Abstract
The role of dendritic cells (DCs) in irritable bowel syndrome (IBS) is unclear. This study tested the hypothesis that intestinal DCs induced visceral hypersensitivity in IBS rats through mast cell (MC) activation. The IBS rat model was established by combining colorectal distension with restraint stress. The number of CD103-positive cells in colon was higher in the IBS group. Expression of PAR-2, IL-4 and IL-9 in the colonic mucosa was higher in the IBS group. Mesenteric lymph node DCs (MLNDCs) and splenic CD4+/CD8+ T cells were isolated and purified by a magnetic labeling-based technique; they were cultured alone or co-cultured (T4+DC/T8+DC). The coculture of MLNDCs and CD4+ T cells had the highest IL-4 secretion in the IBS group, while IL-9 expression was higher in the cultures containing CD8+ T cells. Our findings indicate that an increased number of DCs in the colon stimulated CD4+ T cells to secrete high levels of IL-4, which led to the activation of MCs and subsequently resulted in visceral hypersensitivity.
Keywords: Immune, dendritic cells, visceral hypersensitivity
Introduction
Irritable bowel syndrome (IBS) is characterized by abdominal pain and discomfort and changes in bowel habit. It affects 6.5-10.1% of the Asian population, and although it does not have a high mortality rate, it is associated with fairly high morbidity in some patients, which affects their quality of life [1]. Dysfunction of brain-gut communication, gut motility, visceral perception, neuroimmune responses, inflammation, and mucosal integrity, as well as psychosocial status may play a role in the pathophysiology of IBS [2]; however, this condition is not fully understood. Several lines of evidence suggest the involvement of systemic or intestinal immune activation in IBS. An increase in the amount of immune cells (e.g., CD3+, CD4+ and CD8+ T cells) and cytokines such as interleukin (IL)-5, IL-13, IL-6, tumor necrosis factor (TNF)-α and IL-1β has been reported in the colonic mucosa of IBS patients [3-5].
Antigen presenting cells (APCs) are initiators and modulators of the immune response, and DCs are the most important APCs, which present the antigens to T and B cells through phagocytosing pathogens and self-antigens. DCs have important significance in promoting inflammatory responses to pathogens, inducing immunotolerance and suppressing overactive immune responses [6]. Intestinal DCs are mainly distributed in intestinal lymphoid tissue, such as the Peyer’s patches (PP), intestinal mucosa lamina propria and mesenteric lymph nodes (MLNs). Oversensitivity or reactivity of APC antigens results in abnormal immune response, which may play an important role in the pathogenesis of IBS. However, research on the role of DCs in the pathogenesis of IBS is limited.
Mast cells (MCs) appear to play a particularly important role in the pathogenesis of IBS. An increased number of MCs has been reported in the terminal ileum in patients with IBS [7]. Moreover, the observation that MCs are often found in close association with enteric neurons in vitro and in vivo animal studies suggests that MC mediators may influence enteric motility [8]. When bound to IgE, MCs get activated and release a series of biological active substances [9]. The secretory products of MCs, such as histamine, prostaglandins, and proteinases, are capable of sensorimotor dysfunction. Tryptase is one of the major inflammatory mediators released during MC degranulation, which like other proteases, signals to cells through proteinase activated receptors (PARs) [10], and tryptase selectively activates PAR-2 from among the four known clones PARs [11]. Tryptase induces activation of PAR-2 located on enteric nerves and visceral afferents, causing long-lasting neuronal hyperexcitability [12].
Considering the important role of DCs and MCs in the mucosal immune system and the potential relationship between mucosal immune activation and visceral sensitivity, we hypothesized that DC-mediated MC activation may contribute to the visceral hyperalgesia in IBS. We used a well established rat model of IBS to elucidate the role of DC-mediated MC activation in the development of this condition.
