Abstract
Background: Compared with non-reversible, indirect P2Y12 inhibitor clopidogrel, ticagrelor is a reversible, direct acting inhibitor. The CYP2C19*2 allele is a common genetic variant in individuals that need given higher clopidogrel in acute coronary syndrome patients. Objective: We aimed to assess a pharmacogenetic approach of doubling dose clopidogrel compare with standard dose of ticagrelor among carriers with the CYP2C19*2 homozygotes. Materials and methods: We compared ticagrelor (180 mg loading dose, 90 mg twice daily thereafter) with clopidogrel (600 mg loading dose, 150 mg daily thereafter) for the prevention of cardiovascular events in CYP2C19*2 homozygotes patients admitted to the hospital with an acute coronary syndrome, with or without ST-segment elevation. Results: After genetic test to identify carriers of the CYP2C19*2 allele from 2295 patients, 224 cases with CYP2C19*2 homozygotes were enrolled into our prospective, randomized trial. Patients were random assignment with colpidogrel group (n = 112) and ticagrelor group (n = 112). The two groups were similar in terms of baseline characteristics. After the first 600 mg loading dose of clopidogrel, patients carrying two CYP2C19*2 allele had weaker PRU inhibition (39.8±37.4 vs 27.9±12.4; P = 0.001) and more bleeding adverse events (20.5% vs. 7.1%; hazard ratio = 2.88; 95% [CI], 1.34-6.15; P = 0.001) compared to those taking standard dose of ticagrelor. Conclusions: In CYP2C19*2 carriers with ACS, ticagrlor is as effective as high clopidogrel in reducing platelet reactivity, particularly in first days. This study suggests that ticagrelor may be much better than doubling dose clopidogrel in patients with CYP2C19*2 in according to platelet reactivity monitoring. Use of ticagrelor instead of clopidogrel may eliminate the need for genetic testing and lead to less mild bleeding adverse.
Keywords: Acute coronary syndrome, clopidgrel, ticagrelor, platelet aggregation inhibitors, randomized controlled trial
Introduction
Platelets play a major pathogenic role in thrombus formation. Clopidogrel treatment in combination with aspirin can prevent recurrent ischaemic events after an acute coronary syndrome (ACS) [1,2]. However, many ACS patients on current standard dual antiplatelet therapy still have thrombotic events because clinical responses to clopidogrel are highly variable between patients. Those patients are clinically designated as clopidogrel resistant or non-responders [3,4]. Clopidogrel is a prodrug that has to be converted into an active metabolite by the hepatic cytochrome P450 (CYP) system [5,6]. The polymorphisms of CYP2C19 might be one of the factors influencing the individual susceptibility. Moreover, CYP2C19 is a key enzyme in this activation process, and the presence of the loss-of-function CYP2C19 681G>A polymorphism (CYP2C19*2) is associated with reduced clopidogrel responsiveness in healthy people [7] and in patients with ACS [8] or percutaneous coronary intervention (PCI) [9]. It has been proved that the CYP2C19*2 variant was associated with a significant increase in the rate of cardiovascular events among patients who under clopidogrel treatment [10]. Then, a strategy of raising the dose of clopidogrel or changing for more potent antiplatelet drugs (such as ticagrelor) may help overcome poor response and improve clinical outcome.
In contrast, CYP2C19 genotype was shown no effect on pharmacodynamics in patients during ticagrelor therapy [11]. And ticagrelor have shown more effective that clopidogrel in the treatment of acute coronary syndrome, it can significantly reduce the rate of death as compared with clopidogrel [12]. However, ticagrelor as a new antiplatelet drug is more expensive than clopidogrel. It needs take twice daily for its pharmacokinetic characteristics and will conduct to less compliance in patients. Recently, it suggested that people carries the risk of increased thrombotic events if patients miss a ticagrelor dose [13,14].
