Abstract
To investigate the association of programmed death-1 gene polymorphism rs2227981 with tumor risk. The PubMed, Medline, Ovid Medline, EMBASE, Web of Knowledge were searched. Meta-analyses were conducted using RevMan 5.2.2 software. Total six researches involving in a total of 1427 tumor patients and 1811 healthy control people were included into this meta analysis. There was no association of PD-1 gene polymorphism with total tumor risk under four genetic models. (CT+TT vs CC, OR=1.09, 95% CI=0.80-1.49, P=0.59; CT+CC vs TT, OR=0.93, 95% CI=0.52-1.66, P=0.61; TT vs CC, OR=0.99, 95% CI=0.57-1.72, P=0.97; CT vs CC, OR=1.16, 95% CI=0.80-1.70, P=0.43). The sub-group analysis shown there were a significantly difference on association of PD-1 gene polymorphism with digestive system tumor risk between tumor patients and healthy control people, except recessive model. (CT+TT vs CC, OR=1.57, 95% CI=1.20-2.07, P=0.001; TT vs CC, OR=1.67, 95% CI=1.12-2.49, P=0.01; CT vs CC, OR=1.51, 95% CI=1.13-2.01, P=0.005). Moreover, the meta analysis results shown that there were association of PD-1 gene polymorphism with tumor risk under two models for the tumor specific occurring only in women. (CT+TT vs CC, OR=0.80, 95% CI=0.67-0.95, P=0.01; TT vs CC, OR=0.61, 95% CI=0.44-0.83, P=0.002). This study suggests that PD-1 gene polymorphism rs2227981 is associated with specific tumor types, including digestive system tumor and tumor specific occurring in woman.
Keywords: Programmed death-1, polymorphism, tumor, genetic models, RevMan, meta analysis
Introduction
Lots of factors involves in cause of tumor, including infectious aspects, environment factors, and genetic factors [1-3]. Human immune system plays an important role in resisting tumors, of which the T lymphocyte is the most important anti-tumor immune regulator. Therefore, it is suggest that some immune response-related genes polymorphisms may contribute to tumor pathogenesis.
Programmed death-1 (PD-1), a 55 KDa type I transmembrane glycoprotein, is a potent immunoregulatory molecule expressed on lots of cells, including natural killer T cells (NKTs), monocytes, activated T and B lymphocytes [4]. As we know, PD-1 plays a crucial role in assisting cancer cell to escape the host immune system. Inhibition of PD-1 can improve the anti-tumor activity of immune system [5]. Other study reported that PD-1 is involved in the arthritis, which indicated that PD-1 may play a center role in development of autoimmune disease [6]. Moreover, there was a evidence that PD-1 gene polymorphism is associated with susceptibility of systemic lupus erythematosus [7]. Moreover, more and more studies have focus on the rheumatoid arthritis [8], type 1 diabetes [9] and ankylosing spondylitis [10], however, little is known the relationship between PD-1 gene polymorphism with tumor susceptibility, especially PD-1 rs2227981 (+7785).
The aim of this meta analysis was to comprehensively and quantitatively summarize the all world results to evaluated PD-1 gene polymorphism rs2227981 with tumor risk.
Methods
Lots of databases included PubMed, Medline, Ovid Medline, EMBASE, Web of Knowledge were searched form 1990 to present. The main terms “programmed death-1”, “PD-1”, “polymorphism”, “cancer”, “tumor”, “carcinoma” in the title and/or abstract were used. References cited in the retrieved articles were also searched for relevant titles.
Data extraction and quality assessment
Two investigators independently reviewed all potentially eligible studies and collected data. The discussion and consensus were considered if the there were discrepancies. The quality of all collected data was ranked in accordance with the score of the non-randomized controlled clinical trial quality evaluation standard.
Statistical methods
The all collected data were included into Review Manager software (5.2.2). Heterogeneity was evaluated by Χ2 and I2. The dichotomous variables was analyzed via estimation of odds ratios (OR) with 95% confidence interval (95% CI). Heterogeneity was considered if the I2 statistic was >50% or P<0.05.