Materials and methods
Experimental animals and generation of the IBS rat model
Twenty adult Sprague-Dawley rats (weighing 220-250 g; Animal Centre of Zhejiang Chinese Medical University, Hangzhou, China) were individually housed in a temperature-controlled room (22 ± 1°C). All the rats were housed in a 12-h light/dark cycle with a room humidity of 65-70%, water and food were provided ad libitum. All experimental protocols were conformed to the requirements of the State Authority for Animal Research Conduct. The rats were randomly separated into control group (n = 10) and IBS group (n = 10). The IBS rat model was generated according to the method reported by Plourde et al [13] and Agostini et al [14]. Briefly, an infant catheter balloon (8F, Bard, USA) was slowly placed 1 cm into the rectum of rats and secured by taping the attached tube to the tail. The rats then underwent colorectal distension (CRD) twice at a pressure of 60 mm Hg for 1 min, with a 30-min between each distension, for 14 days. After CRD, plastic restrainers with a close fit to shoulder, upper limbs and chest of rats were used for immobilization. Restraint stress was induced by 2 h of immobilization starting at 9 AM everyday for five consecutive days. The control group did not undergo any of these treatments.
Abdominal withdrawal reflex recording
The level of visceral sensitivity was determined by recording the abdominal withdrawal reflex (AWR). The rats were evaluated using the semi-quantitative AWR test as follows: 0, no behavioral response to CRD; 1, brief head movement only; 2, contraction of abdominal muscles; 3, lifting of the abdomen; 4, arching of the body and lifting of the pelvis. The stimulus intensity that evoked visually identifiable contraction of the abdominal wall was recorded as the threshold intensity of CRD (AWR score of 3) [15]. During the measurements, CRD was performed for 30 s every 5 min. Each measurement was taken three times to ensure accuracy.
Tissue and blood collection
Rats were euthanized. Blood from the abdominal aorta (5 ml) and tissues from the ileocecal junction were collected under sterile conditions. Tissues were cut, washed with phosphate-buffered saline (PBS) and opened longitudinally, placed immediately in 10% formaldehyde or in liquid nitrogen, and stored at -80°C until they were assayed.
Cell isolation
Isolation of splenic CD4+/CD8+ T cells
A splenic single-cell suspension was prepared by gently rubbing tissues between frosted glass slides, and it was placed on bandicoot percoll (Hao Yang Biological Company, Tianjin) for further purification. Then, splenic CD4+/CD8+ T cells were isolated using CD4/CD8 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) in a MACS system (Miltenyi Biotec, Bergisch Gladbach, Germany). The surface phenotype of the isolated cells was analyzed within a few hours after cell preparation by flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA).
Isolation of mesenteric lymph node DCs
Mesenteric lymph node DCs (MLNDCs) were isolated by gently rubbing tissues between frosted glass slides. The cell suspension was filtered through a 200-mm filter and washed. The MLNDCs were then incubated with OX62 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). Selection was performed on MACS columns (Miltenyi Biotec, Bergisch Gladbach, Germany) twice to achieve higher purity. The surface phenotype of the isolated cells was analyzed within a few hours after cell preparation by flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA).
Immunohistochemistry
To determine the amount of DCs, we conducted immunohistochemical analysis for CD103, which is a marker for DCs. Paraffin-embedded samples from the ileocecal junction were cut in 4-µm sections, the paraffin was removed with xylene, and the samples were rehydrated. Endogenous peroxidase activity was blocked with 0.2% hydrogen peroxidase solution, and non-specific labeling was blocked using a serum-blocking solution. Sections were incubated in complete medium for 1 h at room temperature with rabbit polyclonal anti-CD103 (Wuhan Boster Bio-Engineering Company, China) at a dilution of 1:50. In the negative control, the primary antibody was replaced with PBS. Following incubation with the primary antibody, the sections were incubated for 10 min with a biotin-goat anti-rabbit antibody (Kangwei Biotech Company, China) at room temperature. The avidin-biotin complex was used for immunochemical staining (Kangwei Biotech Company, China) for 10 min at room temperature, which was followed by staining with diaminobenzidine (Kangwei Biotech Company, China). Sections were mounted on gelatin-coated slides, dehydrated in a graded alcohol series, and covered with a cover slip. The sections were rinsed in 0.01 mol/L of PBS three times for 5 min each between steps.