The United States FDA has issued a black box warning about carriers of CYP2C19*2 alleles (http://products.sanofi.us/plavix/plavix.html) and they suggested that these carriers should receive a higher dose of clopidogrel or another drug. Evidence suggests that carriers of at least one CYP2C19*2 allele had a relative reduction of 32.4% in plasma exposure to the active metabolite of clopidogrel, as compared with noncarriers [15]. What’s more, patients with two copies of CYP2C19*2 allele had a higher risk of cardiovascular system event than patients with one or none allele [16]. Similar findings have been noted for ACS patients with the 2-flod maintenance dose of clopidogrel, which has possible better prognostic implications [17,18]. Current guidelines offer a choice among clopidogrel or ticagrelor on top of aspirin for dual antiplatelet ACS therapy. Thus, with the availability of ticagrelor, doctors are challenged to determine the right treatment strategies for individual with CYP2C19*2 allele while minimizing the risk of bleeding and maximizing the efficacy. So we assessed the effect of the twice dosage of clopidogrel or ticagrelor in two cohort of patients who were identified with two copies of CYP2C19*2 loss-of-function polymorphism.
Methods
Study design
It was a single center, randomized trial. A computerized random-number generator was used to draw up an allocation schedule by the first. This prospective study enrolling consecutive patients undergoing non-emergent PCI was performed. An independent data and safety monitoring board monitored the trial and had access to all data. The sponsor coordinated the data management. This study protocol was approved by the local ethic committee in China. All patients that gave informed consent for genetic testing were prospectively included.
Inclusion/exclusion criteria
The study randomized 224 patients who carries CYP2C19*2 homozygotes within 24 hours of an acute coronary syndrome (either ST-elevation or non-ST-elevation) to ticagrelor (n = 112) or clopidogrel (n = 112). Patients were excluded if they had periprocedural P2Y12 inhibitors administration, a history of stroke/transient ischemic attack, bleeding diathesis, chronic oral anticoagulation treatment, PCI, hemodynamic instability, platelet count less than 100 g/L, hematocrit 30%, creatinine clearance 30 ml/min, severe hepatic dysfunction, history of bleeding diathesis, and concurrent severe illness with expected survival of 1 month.
Therapeutic strategy
Ticagrelor (AstraZeneca) was administered as a loading dose of 180 mg followed by 90 mg twice daily, and clopidogrel (Bristol-Myers Squibb and Sanofi) was given as a 600 mg loading dose followed by 150 mg daily of maintenance therapy (for patients who had not been previously taking clopidogrel). In contrast, clopidogrel (300 mg) was administered in the first day and followed by 75 mg daily. Usually, platelet inhibition is dose-related up to a single dose of 400 mg of clopidogrel, then a dose of 75 mg per day produces the same level of inhibition after one week [19]. All patients received background aspirin therapy at a dose of 75 mg per day.
Laboratory test
Blood sample collection
Whole blood for clinical laboratory test and genotyping were obtained from the arterial sheath of all patients directly after diagnostic angiography. DNA was extracted from peripheral blood lymphocytes by standard procedures. All tests were done by nurses and the staff of clinical examination.
CYP2C19*2 genotyping
Genotyping for known variants *2 of the CYP2C19 gene was performed by PCR amplification using primer oligonucleotides designed with Primer3 software tool. Amplification was done with two oligonucleotide primers: Forward, 5’-TGCAATAATTTTCCCACTATCATTG-3’; Reverse, 5’-CCAAAATATCACTTTCCATAAAAGCA-3’.
Polymorphism screening was undertaken with the ABI PRISM dye terminator method. DNA sequencing gave identical genotypes by cycle sequencing kit procedure and loaded on to a capillary automatic sequencer ABI3130xl (Applied Biosystems, USA).
PRU analysis
The initial blood drawn was discarded to avoid measuring platelet activation induced by needle puncture; blood was collected into a Vacutainer containing 3.8% trisodium citrate and filled to 8 ml. The Vacutainer was inverted 3 to 5 times for gentle mixing and sent immediately to the hemostasis laboratory. For the VerifyNow P2Y12 assay, blood sample was performed as described by the manufacturer (Accumetrics, USA). Reported measures included P2Y12 Reaction Units (PRU) and P2Y12 device-reported percent inhibition.
