactivity in gastric cancer cell lines in vivo and in vitro is mainly found in the nucleus, and can infer that the continuous expression of Nrf2 in gastric cancer cell lines can cause the production of antioxidants. Thus this gives the cancer cell lines a higher activity of anti reactive oxygen species (ROS). Ma and other person report that there also exists a similar phenomenon in cervical cancer tissue. With the increase of cervical cancer types, the expression of Nrf2 increases gradually in the nucleus, and the protein concentration of downstream antioxidant response are also increased. Therefore, the level of Nrf2 expression in gastric cell nucleus is closely related to the malignant potential of tumor.
Immunohistochemical analysis shows that the expression level of Nrf2 increases in gastric cancer tissues, and 61.7% specimens are from Nrf2 positive patients which is higher than the reported data on non small cell lung cancer (26%) and carcinoma of gallbladder (23%). At the same time tumor depth of invasion, volume of tumor, lymph node metastasis, histological type, lymphatic invasion are significantly related factors which significantly influence the prognosis of patients. Similar to the report of Wang and others, they find the expression of Nrf2 in gallbladder cancer is related to the tumor differentiation, staging and lymph node metastasis [23]. In the recently report of Hu and others, the significant effect on prognosis by the expression of Nrf2 in gastric cancer is also consistent with the results of this study. But they only detect the immunoreactivity of Nrf2 in the cytoplasm and find nothing in the nucleus, and may because of using different antibodies [26]. Many studies have confirmed that Nrf2 exerts its antioxidant activity must be completed from the cytoplasm to the nucleus [27]. The immunoblot analysis of this study shows that Nrf2 in gastric cancer cell lines mainly exists in the nucleus. The continuous and over expression of Nrf2 in cancer cell nucleus may be regarded as antioxidants to protect cancer cells from ROS damage. At the same time, the expression is also the performance of tumor invasive enhancement. Based on this theory, it is necessary to test and evaluate Nrf2 in the nucleus of gastric cancer tissue specimen, rather than the cytoplasm.
In this study, we evaluate the prognostic value of the expression of Nrf2 by univariate analysis. Although Nrf2 positive is not regarded as an evaluated factor of independent prognosis to be added to the multivariable evaluation, the result shows lymph node involvement as a marker for poor prognostic factor which can significantly affect the positive rate of Nrf2. Solis and Wang et al all report that there is a certain correlation between the expression of Nrf2 in small cell lung cancer and in gallbladder cancer and poor prognosis of patients, and they all support the view that the expression of Nrf2 can be regarded as the factor of evaluated prognosis to evaluate the survival time of patients after operation.
At present, some studies show that Nrf2 expression increases the survival rate of cancer cell by increasing the resistance of cancer cells to chemotherapy and radiotherapy [28-33]. This study shows the expression of Nrf2 is significantly related to the adjuvant chemotherapy on the basis of 5FU. So by evaluating the expression level of Nrf2 in gastric cancer nucleus of patients can assistant decision-making the chemotherapy program. Nrf2 gene or inhibition can lead the antioxidant of cell which is regulated by Nrf2 to dysfunction, including glutathione, thioredoxin, non-protein thiols etc. Finally, cause cancer cells to restore the sensitivity to anticancer drugs and radiation. Cho and others find the inhibition of Nrf2 can increase the sensitivity of cancer cells to alkylating anticancer drugs [31]. Ma et al report the in vivo can significantly inhibit the growth of tumor by knocking out the Nrf2 and using in combination with cisplatin at the same time [22]. Therefore, the new chemotherapy program which is involved in Nrf2 regulation of antioxidants will provide better curative effect for the clinic diagnosis and treatment of tumor, and improve the survival prognosis of patients.
In summary, there is a positive correlation between the expression of Nrf2 and the invasion of gastric cancer, which can be used as a potential indicator of patients’ poor prognosis.