To measure the intensity of CD103 immunoreactivity, CD103-positive cells were counted under × 200 magnification in five slides (for each animal).
Toluidine blue staining for MCs
Toluidine blue was used for staining MCs. The slides from the ileocecal junction were oven dried, dipped in alcohol for a few seconds, placed in xylol (xylene) solution for 2 s, dipped once more in alcohol for a few seconds, and stained using 1% aqueous toluidine blue for 30 min. To complete the staining process, the specimens were washed in distilled water. Finally, after three washes with distilled water, the specimens were mounted as described above. The MCs appeared violet/red against the blue background [16].
MCs were counted under a microscope at × 200 magnification. The average number of MCs in five fields was considered as the index for the corresponding lesion. The counting was performed blindly and in duplicate.
Western blot analysis
Western blotting was used to determine the expression of PAR-2, a marker for MC activation. In brief, proteins were separated using 12% sodium dodecyl sulfate, and the proteins were transferred onto polyvinylidene fluoride membranes (Amersham Biosciences, Piscataway, New Jersey, USA). Membranes were blocked with 5% nonfat milk in PBS containing 0.1% Tween-20 (Sigma, St. Louis, MO, USA) at 4°C overnight with gentle rocking, and then probed with antibodies. The membranes were incubated with the primary antibody, rabbit polyclonal anti-PAR-2 (Abcam, Cambridge, UK), diluted to 1:1200 at 4°C. The membranes were then washed and incubated with HRP-conjugated secondary antibody (1:10000 diluted; Abcam, Cambridge, UK) for 2 h at room temperature. After incubation with the secondary antibodies, immunoblots were visualized using an ECL detection kit (Amersham Biosciences) and exposed to an X-ray film.
ELISA for IL-4/IL-9
MLNDCs at a concentration of 5 × 105/ml were cultured with purified CD4+/CD8+ T cells at a concentration of 1.5 × 106/ml for 7 days. The control wells contained MLNDCs or CD4+/CD8+ T cells alone. The concentration of IL-4 and IL-9 (both in the serum and mucosa cell samples) was measured using commercially available ELISA kits (BENDER, USA; GUSABIO, USA). Samples were assayed in duplicate according to the manufacturers’ instructions.
Statistical analysis
Data were expressed as mean ± SD and compared using the independent samples t-test. One-way ANOVA was performed for comparison between multiple groups, and was followed by least significant difference (LSD) multiple range analysis. Statistical analyses were performed using SPSS17.0. P < 0.05 was considered to indicate statistical significance.
Results
Reliability of the IBS model
The balloon pressure required to reach the AWR score of 3 was significantly lower in the IBS group compared with the control group (48.00 ± 5.50 vs. 80.00 ± 8.57 mmHg; P < 0.05). This was indicative of visceral hypersensitivity in the IBS model rats; the IBS model was therefore successfully established.
Immunohistochemistry results
The number of CD103-positive cells in the lamina propria was significantly higher in the IBS model rats than in the control rats (8.91 ± 2.88 vs. 6.34 ± 0.94; P < 0.05) (Figure 1).
Figure 1.