Statistical analysis
Continuous data are expressed as mean ± SD. Categorical data are expressed as frequencies. Means of age, body weight and platelet reactivity unit are compared with Student t tests at a two-tailed 5% level. Frequencies of dyslipidemia, hypertension, diabetes mellitus, smoking, history of myocardial infarction, proportion of ST elevation myocardial infarction and creatinine clearance < 60 ml/min are compared using chi-square test at a two-tailed 5% level. All statistical analyses were performed using SPSS 19.0 software.
Results
We recruited 224 patients from October 2012 through September 2013 by the flow chart (Figure 1). Of 2295 patients with genotype assessment, 224 (9.8%) were identified to have CYP2C19*2 homozygote variant (Table 1) and were all randomized to two group. 1093 (47.6%) were wild-type homozygotes (*1/*1), 978 (42.6%) were heterozygotes (*1/*2), and 224 (9.8%) were homozygous (*2/*2) with respect to the *2 allelic variant. The allelic frequency of CYP2C19*2 was 31.1% (Table 1). We noted no deviation from the expected proportions of genotypes in the selected population predicted by the Hardy-Weinberg equilibrium for polymorphisms.
Figure 1.
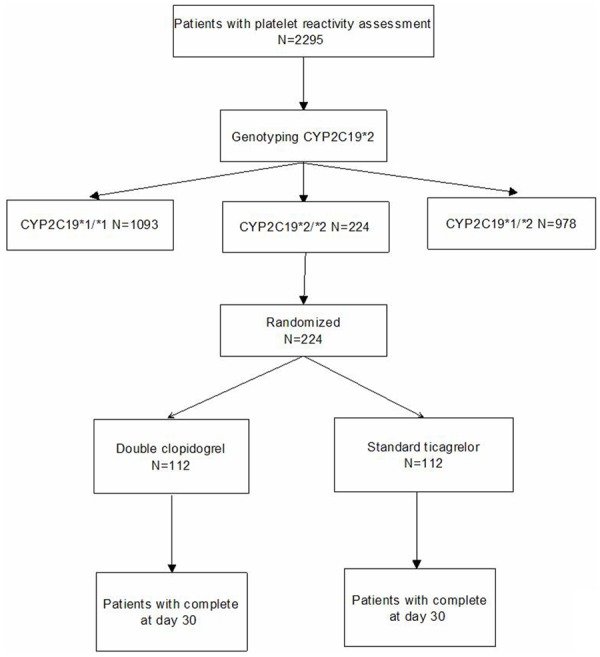
Study Flow Chart.
Table 1.
CYP2C19*2 genotype frequencies in patients
| SNPs | Allele frequencies | Genotype frequencies | Total | |||
|---|---|---|---|---|---|---|
|
| ||||||
| CYP2C19 mutation | *1 | *2 | *1/*1 | *1/*2 | *2/*2 | |
| Number | 2071 | 1202 | 1093 | 978 | 224 | 2295 |
| Frequencies (%) | 68.9 | 31.1 | 47.6 | 42.6 | 9.8 | |
Baseline characteristics, such as comorbidities, previous myocardial infarction, drugs, and procedural characteristics, were similar in both groups. In short, Baseline characteristics were well balanced between the two groups defined by CYP2C19*2 genotype (Table 2). Until day 30, there was no obvious side effects leading to study drug discontinuation, every person completed this study. 224 patients had complete follow-up: 112 patients in the doubling clopidogrel group and 112 were given standard ticagrelor treatment (Figure 1).
Table 2.
