Abstract
Lipopolysaccharide (LPS) is known to induce acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Accumulating data suggest the crucial role of RAGE in the pathogenesis of ALI/ARDS. However, the mechanism by which RAGE mediates inflammatory lung injury in the neonates remains elusive. In this study we established LPS-induced ALI model in neonate rats, and investigated the role of RAGE/NF-κB signaling in mediating ALI. We found that RAGE antibody or bortezomib reduced LPS-induced histopathological abnormalities in the lung and lung damage score. RAGE antibody or bortezomib also reduced TNF-α level in both serum and BALF of the rats. Furthermore, RAGE antibody or bortezomib significantly reduced LPS-induced upregulation of RAGE and NF-κB expression in the lung. In conclusion, we established ALI model in neonate rats to demonstrate that LPS induced inflammatory lung injury via RAGE/NF-κB signaling. Interference with RAGE/NF-κB signaling is a potential approach to prevent and treat sepsis-related ALI/ARDS.
Keywords: Acute lung injury, neonate, RAGE, NF-κB, bortezomib
Introduction
Acute lung injury (ALI) remains a devastating disorder for intensive care medicine due to the high morbidity and mortality. Although the pathogenesis of ALI remains not completely understood, recent evidence suggests that ALI is characterized by a local inflammatory response that involves a wide variety of inflammatory mediators [1-3]. Neonates are susceptible to ALI, especially under several conditions such as the infection, hypoxia and shock [4]. Upon the infection, bacterial components such as lipopolysaccharide (LPS) are known to activate the inflammatory cascade, resulting in the release of inflammatory mediators which induces the development of ALI and acute respiratory distress syndrome (ARDS). In the clinical, sepsis-related ARDS has higher overall disease severity and mortality than non-sepsis-related ARDS [5]. Therefore, LPS-induced ALI/ARDS animal models are important tools to explore the mechanisms of ALI/ARDS and identify novel biomarkers and therapeutic targets for these diseases [6].
Receptor for Advanced Glycation End-products (RAGE) is a member of the cell surface receptors of the immunoglobulin superfamily. Accumulating data suggest the crucial role of RAGE in the pathogenesis of ALI and ARDS, and indicate the potential of RAGE as an important therapeutic target for ALI/ARDS [7]. Moreover, our recent studies demonstrated that hyperoxia induced acute damage in the lung of neonatal rats via RAGE/NF-κB signaling and RAGE mediates inflammatory response in alveolar type I epithelial cells (AECIs) via activating NF-κB [8,9]. In this study we established LPS induced ALI neonate rat model, and investigated the role of RAGE/NF-κB signaling in mediating ALI in the neonates.
Materials and methods
ALI model
This study was approved by Animal Care and Use Committee of Nanjing Medical University. Newborn Sprague-Dawley rats were purchased from the Animal Center of Jiangsu Province (Nanjing, China) and housed in individual cages with free access to water and laboratory chow. The rats were anesthetized with pentobarbital sodium and then randomly divided into 4 groups (n = 8): control group, LPS group, LPS + RAGE antibody (Ab) group, and LPS + bortezomib group. The rats in the control group were intraperitoneally injected with normal saline, while the rats in other groups were intraperitoneally injected with 3 mg/kg LPS (Sigma-Aldrich, St. Louis, MO, USA). The rats in LPS + Ab group and LPS + bortezomib group were also intraperitoneally injected with RAGE Ab (R&D, USA, 15 mg/kg) and bortezomib (Xian-Janssen Pharmaceutical, Xi’an, China, 0.2 mg/kg), respectively, 1 h prior to the injection of LPS.
Sample collection
Twenty-four hours after the injection of LPS, the rats were sacrificed via an intraperitoneal injection of 120 mg/kg pentobarbital, and blood samples were collected from the right atrium. The bronchoalveolar lavage fluid (BALF) was harvested as described previously [10]. The lungs were excised from the rats by opening the chest via median sternotomy. The left upper lobe was removed for the extraction of total RNA, the left lower lobe was removed for the extraction of total protein, and the right lobe was removed and fixed in 10% buffered formalin for 24 h.