Results
Study characteristics
The characteristics of all included studies were summarized in Table 1. We collected the data from six studies [11-16], involving in a total of 1427 tumor patients and 1811 healthy control people. Two studies involved in colorectal cancer [14,16], of which one study involved in rectal cancer [14], one research involved in gastric cancer [15], two papers involved in breast cancer [12,13], and one article involved in gestational trophoblastic neoplasms [11]. Five studies were from Iran [11,13-16], only on from China [13]. More detail can be found in Table 1.
Table 1.
Charateristics of Studies Included in the Meta-analysis
| Study | Year | Ethnicity | Tumor type | Case | Control | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||
| CC | CT | TT | CC+CT | CC+TT | CT+TT | N | CC | CT | TT | CC+CT | CC+TT | CT+TT | N | ||||
| Dehaghani | 2009 | Iran | gestational trophoblastic neoplasms | 42 | 37 | 13 | 79 | 55 | 50 | 92 | 118 | 56 | 121 | 174 | 239 | 177 | 295 |
| Haghshenas | 2011 | Iran | breast cancer | 194 | 191 | 50 | 385 | 244 | 241 | 443 | 137 | 145 | 46 | 282 | 183 | 191 | 328 |
| Hua | 2011 | China | breast cancer | 295 | 169 | 22 | 464 | 317 | 191 | 490 | 244 | 210 | 24 | 454 | 268 | 234 | 512 |
| Mojtahedi | 2012a* | Iran | colon cancer | 47 | 102 | 26 | 149 | 73 | 128 | 175 | 75 | 89 | 36 | 164 | 111 | 125 | 200 |
| 2012b* | Rectal cancer | 12 | 7 | 6 | 19 | 18 | 13 | 25 | 75 | 89 | 36 | 164 | 111 | 125 | 200 | ||