Baseline characteristics of the patients, according to treatment group
| Baseline Characteristic | Ticagrelor Group (N = 112) | Clopidogrel Group (N = 112) | Two-sided p-value |
|---|---|---|---|
| Race | 100% (Han Chinese) | 100% (Han Chinese) | 1.00 |
| Male | 70% (86) | 71% (87) | 0.98 |
| Age, yrs | 66±8 | 67±9 | 0.36 |
| Body weight, kg ± SD | 71±18 | 70±19 | 0.67 |
| Dyslipidemia | 71% (87) | 72% (88) | 0.98 |
| Hypertension | 30% (36) | 32% (39) | 0.78 |
| Diabetes mellitus | 22% (27) | 23% (28) | 0.98 |
| Smoking | 55% (67) | 50% (61) | 0.98 |
| Previous myocardial infarction | 27% (33) | 29% (35) | 0.89 |
| ST elevation myocardial infarction | 41% (50) | 39% (47) | 0.79 |
| CrCl < 60 mL/min | 12% (15) | 10% (12) | 0.68 |
Individual platelet reactivity units (PRU) according to treatment and baseline platelet reactivity values are depicted in Figure 2. Present study showed that the baseline of platelet function was 283.2±12 PRU on aspirin + clopidogrel therapy versus 279.5±16.2 PRU on aspirin + ticagrelor therapy) in CYP2C19*2 homozygotes. After that, the PRU was significantly lower for ticagrelor (34.5±21.0 PRU) compared with clopidogrel (76.6±55.2 PRU) at day 15 (P = 0.000, and their difference is 42.1 (95% confidence interval [CI], 31.1 to 53.1). However, individual PRU at the end day of treatment period are depicted (Clopidogrel PRU 39.8±37.4, 95% [CI]: 32.8 to 46.8 vs. ticagrelor PRU 27.9±12.4, 95% [CI]: 25.6 to 30.2, P = 0.001) in Figure 3.
Figure 2.
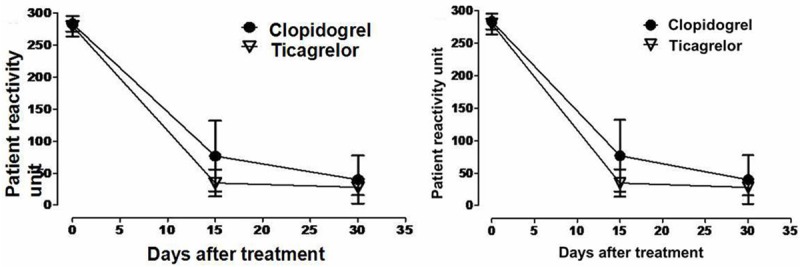
Platelet reactivity units (PRU) after treatment sequence.
Figure 3.
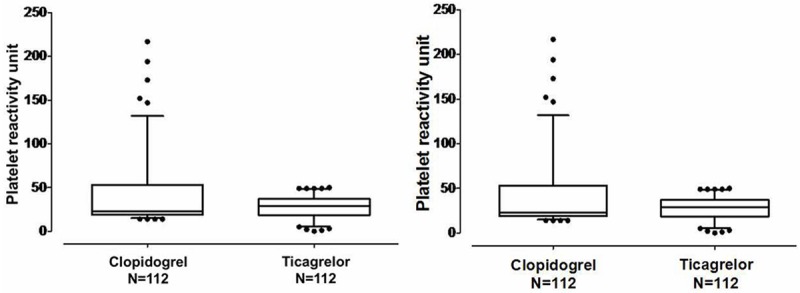
Individual platelet reactivity unit values at day 30 after treatment.
No patient exhibited a major adverse cardiovascular event or a major bleeding event at either treatment group. There was no difference in major bleeding. But there were some differences between ticagrelor and clopidogrel in minor bleeding. 23 patients under clopidogrel during the first period reported minimal bleeding events. On the contrary, only 8 patients under ticagrelor reported a mild bleeding event. Thus, patients who treated by clopidogrel were at significantly higher risk for the safety outcome of minimal bleeding events (20.5% vs. 7.1%; hazard ratio for ticagrelor, 2.88; 95% confidence interval [CI], 1.34 to 6.15; P = 0.001).