Enzyme-linked immunosorbent assay (ELISA)
TNF-α level in the serum was measured using ELISA kits according to the manufacturer’s protocol (Abcam, Cambridge, MA, USA).
PCR
Total RNA was extracted from the left upper lobe by using TRIzol (Invitrogen, USA) and used to synthesize cDNA with reverse transcription system kits (Promega, Madison, WI, USA). Real-time PCR was performed using the following primers: RAGE 5’GGTGCTGGTTCTTGCTC 3’ and 5’TCCCTCGCCTGTTAGTT 3’; NF-κB 5’-GAAGAAGCGAGACCTGGAG-3’ and 5’-TCCGGAACACAATGGCCAC-3’; β-actin 5’-TCCTAGCACCATGA AGATC-3’ and 5’-AAACGCAGCTCAGTAACAG-3’. Amplification conditions were as follows: 5 min at 95°C (one cycle); 20 sec at 94°C; 20 sec at 58°C and 20 sec at 72°C (40 cycles); and 72°C for 5 min (one cycle). RT-PCR was performed three times in triplicate. The relative RAGE and NF-κB mRNA levels were compared to that of β-actin and calculated by the 2-ΔΔCt method. Each Ct value used for these calculations was the mean of the triplicate for each reaction.
Western blot analysis
Total protein was extracted from the left lower lobe using lysis buffer (Pierce, Rockford, IL, USA) and protein concentration was determined using BSA method. Then equal amounts of protein were separated in 10% SDS-PAGE and transferred into polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The membranes were incubated with antibody against RAGE, NF-κB, or β-actin (Millipore, Billerica, MA, USA) overnight at 4°C, then incubated with peroxidase-coupled IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 h at 37°C. The membranes were developed using ECL kit (Pierce, Rockford, IL, USA). β-actin was used as loading control.
Histological analysis
The morphological changes of lung tissue were evaluated by staining the sections with hematoxylin and eosin, and observed under light microscopy from ten randomly chosen areas for each section. Each slide was evaluated by two investigators in a blinded manner. Lung injury was scored according to the following four categories: alveolar congestion, hemorrhage, neutrophil infiltration into the airspace or vessel wall, and alveolar wall thickness/hyaline membrane formation. Each category was graded on a five point scale: 0 = minimal injury, 1 = injury up to 25% of the field, 2 = injury up to 50% of the field, 3 = injury up to 75% of the field, and 4 = diffuse injury.
Statistical analysis
The data were expressed as means ± standard deviations (SD) and analyzed by using SSPS 11.5 software (SPSS Inc., Chicago, IL, USA). Differences among multi-groups were analyzed with One-Way ANOVA, and differences between two groups were analyzed with the Student’s t-test. P value <0.05 was considered statistically significant.
Results
RAGE Ab and bortezomib alleviate LPS-induced ALI in neonate rats
We observed the general conditions of the rats at 24 h after the injection of LPS, just before their sacrifice. In contrast to the normal activities of the rats in control group, the rats in LPS group exhibited less activity, dull reaction, difficult breath, and perioral cyanosis. In LPS + Ab group and LPS + bortezomib group, the rats showed normal activities, had some difficulty in breath but no obvious perioral cyanosis.
By histological analysis, we observed clear alveolar structures and found no hemorrhage or effusion in alveolar spaces in control group (Figure 1A). As expected, in LPS group we observed typical histopathological abnormalities characterized by hemorrhage and effusion in alveolar spaces, alveolar wall thickening, and the infiltration of inflammatory cells (Figure 1B). In LPS + Ab group and LPS + bortezomib group, the rats showed less histopathological abnormalities in the lung (Figure 1C, 1D). The lung damage score was 0.47±0.12, 4.10±0.45, 3.06±0.42, and 2.69±0.21 in control group, LPS group, LPS + Ab group and LPS + bortezomib group, respectively. The lung damage score was significantly lower in LPS + Ab group and LPS + bortezomib group, compared to LPS group (P<0.05). Taken together, these results indicate that RAGE Ab and bortezomib alleviate LPS-induced lung injury in neonate rats.
Figure 1.