Disclosure of conflict of interest
None.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467–77. doi: 10.1007/978-1-60327-492-0_23. [DOI] [PubMed] [Google Scholar]
- 3.Warneke VS, Behrens HM, Hartmann JT, Held H, Becker T, Schwarz NT, Röcken C. Cohort study based on the seventh edition of the TNM classification for gastric cancer: proposal of a new staging system. J. Clin. Oncol. 2011;29:2364–71. doi: 10.1200/JCO.2010.34.4358. [DOI] [PubMed] [Google Scholar]
- 4.Lagarde SM, ten Kate FJ, Reitsma JB, Busch OR, van Lanschot JJ. Prognostic factors in adenocarcinoma of the esophagus or gastroesophageal junction. J. Clin. Oncol. 2006;24:4347–55. doi: 10.1200/JCO.2005.04.9445. [DOI] [PubMed] [Google Scholar]
- 5.Ni J, Mei M, Sun L. Oxidative DNA damage and repair in chronic atrophic gastritis and gastric cancer. Hepatogastroenterology. 2012;59:671–5. doi: 10.5754/hge12177. [DOI] [PubMed] [Google Scholar]
- 6.Engin AB, Karahalil B, Engin A, Karakaya AE. DNA repair enzyme polymorphisms and oxidative stress in a Turkish population with gastric carcinoma. Mol Biol Rep. 2011;38:5379–86. doi: 10.1007/s11033-011-0690-9. [DOI] [PubMed] [Google Scholar]
- 7.Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H: quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960–5. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–89. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 9.Shibata T, Saito S, Kokubu A, Suzuki T, Yamamoto M, Hirohashi S. Global downstream pathway analysis reveals a dependence of oncogenic NF-E2- related factor 2 mutation on the mTOR growth signaling pathway. Cancer Res. 2010;70:9095–105. doi: 10.1158/0008-5472.CAN-10-0384. [DOI] [PubMed] [Google Scholar]
- 10.Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redoxregulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941–53. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem. 2000;275:16023–9. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- 12.Katoh Y, Itoh K, Yoshida E, Miyagishi M, Fukamizu A, Yamamoto M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells. 2001;6:857–68. doi: 10.1046/j.1365-2443.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- 13.Banning A, Deubel S, Kluth D, Zhou Z, Brigelius-Flohe R. The GI-GPx gene is a target for Nrf2. Mol Cell Biol. 2005;25:4914–23. doi: 10.1128/MCB.25.12.4914-4923.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohta T, Iijima K, Miyamoto M, Nakahara I, Tanaka H, Ohtsuji M, Suzuki T, Kobayashi A, Yokota J, Sakiyama T, Shibata T, Yamamoto M, Hirohashi S. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res. 2008;68:1303–9. doi: 10.1158/0008-5472.CAN-07-5003. [DOI] [PubMed] [Google Scholar]
- 15.Kim HR, Kim S, Kim EJ, Park JH, Yang SH, Jeong ET, Park C, Youn MJ, So HS, Park R. Suppression of Nrf2-driven heme oxygenase-1 enhances the chemosensitivity of lung cancer A549 cells toward cisplatin. Lung Cancer. 2008;60:47–56. doi: 10.1016/j.lungcan.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 16.Kim JH, Bogner PN, Ramnath N, Park Y, Yu J, Park YM. Elevated peroxiredoxin 1, but not NF-E2-related factor 2, is an independent prognostic factor for disease recurrence and reduced survival in stage I non-small cell lung cancer. Clin Cancer Res. 2007;13:3875–82. doi: 10.1158/1078-0432.CCR-06-2893. [DOI] [PubMed] [Google Scholar]
- 17.Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, Fujisawa T, Feng Z, Roth JA, Herz J, Minna JD, Gazdar AF. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–46. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 18.Stacy DR, Ely K, Massion PP, Yarbrough WG, Hallahan DE, Sekhar KR, Freeman ML. Increased expression of nuclear factor E2 p45-related factor 2 (NRF2) in head and neck squamous cell carcinomas. Head Neck. 2006;28:813–8. doi: 10.1002/hed.20430. [DOI] [PubMed] [Google Scholar]