| Savabkar | 2013 | Iran | gastric cancer | 50 | 66 | 6 | 116 | 56 | 72 | 122 | 89 | 70 | 7 | 159 | 96 | 77 | 166 |
| Yousefi | 2013 | Iran | Colorectal Cancer | 18 | 27 | 35 | 45 | 53 | 62 | 80 | 43 | 45 | 22 | 88 | 65 | 67 | 110 |
From the same study.
No association of PD-1 gene polymorphism with tumor risk
As shown in Figure 1, the meta analysis results shown that there was no association of PD-1 gene polymorphism with tumor risk under four genetic models. (CT+TT vs CC, OR=1.09, 95% CI=0.80-1.49, P=0.59; CT+CC vs TT, OR=0.93, 95% CI=0.52-1.66, P=0.61; TT vs CC, OR=0.99, 95% CI=0.57-1.72, P=0.97; CT vs CC, OR=1.16, 95% CI=0.80-1.70, P=0.43).
Figure 1.
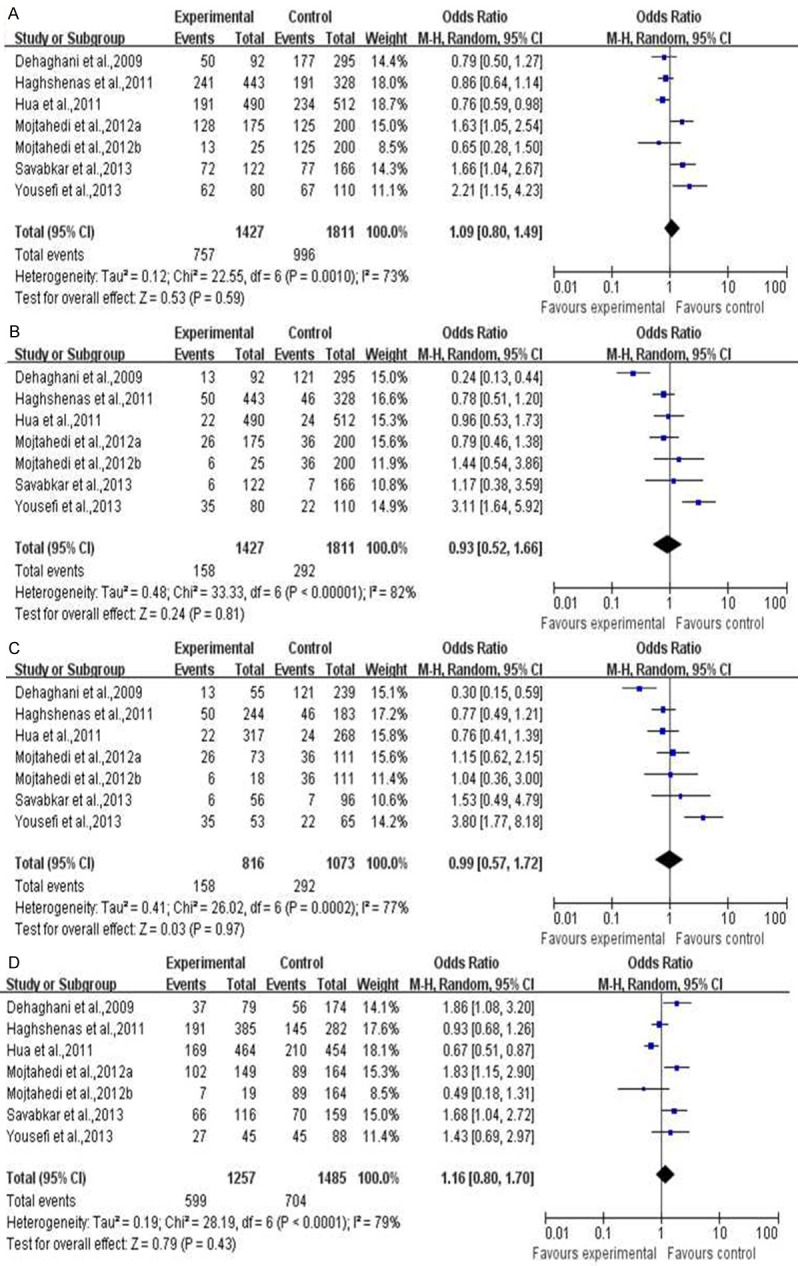
The meta analysis of PD-1 gene polymorphism with total tumor. A. CT+TT vs CC; B. CT+CC vs TT; C. TT vs CC; D. CT vs CC.
Sub-group analysis
We further explored potential causes of the PD-1 gene polymorphism with different tumors. As shown in Figure 2, there were a significantly difference on association of PD-1 gene polymorphism with digestive system tumor risk between tumor patients and healthy control people, except recessive model. (CT+TT vs CC, OR=1.57, 95% CI=1.20-2.07, P=0.001; TT vs CC, OR=1.67, 95% CI=1.12-2.49, P=0.01; CT vs CC, OR=1.51, 95% CI=1.13-2.01, P=0.005).
Figure 2.
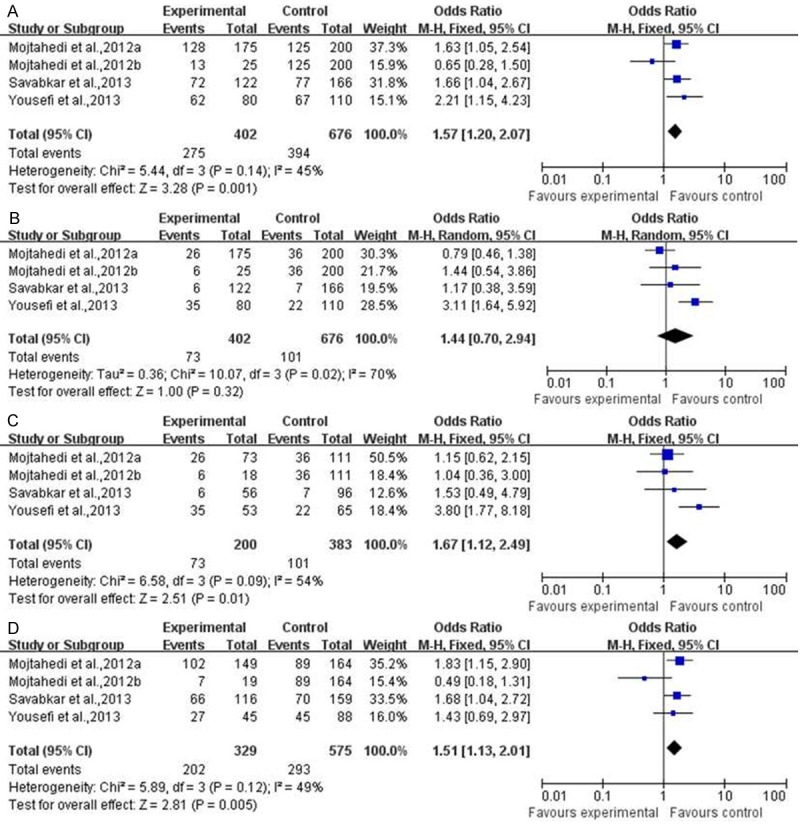
The meta analysis of PD-1 gene polymorphism with digestive system tumor. A. CT+TT vs CC; B. CT+CC vs TT; C. TT vs CC; D. CT vs CC.