Discussions
The present study first aimed to compare ticagreor with clopidogrel in ACS patients with two copies of CYP2C19*12 mutation. We hypothesized that persistence of high dose clopidogrel on treatment platelet reactivity could block the P2Y12 receptor as same as ticagrelor. In the present study no significance difference at major events was observed between clopidogrel and ticagrelor group during treatment period. We have demonstrated that clopidgrel provides a little stronger than ticagrelor platelet inhibition and more minor bleeding adverse at last. The results support that people with CYP2C19*2 alleles need rising the dose of clopidogrel to induce an optimal platelet reactivity inhibition. However, the only significant difference between two groups was platelet reactivity inhibition after the first loading dose of clopidogrel at day 15. It means ticagrelor was a faster onset of action in the platelet inhibition and an approximately 2-fold higher potency than clopidogrel. These findings would suggest that ticagrelor is a more rapid and efficacious treatment for ACS than is clopidogrel, irrespective of CYP2C19*2 polymorphisms.
We have further demonstrated that using this kind of doubling clopidogrel loading dose in patients with high on treatment platelet reactivity was associated with higher bleeding adverse. However, previous studies have shown that compared with 300 mg loading dose of clopidogrel, treatment with a 600 mg loading dose can reduced nearly 4 times of non-responsiveness [20] and significance lower rate of cardiovascular death or non-fatal myocardial infarction [21]. It implied that simple doubling dose was not a good choice for all patients with *2 alleles. There may be other CYPs mutations should be considered. CYP2C19*2 polymorphism is not the sole determinant of the response to clopidogrel: implications for its monitoring [22]. The variant allele CYP2C9*3 and *17 play an important role in the response to clopidogrel in patients on dual antiplatelet therapy undergoing coronary stenting [23,24].
First originality is that we have used the VerifyNow as a specific platelet assay to assess platelet reactivity after doubling clopidogrel loading dose (600 mg). In addition, we selected the subjects with two copies of CYP2C19 for the clinical trial at the first time. Only patients who were identified CYP2C19*2 homozygotes were assessed in this comparative study. The CYP2C19 gene polymorphisms are differently distributed according to ethnicity in world population: African (20.2%); Caucasian (16.9%); Mulatto (16.5%); and Amerindian (10.4%) individuals, respectively [25]. In fact, it has been demonstrated that Asian populations had a much higher proportion (> 50%) than caucasian or others [26]. In conclusion, the CYP2C19*2 induced clopidogrel resistance can be managed in some patients by increasing the given dose. But the twice dose of clopidogrel is not responsible for all persons according to platelet reactivity. The comparative risks of long-term cardiovascular events during clopidogrel treatment are not clear. Long-term prognosis should be considered during clopidogrel or ticagrelor exposure. Ticagrelor may be useful in these patients to improve platelet reactivity inhibition. Therefore, for CYP2C19*2 cases, physician use the ticagrelor instead of clopidogrel is a more effective and safe solution for ACS patients.
Acknowledgements
This study was supported by a grant from the Beijing Natural Science Foundation (No, 7112044).
Disclosure of conflict of interest
None.
References
- 1.Barilla F, Pulcinelli FM, Mangieri E, Torromeo C, Tanzilli G, Dominici T, Pellicano M, Paravati V, Acconcia MC, Gaudio C. Clopidogrel plus indobufen in acute coronary syndrome patients with hypersensitivity to aspirin undergoing percutaneous coronary intervention. Platelets. 2013;24:183–188. doi: 10.3109/09537104.2012.686072. [DOI] [PubMed] [Google Scholar]