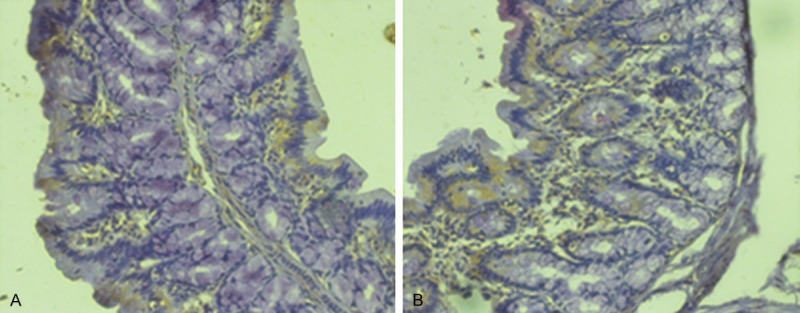
Expression of CD103 in the colon of rats (× 200). A. Control group. B. Model group. The number of CD103-positive cells in the lamina propria was significantly higher in the IBS model rats than in the control rats.
MC count
The amount of MCs was markedly increased in the jejunal mucosa of IBS rats compared to the control rats (2.73 ± 0.21 vs. 1.13 ± 0.10; P < 0.05) (Figure 2), and the majority of these cells were localized within the lamina propria.
Figure 2.
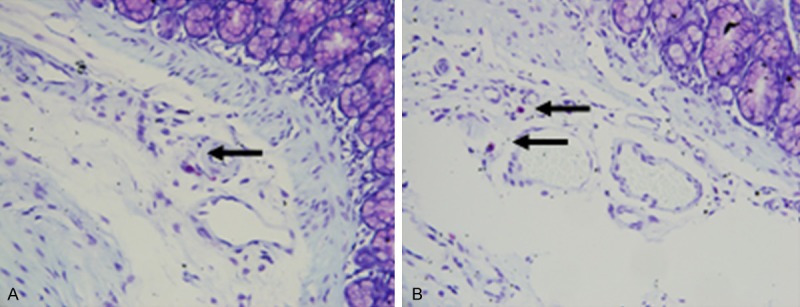
Mast cells in the colon of rats (× 200). A. Control group. B. Model group. The amount of MCs was markedly increased in the jejunal mucosa of IBS rats compared to the control rats.
Western blot results
Protein expression of PAR-2 was significantly increased in the IBS group (2.13 ± 0.81 vs. 0.42 ± 0.29; P < 0.05) (Figure 3).
Figure 3.
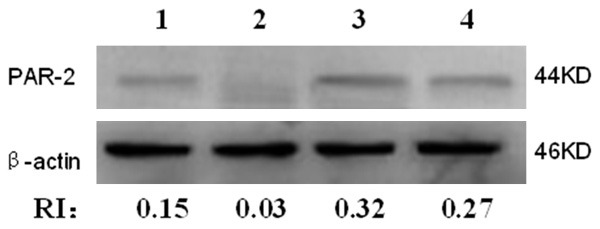
Western blot analysis of PAR-2 protein expression. 1~2: Control group; 3~4: Model group. Protein expression of PAR-2 was significantly increased in the IBS group.
Expression of IL-4 and IL-9 in the serum and mucosa
The median value of serum IL-4 and IL-9 levels was 3.68 pg/ml and 30.60 pg/ml respectively in the IBS group, while it was 2.19 pg/ml and 21.85 pg/ml respectively in the control group. The median value of mucosal IL-4 and IL-9 levels was 7.15 pg/ml and 7.30 pg/ml respectively in the IBS group, while it was 3.29 pg/ml and 5.21 pg/ml respective in the control group. The differences between the two groups were statistically significant (Table 1).
Table 1.
Expression of IL-4 and IL-9 in the serum and colon mucosa (pg/ml)
| Groups | IL-4 (serum) | IL-4 (mucosa) | IL-9 (serum) | IL-9 (mucosa) |
|---|---|---|---|---|
| Control | 2.19 ± 0.38 | 3.29 ± 1.01 | 21.85 ± 1.57 | 5.21 ± 0.63 |
| Model | 3.68 ± 0.61* | 7.15 ± 1.17* | 30.60 ± 4.63* | 7.30 ± 1.30* |
P < 0.05 versus the control group.