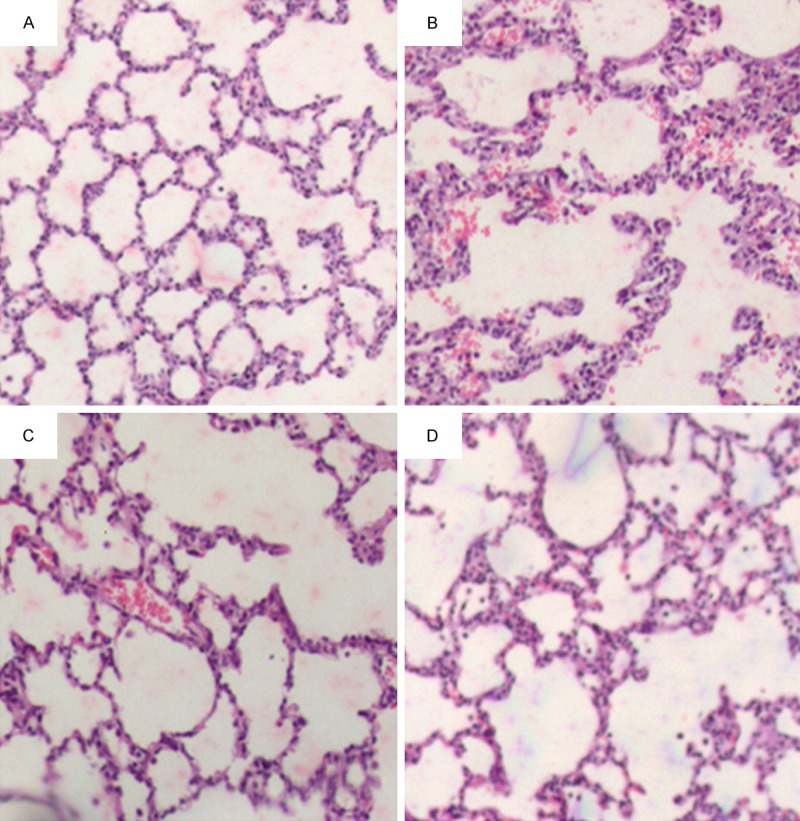
Representative images of lung histology in the rats of four groups (HE staining, ×100). A. Control group, we observed normal alveolar structures and no hemorrhage or effusion in alveolar spaces. B. LPS group, we observed abnormal alveolar structures, and hemorrhage and effusion in alveolar spaces. We also observed the infiltration of inflammatory cells. C. LPS + Ab group, D. LPS + bortezomib group, we observed much less abnormal alveolar structures, hemorrhage and effusion than in LPS group.
RAGE Ab and bortezomib inhibit LPS-induced upregulation of TNF-α in neonate rats
Based on ELISA we found that LPS increased the levels of TNF-α in both serum and BALF. However, the levels of TNF-α in both serum and BALF were significantly lower in LPS + Ab group and LPS + bortezomib group, compared to LPS group (Table 1). These results suggest that RAGE Ab and bortezomib inhibit LPS-induced upregulation of TNF-α in neonate rats.
Table 1.
Analysis of TNF-α levels in the serum and BALF in neonate rats (x̅±s, n = 8)
| Group | TNF-α (ng/L) | |
|---|---|---|
|
| ||
| Serum | BALF | |
| LPS + Ab | 210.67±27.61 | 114.58±13.46 |
| LPS + bortezomib | 175.40±14.93 | 127.50±10.41 |
| LPS | 377.92±33.85 | 191.03±11.01 |
| Control | 75.69±16.78 | 46.56±3.48 |
| F | 150.70 | 165.83 |
| P | 0.000 | 0.000 |
RAGE Ab and bortezomib inhibit LPS-induced upregulation of RAGE and NF-κB in neonate rats
TNF-α is known to induce the activation of NF-κB, an important mediator of inflammatory response downstream of RAGE. Therefore, we detected the expression of RAGE and NF-κB mRNA levels in each group of neonate rats. Real-time PCR analysis showed that LPS increased the mRNA levels of RAGE and NF-κB in the lungs. However, mRNA levels of RAGE and NF-κB in the lungs were significantly lower in LPS + Ab group and LPS + bortezomib group, compared to LPS group (Figure 2A, 2B).
Figure 2.