- 2.Fox KA, Mehta SR, Peters R, Zhao F, Lakkis N, Gersh BJ, Yusuf S Clopidogrel in Unstable angina to prevent Recurrent ischemic Events Trial. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation. 2004;110:1202–1208. doi: 10.1161/01.CIR.0000140675.85342.1B. [DOI] [PubMed] [Google Scholar]
- 3.Esposito G. Responsiveness to P2Y12 receptor inhibitors. Curr Opin Cardiol. 2011;26(Suppl 1):S31–37. doi: 10.1097/01.hco.0000409965.20588.c5. [DOI] [PubMed] [Google Scholar]
- 4.Siller-Matula JM, Krumphuber J, Jilma B. Pharmacokinetic, pharmacodynamic and clinical profile of novel antiplatelet drugs targeting vascular diseases. Br J Pharmacol. 2010;159:502–517. doi: 10.1111/j.1476-5381.2009.00555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurbin F, Boulenc X, Daskalakis N, Farenc C, Taylor T, Bonneau D, Lacreta F, Cheng S, Sultan E. Clopidogrel pharmacodynamics and pharmacokinetics in the fed and fasted state: a randomized crossover study of healthy men. J Clin Pharmacol. 2012;52:1506–1515. doi: 10.1177/0091270011419852. [DOI] [PubMed] [Google Scholar]
- 6.Kelly RP, Close SL, Farid NA, Winters KJ, Shen L, Natanegara F, Jakubowski JA, Ho M, Walker JR, Small DS. Pharmacokinetics and pharmacodynamics following maintenance doses of prasugrel and clopidogrel in Chinese carriers of CYP2C19 variants. Br J Clin Pharmacol. 2012;73:93–105. doi: 10.1111/j.1365-2125.2011.04049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H, Yu G, Sun H, Wu X, Wang H. Comparison of Adjunctive Naoxintong versus Clopidogrel in Volunteers with the CYP2C19*2 Gene Mutation Accompanied with Qi Deficiency and Blood Stasis Constitution. Evid Based Complement Alternat Med. 2011;2011:207034. doi: 10.1155/2011/207034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cano P, Consuegra-Sanchez L, Conesa P, Torres-Moreno D, Jaulent L, Dau D, Pico F, Villegas M. [Relevance of CYP2C19 2 regarding platelet reactivity in patients with acute coronary syndrome treated with clopidogrel] . Med Clin (Barc) 2014;143:6–12. doi: 10.1016/j.medcli.2013.04.042. [DOI] [PubMed] [Google Scholar]
- 9.Bernlochner I, Mayer K, Morath T, Braun S, Schulz S, Schomig A, Koch W, Kastrati A, Sibbing D. High frequency of CYP2C19*2 carriers in PCI-treated patients switched over from clopidogrel to prasugrel based on platelet function monitoring. Platelets. 2013;24:500–502. doi: 10.3109/09537104.2012.715218. [DOI] [PubMed] [Google Scholar]
- 10.Collet JP, Hulot JS, Pena A, Villard E, Esteve JB, Silvain J, Payot L, Brugier D, Cayla G, Beygui F, Bensimon G, Funck-Brentano C, Montalescot G. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009;373:309–317. doi: 10.1016/S0140-6736(08)61845-0. [DOI] [PubMed] [Google Scholar]
- 11.Tantry US, Bliden KP, Wei C, Storey RF, Armstrong M, Butler K, Gurbel PA. First analysis of the relation between CYP2C19 genotype and pharmacodynamics in patients treated with ticagrelor versus clopidogrel: the ONSET/OFFSET and RESPOND genotype studies. Circ Cardiovasc Genet. 2010;3:556–566. doi: 10.1161/CIRCGENETICS.110.958561. [DOI] [PubMed] [Google Scholar]
- 12.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Investigators P, Freij A, Thorsen M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 13.Schomig A. Ticagrelor--is there need for a new player in the antiplatelet-therapy field? N Engl J Med. 2009;361:1108–1111. doi: 10.1056/NEJMe0906549. [DOI] [PubMed] [Google Scholar]
- 14.Sabatine MS. Novel antiplatelet strategies in acute coronary syndromes. Cleve Clin J Med. 2009;76(Suppl 1):S8–15. doi: 10.3949/ccjm.76.s1.02. [DOI] [PubMed] [Google Scholar]