For the tumor occurring only in women, the meta analysis results shown that there were association of PD-1 gene polymorphism with tumor risk under two models. (CT+TT vs CC, OR=0.80, 95% CI=0.67-0.95, P=0.01; TT vs CC, OR=0.61, 95% CI=0.44-0.83, P=0.002) However, the results under other two models indicated there were no correlation. (CT+CC vs TT, OR=0.57, 95% CI=0.26-1.25, P=0.16; CT vs CC, OR=0.99, 95% CI=0.61-1.62, P=0.98) (Figure 3).
Figure 3.
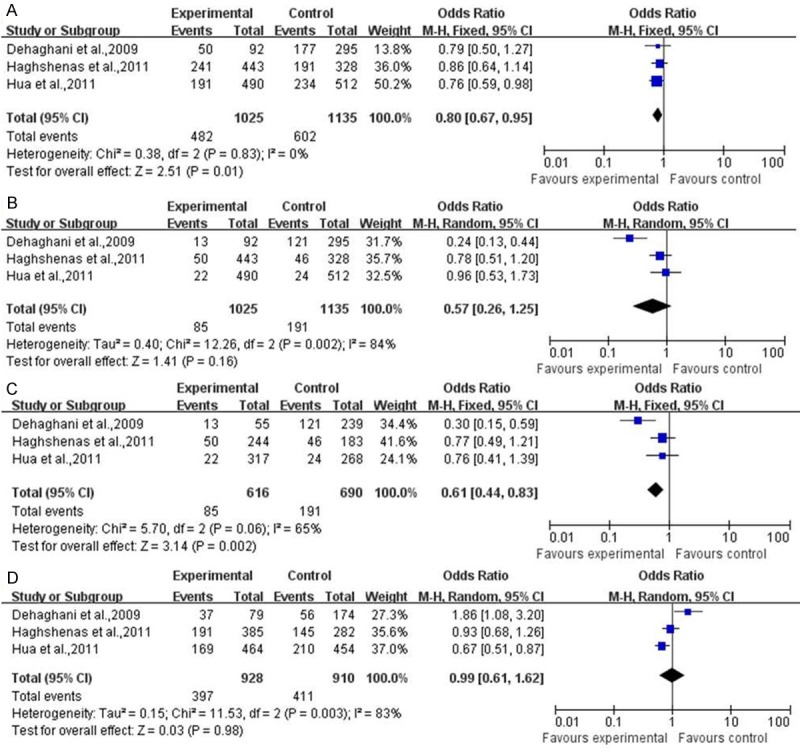
The meta analysis of PD-1 gene polymorphism with tumor specific occurring in woman. A. CT+TT vs CC; B. CT+CC vs TT; C. TT vs CC; D. CT vs CC.
Discussion
In this meta analysis, the results shown that there were significantly difference on association of PD-1 rs2227981 gene polymorphism with special tumor type, including digestive system tumor and tumor occurring in woman under specific genetic models.
Human tumors have lots of genetic and epigenetic changes, which can produce some immune system-recognizing tumor antigens [17]. PD-1, liking CTLA-4, is a member of the B7-CD28 family and is potential therapeutic anticancer target based on its role of regulating T cell activation. PD-1 is a key immune-checkpoint receptor, which regulates immunosuppression by expression on activated T cell. Therefore, the PD-1 maybe a potential molecular target for tumor progression in tumors. The relative studies revealed that the association of CD28 and CTLA-4 gene polymorphisms with tumor risk is present [18-20]. Compared to the CTLA-4, the PD-1 is involve in the later of a T cell response in order to limit the T cell activity in the tumor microenvironment [21].