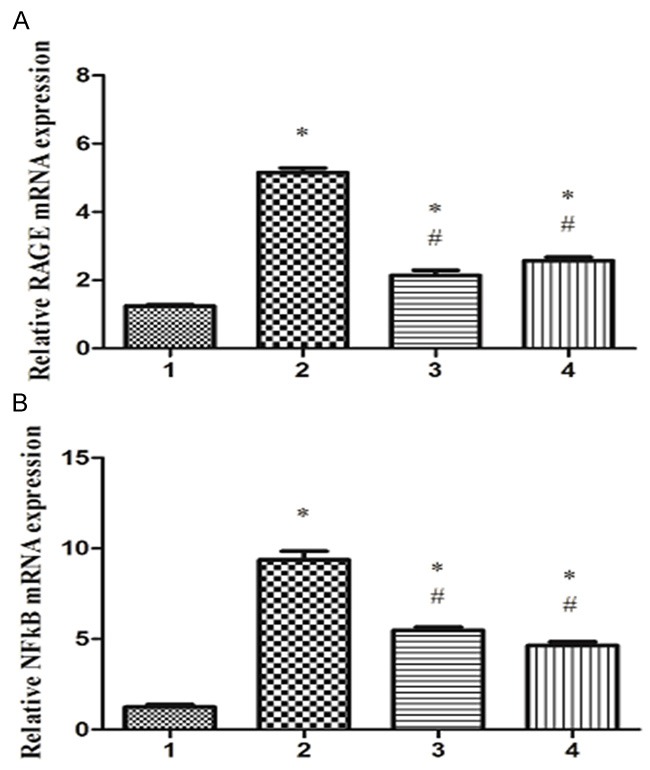
RAGE Ab and bortezomib reduce LPS-induced upregulation of RAGE and NF-κB mRNA expression in the lungs. Total RNA was extracted from the lung tissues of different groups, and RAGE mRNA level (A) and NF-κB mRNA level (B) were detected by real-time PCR analysis. 1. Control group; 2. LPS group; 3. LPS + Ab group; 4. LPS + bortezomib group. *P<0.05 vs. control group; #P<0.05 vs. LPS group.
Furthermore, we performed Western blot analysis to detect the expression of RAGE and NF-κB protein levels in each group of neonate rats. We found that LPS increased the protein levels of RAGE and NF-κB in the lungs. However, protein levels of RAGE and NF-κB in the lungs were significantly lower in LPS + Ab group and LPS + bortezomib group, compared to LPS group (Figure 3A, 3B). Collectively, these data suggest that RAGE Ab and bortezomib inhibit LPS-induced upregulation of RAGE and NF-κB in neonate rats.
Figure 3.
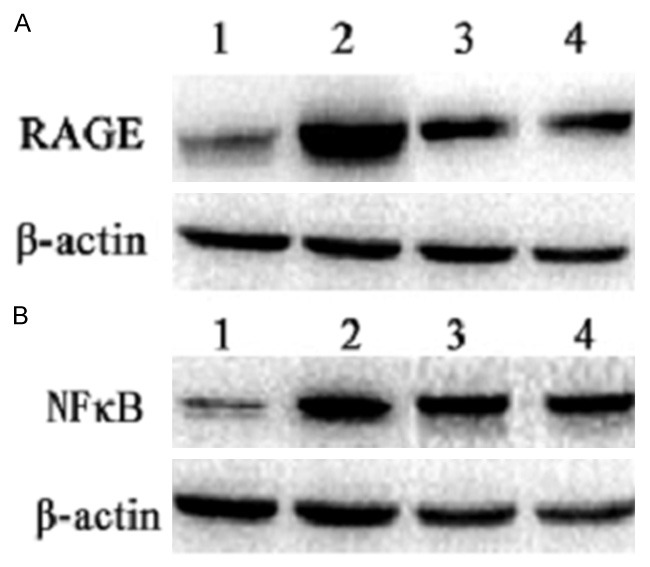
RAGE Ab and bortezomib reduce LPS-induced upregulation of RAGE and NF-κB protein expression in the lungs. Total protein was extracted from the lung tissues of different groups, and RAGE protein level (A) and NF-κB protein level (B) were detected by Western blot analysis. 1. Control group; 2. LPS group; 3. LPS + Ab group; 4. LPS + bortezomib group. β-actin was loading control.