Cytokine production of T cells in the presence of MLNDCs
Flow cytometric analysis showed that the amount of DCs was 85.57% ± 7.67% higher in the IBS group, and the amount of CD4+ T cells and CD8+ T cells was 92.46% ± 6.87% and 90.21% ± 7.32% higher. Increased secretion of IL-4 was observed when CD4+ T cells were cultured with MLNDCs, compared to cultures of MLNDCs or T cells alone, with the IBS group showing significantly higher production of IL-4 than the control group (P < 0.05). IL-9 production was found to be higher in the DC and CD8+ T cell coculture as well as the only CD8+ T cell culture; the difference in IL-9 values between the two groups was not significant (Table 2).
Table 2.
Cytokine release from CD4+/CD8+ T cells cocultured with dendritic cells (pg/ml)
| Groups | IL-4 | IL-9 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| T4 alone | T4+DC | T8 alone | T8+DC | DC alone | T4 alone | T4+DC | T8 alone | T8+DC | DC alone | |
| Control | 0.08 ± 0.063a | 0.80 ± 0.48 | 0.08 ± 0.063a | 0.08 ± 0.083a | 0.09 ± 0.095a | 0.50 ± 0.13c,d | 9.9 0± 3.67c,d | 173.75 ± 44.22 | 200.48 ± 57.17 | 0.20 ± 0.08c,d |
| Model | 0.03 ± 0.046a | 1.22 ± 0.33b | 0.08 ± 0.067a | 0.06 ± 0.053a | 0.08 ± 0.067a | 0.43 ± 0.20c,d | 14.54±7.15c,d | 210.31 ± 67.33 | 213.41 ± 91.23 | 0.16 ± 0.08c,d |
| F | 32.811 | 47.738 | ||||||||
| P | 0.000 | 0.000 | ||||||||
T4 alone: culture of CD4+ T cells alone; T4+DC: coculture of CD4+ T cells and DCs; T8 alone: culture of CD8+ T cells alone; T8+DC: coculture of CD8+ T cells and DCs; DC alone: culture of DCs alone;
P < 0.05 versus T4+DC;
P < 0.05 versus the control group;
P < 0.05 versus T8 alone;
P < 0.05 versus T8+DC, P < 0.05.
Discussion
From the findings in this study, we think that increase in the amount of DCs in the colon stimulated CD4+ T cells to secrete high levels of the cytokine IL-4, which led to MC degranulation, and this subsequently resulted in visceral hypersensitivity in the IBS model rats. To our knowledge, this is the first study to demonstrate a relationship between DC-mediated abnormal MC function and visceral hypersensitivity in a rat model of IBS.
There is very little evidence for the role of DCs in IBS. One study found that the plasma concentration of kynurenic acid, which is produced via the tryptophan degradation pathway regulated by indoleamine 2,3-dioxygenase (IDO), is increased in male patients with IBS [17]. Since IDO is a ke y enzyme that regulates the metabolism of tryptophan, which plays an important role in DC function, this finding suggests that the function of DCs is altered in patients with IBS. Later on, Long and his team went on to show that LPDCs (lamina propria dendritic cells) displayed increased CD86 and MHC-II expression on enhanced induction of T cell proliferation in the PI-IBS (post infectious IBS) phase. Moreover, co-culture of CD4+ T cells with LPDCs in the post-infectious phase induced Th1 and Th17 responses. These results demonstrate that phenotypical and functional alterations of LPDCs contribute to the development of PI-IBS [18]. However, the specific mechanism of DC-mediated abnormal immune functions is not very clear, also with regard to the key target cells and molecules. In our study, we found high expression of TH2-type cytokine IL-4 when MLNDCs were cultured with splenic CD4+ T cells from IBS rats, which is inconsistent with the findings of Long and his team; we believe that this difference may be attributable to the difference in the models used. Nonetheless, our findings do indicate that increase in the number of DCs play a role in activating the mucosal immune system and in turn effect visceral sensitivity and subsequently IBS by activating MCs via IL-4 secretion from CD4+ T cells.