In our study, we demonstrated that PD-1 gene polymorphism rs2227981 is not relevant to total tumor types risk. So, we performed a sub-group analysis. The meta analysis results shown that an association of PD-1 gene polymorphism rs2227981 with digestive system tumors or tumors only occurring in woman under specific genetic models. Savabkar et al. [15] and Mojtahedi et al. [14] reported that significant association in CT genotype and gastric cancer and colon cancer risk. Similarly, other study demonstrated association of CT genotype of the PD-1 gene polymorphism and rheumatoid arthritis [22]. Moreover, PD-1 gene polymorphism CC genotype and C allele may play a potential risk in tumor specific occurring in woman, including breast cancer [13], which all are consistent with our results.
In here, five studies involved in Iran, only research is from China, which limited the meta analysis results. Moreover, other limitation in this meta analysis should be addressed. Firstly, a relatively small number of studies and sample size were included into this study, which may influence the statistical power of the analysis. Secondly, our meta analysis results were based on unadjusted estimates, while a more precise analysis could be conducted if individual data were available.
In conclusion, this study suggested that PD-1 gene polymorphism rs2227981 is associated with specific tumor types, including digestive system tumor and tumor specific occurring in woman. However, more studies needed to be included into this meta analysis.
Disclosure of conflict of interest
None.
References
- 1.Fan X, Yu K, Wu J, Shao J, Zhu L, Zhang J. Correlation between squamous cell carcinoma of the lung and human papillomavirus infection and the relationship to expression of p53 and p16. Tumour Biol. 2015;36:3043–9. doi: 10.1007/s13277-014-2940-7. [DOI] [PubMed] [Google Scholar]
- 2.Kukulj S, Popović F, Budimir B, Drpa G, Serdarević M, Polić-Vižintin M. Smoking behaviors and lung cancer epidemiology: a cohort study. Psychiatr Danub. 2014;26:485–9. [PubMed] [Google Scholar]
- 3.Romani C, Calza S, Todeschini P, Tassi RA, Zanotti L, Bandiera E, Sartori E, Pecorelli S, Ravaggi A, Santin AD, Bignotti E. Identification of optimal reference genes for gene expression normalization in a wide cohort of endometrioid endometrial carcinoma tissues. PLoS One. 2014;9:e113781. doi: 10.1371/journal.pone.0113781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–45. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 5.Shindo Y, Yoshimura K, Kuramasu A, Watanabe Y, Ito H, Kondo T, Oga A, Ito H, Yoshino S, Hazama S, Tamada K, Yagita H, Oka M. Combination Immunotherapy with 4-1BB Activation and PD-1 Blockade Enhances Antitumor Efficacy in a Mouse Model of Subcutaneous Tumor. Anticancer Res. 2015;35:129–36. [PubMed] [Google Scholar]
- 6.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–51. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 7.Prokunina L, Castillejo-López C, Oberg F, Gunnarsson I, Berg L, Magnusson V, Brookes AJ, Tentler D, Kristjansdóttir H, Gröndal G, Bolstad AI, Svenungsson E, Lundberg I, Sturfelt G, Jönssen A, Truedsson L, Lima G, Alcocer-Varela J, Jonsson R, Gyllensten UB. A regulatory polymorphism in PDCD1 is associated with susceptibility to systemic lupus erythematosus in humans. Nat Genet. 2002;32:666–9. doi: 10.1038/ng1020. [DOI] [PubMed] [Google Scholar]