Discussion
Sepsis is one of the most common causes of ALI in neonates. LPS is the main component of the cell wall of gram-negative bacteria and plays an important role in the development of ALI. LPS-induced ALI is a well established experimental model to screen novel drugs for ALI [11]. Therefore, in this study we established LPS-induced ALI model in neonate rats. We found that LPS induced histopathological abnormalities in the lung, increased the levels of TNF-α in both serum and BALF, and induced the upregulation of RAGE and NF-κB in the lung. These data demonstrate that LPS could induce injury and inflammatory response in the lung, suggesting that LPS-induced ALI was successfully established in neonate rats.
To investigate the role of RAGE/NF-κB signaling in mediating LPS-induced inflammatory response in the lung, we employed a loss-of-function approach to inhibit the activity of RAGE by monoclonal antibody against RAGE, and inhibit the activity of NF-κB by bortezomib [12]. Our results showed that RAGE antibody or bortezomib reduced histopathological abnormalities in the lung of neonate rats. Furthermore, the lung damage score was significantly lower in the rats pretreated with RAGE antibody or bortezomib than in the rats treated with LPS alone. These data indicate that the inhibition of RAGE or NF-κB alleviates LPS-induced lung injury in neonate rats.
NF-κB is one of the most important signaling pathways downstream of RAGE to mediate inflammatory response in the body [13]. Next we performed ELISA assay to examine the levels of TNF-α in both serum and BALF of the rats. As expected, LPS increased TNF-α level in both serum and BALF. Notably, TNF-α levels in both serum and BALF were significantly reduced in the rats pretreated with RAGE antibody or bortezomib, compared to the rats treated with LPS alone. These results suggest that the inhibition of RAGE or NF-κB inhibits LPS-induced upregulation of TNF-α in neonate rats.
It has been proposed that the activation of RAGE/NF-κB signaling promotes the transcription of RAGE itself. Consequently, RAGE expression is drastically augmented to mediate downstream processes to cause lung inflammatory injury [14,15]. In this study, we pretreated the neonate rats with RAGE antibody or NF-κB inhibitor bortezomib. We found that RAGE antibody or bortezomib significantly reduced LPS-induced upregulation of RAGE expression in the lung at both protein and mRNA levels. Furthermore, RAGE antibody or bortezomib significantly reduced LPS-induced upregulation of NF-κB expression in the lung at both protein and mRNA levels. Collectively, these data suggest that RAGE/NF-κB pathway forms a positive feedback loop to promote LPS-induced inflammatory response in the lung. Our data are consistent with recent study showing that sRAGE attenuated LPS-induced inflammation, NF-κB activation and pathologic changes in the lung [16].
In summary, in this study we established ALI model in neonate rats to demonstrate that LPS induced inflammatory lung injury via RAGE/NF-κB signal pathway. Inhibition of RAGE/NF-κB signaling by RAGE antibody or NF-κB inhibitor bortezomib alleviates LPS-induced inflammatory lung injury, accompanied by the downregulation of TNF-α, RAGE and NF-κB expression. Interference with RAGE/NF-κB signaling is a potential approach to prevent and treat sepsis-related ALI/ARDS.
Acknowledgements
This study was supported by Clinical Medicine Specialty Fund of Jiangsu Province (No. BL2014063), and Applied Science and Technology Project of Huai’an (No. HAS2014010).
Disclosure of conflict of interest
None.