- 19.Shibata T, Kokubu A, Gotoh M, Ojima H, Ohta T, Yamamoto M, Hirohashi S. Genetic alteration of Keap1 confers constitutive Nrf2 activation and resistance to chemotherapy in gallbladder cancer. Gastroenterology. 2008;135:1358–68. doi: 10.1053/j.gastro.2008.06.082. [DOI] [PubMed] [Google Scholar]
- 20.Lau A, Villeneuve NF, Sun Z, Wong PK, Zhang DD. Dual roles of Nrf2 in cancer. Pharmacol Res. 2008;58:262–70. doi: 10.1016/j.phrs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solis LM, Behrens C, Dong W, Suraokar M, Ozburn NC, Moran CA, Corvalan AH, Biswal S, Swisher SG, Bekele BN, Minna JD, Stewart DJ, Wistuba II. Nrf2 and Keap1 Abnormalities in Non-Small Cell Lung Carcinoma and Association with Clinicopathologic Features. Clin Cancer Res. 2010;16:3743–53. doi: 10.1158/1078-0432.CCR-09-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma X, Zhang J, Liu S, Huang Y, Chen B, Wang D. Nrf2 knockdown by shRNA inhibits tumor growth and increases efficacy of chemotherapy in cervical cancer. Cancer Chemother Pharmacol. 2012;69:485–94. doi: 10.1007/s00280-011-1722-9. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Zhang M, Zhang L, Cai H, Zhou S, Zhang J, Wang Y. Correlation of Nrf2, HO-1, and MRP3 in gallbladder cancer and their relationships to clinicopathologic features and survival. J Surg Res. 2010;164:e99–105. doi: 10.1016/j.jss.2010.05.058. [DOI] [PubMed] [Google Scholar]
- 24.Kim YR, Oh JE, Kim MS, Kang MR, Park SW, Han JY, Eom HS, Yoo NJ, Lee SH. Oncogenic NRF2 mutations in squamous cell carcinomas of oesophagus and skin. J Pathol. 2010;220:446–51. doi: 10.1002/path.2653. [DOI] [PubMed] [Google Scholar]
- 25.Grogan TM, Fenoglio-Prieser C, Zeheb R, Bellamy W, Frutiger Y, Vela E, Stemmerman G, Macdonald J, Richter L, Gallegos A, Powis G. Thioredoxin, a putative oncogene product, is overexpressed in gastric carcinoma and associated with increased proliferation and increased cell survival. Hum Pathol. 2000;31:475–81. doi: 10.1053/hp.2000.6546. [DOI] [PubMed] [Google Scholar]
- 26.Hu XF, Yao J, Gao SG, Wang XS, Peng XQ, Yang YT, Feng XS. Nrf2 overexpression predicts prognosis and 5-fu resistance in gastric cancer. Asian Pac J Cancer Prev. 2013;14:5231–5. doi: 10.7314/apjcp.2013.14.9.5231. [DOI] [PubMed] [Google Scholar]
- 27.Li W, Kong AN. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog. 2009;48:91–104. doi: 10.1002/mc.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang P, Singh A, Yegnasubramanian S, Esopi D, Kombairaju P, Bodas M, Wu H, Bova SG, Biswal S. Loss of Kelch-like ECH-associated protein 1 function in prostate cancer cells causes chemoresistance and radioresistance and promotes tumor growth. Mol Cancer Ther. 2010;9:336–46. doi: 10.1158/1535-7163.MCT-09-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Homma S, Ishii Y, Morishima Y, Yamadori T, Matsuno Y, Haraguchi N, Kikuchi N, Satoh H, Sakamoto T, Hizawa N, Itoh K, Yamamoto M. Nrf2 enhances cell proliferation and resistance to anticancer drugs in human lung cancer. Clin Cancer Res. 2009;15:3423–32. doi: 10.1158/1078-0432.CCR-08-2822. [DOI] [PubMed] [Google Scholar]
- 30.Wang XJ, Sun Z, Villeneuve NF, Zhang S, Zhao F, Li Y, Chen W, Yi X, Zheng W, Wondrak GT, Wong PK, Zhang DD. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29:1235–43. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho JM, Manandhar S, Lee HR, Park HM, Kwak MK. Role of the Nrf2- antioxidant system in cytotoxicity mediated by anticancer cisplatin: implication to cancer cell resistance. Cancer Lett. 2008;260:96–108. doi: 10.1016/j.canlet.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Kensler TW, Wakabayash N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Jungsuwadee P, Vore M, Butterfield DA, Clair DKS. Collateral damage in cancer chemotherapy - Oxidative stress in nontargeted tissues. Mol Interv. 2007;7:147–56. doi: 10.1124/mi.7.3.6. [DOI] [PubMed] [Google Scholar]