- 15.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, Walker JR, Antman EM, Macias W, Braunwald E, Sabatine MS. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 16.Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Meneveau N, Steg PG, Ferrieres J, Danchin N, Becquemont L French Registry of Acute STE and Non STEMII. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–375. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen TA, Lordkipanidze M, Diodati JG, Palisaitis DA, Schampaert E, Turgeon J, Pharand C. Week-long high-maintenance dose clopidogrel regimen achieves better platelet aggregation inhibition than a standard loading dose before percutaneous coronary intervention: results of a double-blind, randomized clinical trial. J Interv Cardiol. 2009;22:368–377. doi: 10.1111/j.1540-8183.2009.00486.x. [DOI] [PubMed] [Google Scholar]
- 18.Angiolillo DJ, Welsh RC, Trenk D, Neumann FJ, Conley PB, McClure MW, Stephens G, Kochman J, Jennings LK, Gurbel PA, Wojcik J, Dabrowski M, Saucedo JF, Stumpf J, Buerke M, Broderick S, Harrington RA, Rao SV. Pharmacokinetic and pharmacodynamic effects of elinogrel: results of the platelet function substudy from the intravenous and oral administration of elinogrel to evaluate tolerability and efficacy in nonurgent percutaneous coronary intervention patients (INNOVATE-PCI) trial. Circ Cardiovasc Interv. 2012;5:347–356. doi: 10.1161/CIRCINTERVENTIONS.111.965608. [DOI] [PubMed] [Google Scholar]
- 19.Maree AO, Fitzgerald DJ. Variable platelet response to aspirin and clopidogrel in atherothrombotic disease. Circulation. 2007;115:2196–2207. doi: 10.1161/CIRCULATIONAHA.106.675991. [DOI] [PubMed] [Google Scholar]
- 20.Gurbel PA, Bliden KP, Hayes KM, Yoho JA, Herzog WR, Tantry US. The relation of dosing to clopidogrel responsiveness and the incidence of high post-treatment platelet aggregation in patients undergoing coronary stenting. J Am Coll Cardiol. 2005;45:1392–1396. doi: 10.1016/j.jacc.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 21.Biondi-Zoccai GG, Lotrionte M, Agostoni P, Valgimigli M, Abbate A, Sangiorgi G, Moretti C, Sheiban I. Benefits of clopidogrel in patients undergoing coronary stenting significantly depend on loading dose: evidence from a meta-regression. Am Heart J. 2007;153:587–593. doi: 10.1016/j.ahj.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Aleil B, Leon C, Cazenave JP, Gachet C. CYP2C19*2 polymorphism is not the sole determinant of the response to clopidogrel: implications for its monitoring. J Thromb Haemost. 2009;7:1747–1749. doi: 10.1111/j.1538-7836.2009.03554.x. [DOI] [PubMed] [Google Scholar]
- 23.Grosdidier C, Quilici J, Loosveld M, Camoin L, Moro PJ, Saut N, Gaborit B, Pankert M, Cohen W, Lambert M, Beguin S, Morange PE, Bonnet JL, Alessi MC, Cuisset T. Effect of CYP2C19*2 and *17 genetic variants on platelet response to clopidogrel and prasugrel maintenance dose and relation to bleeding complications. Am J Cardiol. 2013;111:985–990. doi: 10.1016/j.amjcard.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Harmsze A, van Werkum JW, Bouman HJ, Ruven HJ, Breet NJ, Ten Berg JM, Hackeng CM, Tjoeng MM, Klungel OH, de Boer A, Deneer VH. Besides CYP2C19*2, the variant allele CYP2C9*3 is associated with higher on-clopidogrel platelet reactivity in patients on dual antiplatelet therapy undergoing elective coronary stent implantation. Pharmacogenet Genomics. 2010;20:18–25. doi: 10.1097/FPC.0b013e328333dafe. [DOI] [PubMed] [Google Scholar]
- 25.Santos PC, Soares RA, Santos DB, Nascimento RM, Coelho GL, Nicolau JC, Mill JG, Krieger JE, Pereira AC. CYP2C19 and ABCB1 gene polymorphisms are differently distributed according to ethnicity in the Brazilian general population. BMC Med Genet. 2011;12:13. doi: 10.1186/1471-2350-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhatt DL. Tailoring antiplatelet therapy based on pharmacogenomics: how well do the data fit? JAMA. 2009;302:896–897. doi: 10.1001/jama.2009.1249. [DOI] [PubMed] [Google Scholar]