IL-4 is cytokine specific for IgE generation, which is associated with the activation of MCs [19]. IL-9 activates MCs by increasing the expression of FceRIα on the surface of MCs [20]. Research into IL-4 levels in IBS are rare, and only one study has suggested that the intestinal cell in mice with acute infection express high levels of IL-4; after the PI-IBS model was established successfully, the expression returned to normal [21]. Therefore, in the early stage of IBS, IL-4 may play a key role in the activation of MCs. We found that the expression of IL-4 in the colonic mucosa and serum was higher in the model group. This indicates that the high level of expression of IL-4 both in the serum and mucosa may induce MC activation.
So far, there has been no research on the relationship between IL-9 and IBS. Our study is therefore the first to report that the colonic mucosa and serum of IBS rats showed high expression of IL-9, which could indicate its involvement in MC activation. However, high expression of IL-9 was not found in the co-culture of MLNDCs with splenic CD4+ T cells, while the culture of CD8+ T cells alone and coculture of CD8+ T cells and MLNDCs had high levels of IL-9 expression; these findings suggest that the expression of IL-9 may be associated with CD8+ T cells.
In conclusion, we proved that the increased number of DCs in the colon stimulated CD4+ T cells to secrete high levels of the cytokine IL-4, which led to mast cell degranulation and visceral hyperalgesia in IBS. Although we attempted to elucidate the mechanism of DC-mediated abnormal immune function in IBS rats, further studies are still needed to clarify the detailed features and functions of DCs at the physiological level in IBS.
Acknowledgements
This study was supported by Natural Science Foundation of China (NSFC) (81170348) and natural science foundation of Zhejiang province (LZ12H03001).
Disclosure of conflict of interest
None.
References
- 1.Chang FY, Lu CL, Chen TS. The current prevalence of irritable bowel syndrome in Asia. J Neurogastroenterol Motil. 2010;16:389–400. doi: 10.5056/jnm.2010.16.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang XY, Zarate N, Soderholm JD, Bourgeois JM, Liu LW, Huizinga JD. Ultrastructural injury to interstitial cells of Cajal and communication with mast cells in Crohn’s disease. Neurogastroenterol Motil. 2007;19:349–364. doi: 10.1111/j.1365-2982.2006.00894.x. [DOI] [PubMed] [Google Scholar]
- 3.Cremon C, Gargano L, Morselli-Labate AM, Santini D, Cogliandro RF, De Giorgio R, Stanghellini V, Corinaldesi R, Barbara G. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol. 2009;104:392–400. doi: 10.1038/ajg.2008.94. [DOI] [PubMed] [Google Scholar]
- 4.Liebregts T, Adam B, Bredack C, Röth A, Heinzel S, Lester S, Downie-Doyle S, Smith E, Drew P, Talley NJ, Holtmann G. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913–920. doi: 10.1053/j.gastro.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 5.Kindt S, Van Oudenhove L, Broekaert D, Kasran A, Ceuppens JL, Bossuyt X, Fischler B, Tack J. Immune dysfunction in patients with functional gastrointestinal disorders. Neurogastroenterol Motil. 2009;21:389–398. doi: 10.1111/j.1365-2982.2008.01220.x. [DOI] [PubMed] [Google Scholar]