References
- 1.Wang C. Obesity, inflammation, and lung injury (OILI): the good. Mediators Inflamm. 2014;2014:978463. doi: 10.1155/2014/978463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cross LJ, Matthay MA. Biomarkers in acute lung injury: insights into the pathogenesis of acute lung injury. Crit Care Clin. 2011;27:355–377. doi: 10.1016/j.ccc.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu MD, Yang Y, Zhou CX, Li Q, Yi W, Qian GS, Mao M, Xu JC. Pretreatment with anti-flagellin serum delays acute lung injury in rats with sepsis. Inflamm Res. 2012;61:837–44. doi: 10.1007/s00011-012-0475-1. [DOI] [PubMed] [Google Scholar]
- 4.Willson DF, Chess PR, Notter RH. Surfactant for pediatric acute lung injury. Pediatr Clin North Am. 2008;55:545–75. doi: 10.1016/j.pcl.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheu CC, Gong MN, Zhai R, Chen F, Bajwa EK, Clardy PF, Gallagher DC, Thompson BT, Christiani DC. Clinical characteristics and outcomes of sepsis-related vs non-sepsis-related ARDS. Chest. 2010;138:559–567. doi: 10.1378/chest.09-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Bai C, Wang X. The value of the lipopolysaccharide-induced acute lung injury model in respiratory medicine. Expert Rev Respir Med. 2010;4:773–83. doi: 10.1586/ers.10.71. [DOI] [PubMed] [Google Scholar]
- 7.Guo WA, Knight PR, Raghavendran K. The receptor for advanced glycation end products and acute lung injury/acute respiratory distress syndrome. Intensive Care Med. 2012;38:1588–98. doi: 10.1007/s00134-012-2624-y. [DOI] [PubMed] [Google Scholar]
- 8.Tian Z, Li Y, Ji P, Zhao S, Cheng H. Mesenchymal stem cells protects hyperoxia-induced lung injury in newborn rats via inhibiting receptor for advanced glycation end-products/nuclear factor κB signaling. Exp Biol Med (Maywood) 2013;238:242–7. doi: 10.1177/1535370212473706. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Wu R, Zhao S, Cheng H, Ji P, Yu M, Tian Z. RAGE/NF-κB Pathway Mediates Lipopolysaccharide-Induced Inflammation in Alveolar Type I Epithelial Cells Isolated from Neonate Rats. Inflammation. 2014;37:1623–9. doi: 10.1007/s10753-014-9889-y. [DOI] [PubMed] [Google Scholar]
- 10.Jeyaseelan S, Chu HW, Young SK, Worthen GS. Transcriptional profiling of lipopolysaccharide-induced acute lung injury. Infect Immun. 2004;72:7247–7256. doi: 10.1128/IAI.72.12.7247-7256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ni YF, Jiang T, Cheng QS, Gu ZP, Zhu YF, Zhang ZP, Wang J, Yan XL, Wang WP, Ke CK, Han Y, Li XF. Protective effect of magnolol on lipopolysaccharide-induced acute lung injury in mice. Inflammation. 2012;35:1860–6. doi: 10.1007/s10753-012-9507-9. [DOI] [PubMed] [Google Scholar]
- 12.Di Filippo C, Petronella P, Freda F, Scorzelli M, Ferretti M, Canonico S, Rossi F, D’Amico M. Involvement of the ubiquitin-proteasome system in the formation of experimental postsurgical peritoneal adhesions. Mediators Inflamm. 2012;2012:194723. doi: 10.1155/2012/194723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Postina R, Wang Y. Ectodomain shedding of the receptor for advanced glycation end products: a novel therapeutic target for Alzheimer’s disease. Cell Mol Life Sci. 2009;66:3923–35. doi: 10.1007/s00018-009-0121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds PR, Wasley KM, Allison CH. Diesel Particulate Matter Induces RAGE Expression in Pulmonary Epithelial cells and RAGE Signaling Influences NF-kB-Mediated Inflammation. Environ Health Perspect. 2011;119:332–6. doi: 10.1289/ehp.1002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reynolds PR, Kasteler SD, Schmitt RE Hoidal JR. RAGE Signals through Ras during Tobacco Smoke-Induced Pulmonary Inflammation. Am J Respir Cell Mol Biol. 2011;45:411–8. doi: 10.1165/rcmb.2010-0231OC. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Tasaka S, Shiraishi Y, Fukunaga K, Yamada W, Seki H, Ogawa Y, Miyamoto K, Nakano Y, Hasegawa N, Miyasho T, Maruyama I, Ishizaka A. Role of soluble receptor for advanced glycation end products on endotoxin-induced lung injury. Am J Respir Crit Care Med. 2008;178:356–62. doi: 10.1164/rccm.200707-1069OC. [DOI] [PubMed] [Google Scholar]