- 8.Liu C, Jiang J, Gao L, Hu X, Wang F, Shen Y, Yu G, Zhao Z, Zhang X. A Promoter Region Polymorphism in PDCD-1 Gene Is Associated with Risk of Rheumatoid Arthritis in the Han Chinese Population of Southeastern China. Int J Genomics. 2014;2014:247637. doi: 10.1155/2014/247637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pizarro C, García-Díaz DF, Codner E, Salas-Pérez F, Carrasco E, Pérez-Bravo F. PD-L1 gene polymorphisms and low serum level of PD-L1 protein are associated to type 1 diabetes in Chile. Diabetes Metab Res Rev. 2014;30:761–6. doi: 10.1002/dmrr.2552. [DOI] [PubMed] [Google Scholar]
- 10.Yang M, Zou Y, Bai Y, Li M. The programmed cell death 1 gene polymorphisms (PD 1.3 G/A, PD 1.5 C/T and PD 1.9 C/T) and susceptibility to ankylosing spondylitis: a meta-analysis. J Orthop Sci. 2015;20:55–63. doi: 10.1007/s00776-014-0648-6. [DOI] [PubMed] [Google Scholar]
- 11.Dehaghani AS, Kashef MA, Ghaemenia M, Sarraf Z, Khaghanzadeh N, Fattahi MJ, Ghaderi A. PDCD1, CTLA-4 and p53 gene polymorphism and susceptibility to gestational trophoblastic diseases. J Reprod Med. 2009;54:25–31. [PubMed] [Google Scholar]
- 12.Haghshenas MR, Naeimi S, Talei A, Ghaderi A, Erfani N. Program death 1 (PD1) haplotyping in patients with breast carcinoma. Mol Biol Rep. 2011;38:4205–10. doi: 10.1007/s11033-010-0542-z. [DOI] [PubMed] [Google Scholar]
- 13.Hua Z, Li D, Xiang G, Xu F, Jie G, Fu Z, Jie Z, Da P, Li D. PD-1 polymorphisms are associated with sporadic breast cancer in Chinese Han population of Northeast China. Breast Cancer Res Treat. 2011;129:195–201. doi: 10.1007/s10549-011-1440-3. [DOI] [PubMed] [Google Scholar]
- 14.Mojtahedi Z, Mohmedi M, Rahimifar S, Erfani N, Hosseini SV, Ghaderi A. Programmed death-1 gene polymorphism (PD-1.5 C/T) is associated with colon cancer. Gene. 2012;508:229–32. doi: 10.1016/j.gene.2012.07.059. [DOI] [PubMed] [Google Scholar]
- 15.Savabkar S, Azimzadeh P, Chaleshi V, Nazemalhosseini Mojarad E, Aghdaei HA. Programmed death-1 gene polymorphism (PD-1.5 C/T) is associated with gastric cancer. Gastroenterol Hepatol Bed Bench. 2013;6:178–82. [PMC free article] [PubMed] [Google Scholar]
- 16.Yousefi AR, Karimi MH, Shamsdin SA. PD-1 gene polymorphism in iranian patients with colorectal cancer. Lab Medicine. 2013;44:23–6. [Google Scholar]
- 17.Chittenden TW, Howe EA, Culhane AC, Sultana R, Taylor JM, Holmes C, Quackenbush J. Functional classification analysis of somatically mutated genes in human breast and colorectal cancers. Genomics. 2008;91:508–11. doi: 10.1016/j.ygeno.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baek J, Lee H, Hwang KW, Kim E, Min H. The association of CD28 polymorphism, rs3116496, with Cancer: A meta-analysis. Comput Biol Med. 2015;61:172–7. doi: 10.1016/j.compbiomed.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Gao X, Zhang S, Qiao X, Yao Y, Wang L, Dong D, Ma X, Wang T. Association of cytotoxic T lymphocyte antigen-4 +49A/G polymorphism and cancer risk: An updated meta-analysis. Cancer Biomark. 2014;14:287–94. doi: 10.3233/CBM-140403. [DOI] [PubMed] [Google Scholar]
- 20.Lee YH, Song GG. A meta-analysis of the association between CTLA-4 +49 A/G, -318 C/T, and IL-1 polymorphisms and susceptibility to cervical cancer. Neoplasma. 2014;61:481–90. doi: 10.4149/neo_2014_060. [DOI] [PubMed] [Google Scholar]
- 21.Fife BT, Bluestone JA. Control of peripheral T-cell torelance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–82. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 22.Lin SC, Yen JH, Tsai JJ, Tsai WC, Ou TT, Liu HW, Chen CJ. Association of a programmed death 1 gene polymorphism with the development of rheumatoid arthritis, but not systemic lupus erythematosus. Arthritis Rheum. 2004;50:770–5. doi: 10.1002/art.20040. [DOI] [PubMed] [Google Scholar]