- 6.Merad M, Manz MG. Dendritic cell homeostasis. Blood. 2009;113:3418–3427. doi: 10.1182/blood-2008-12-180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guilarte M, Santos J, De Torres I, Alonso C, Vicario M, Ramos L, Martínez C, Casellas F, Saperas E, Malagelada JR. Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum. Gut. 2007;56:203–209. doi: 10.1136/gut.2006.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M, Bunnett NW, Grundy D, Corinaldesi R. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 9.Bugajev V, Bambousková M, Dráberová L, Dráber P. What precedes the initial tyrosine phosphorylation of the high affinity IgE receptor in antigen-activated mast cell? FEBS Lett. 2010;584:4949–4955. doi: 10.1016/j.febslet.2010.08.045. [DOI] [PubMed] [Google Scholar]
- 10.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- 11.Gao C, Liu S, Hu HZ, Gao N, Kim GY, Xia Y, Wood JD. Serine proteases excite myenteric neurons through protease-activated receptors in guinea pig small intestine. Gastroenterology. 2002;123:1554–1564. doi: 10.1053/gast.2002.36581. [DOI] [PubMed] [Google Scholar]
- 12.Reed DE, Barajas-Lopez C, Cottrell G, Velazquez-Rocha S, Dery O, Grady EF, Bunnett NW, Vanner SJ. Mast cell tryptase and proteinase-activated receptor 2 induce hyperexcitability of guinea-pig submucosal neurons. J Physiol. 2003;547:531–542. doi: 10.1113/jphysiol.2002.032011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plourde V, St-Pierre S, Quirion R. Calcitonin gene-related peptide in viscerosensitive response to colorectal distension in rats. Am J Physiol. 1997;273:G191–196. doi: 10.1152/ajpgi.1997.273.1.G191. [DOI] [PubMed] [Google Scholar]
- 14.Agostini S, Goubern M, Tondereau V, Salvador-Cartier C, Bezirard V, Lévèque M, Keränen H, Theodorou V, Bourdu-Naturel S, Goupil-Feuillerat N, Legrain-Raspaud S, Eutamene H. A marketed fermented dairy product containing Bifidobacterium lactis CNCM I-2494 suppresses gut hypersensitivity and colonic barrier disruption induced by acute stress in rats. Neurogastroenterol Motil. 2012;24:376–e172. doi: 10.1111/j.1365-2982.2011.01865.x. [DOI] [PubMed] [Google Scholar]
- 15.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 16.Katayama G, Nabe T, Fujii M, Kohno S. Mast cell hyperplasia induced by multiple challenge with cedar pollen in sensitised guinea pigs. Inflamm Res. 2005;54:370–374. doi: 10.1007/s00011-005-1369-2. [DOI] [PubMed] [Google Scholar]
- 17.Clarke G, Fitzgerald P, Cryan JF, Cassidy EM, Quigley EM, Dinan TG. Tryptophan degradation in irritable bowel syndrome: evidence of indoleamine 2,3-dioxygenase activation in a male cohort. BMC Gastroenterol. 2009;9:1–7. doi: 10.1186/1471-230X-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long Y, Wang W, Wang H, Hao L, Qian W, Hou X. Characteristics of intestinal lamina propria dendritic cells in a mouse model of postinfectious irritable bowel syndrome. J Gastroenterol Hepatol. 2012;27:935–944. doi: 10.1111/j.1440-1746.2011.07046.x. [DOI] [PubMed] [Google Scholar]
- 19.Kashiwada M, Levy DM, McKeag L, Murray K, Schröder AJ, Canfield SM, Traver G, Rothman PB. IL-4-induced transcription factor NFIL3/E4BP4 controls IgE class switching. Proc Natl Acad Sci U S A. 2010;107:821–826. doi: 10.1073/pnas.0909235107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louahed J, Kermouni A, Van Snick J, Renauld JC. IL-9 induces expression of granzymes and high-affinity IgE receptor in murine T helper clones. J Immunol. 1995;154:5061–5070. [PubMed] [Google Scholar]
- 21.Akiho H, Deng Y, Blennerhassett P, Kanbayashi H, Collins SM. Mechanisms underlying the maintenance of muscle hypercontractility in a model of post infective gut dysfunction. Gastroenterology. 2005;129:131–141. doi: 10.1053/j.gastro.2005.03.049. [DOI] [PubMed] [Google Scholar]


