Abstract
Objective: The estrogen receptor alpha (ESR1) gene has been implicated in the etiology of osteoarthritis (OA). However, the results are conflicting. We assessed the association of three common ESR1 polymorphisms, rs2234693, rs9340799 and rs2228480, with OA in this meta-analysis. Methods: A comprehensive search was performed to identify related studies. Pooled odds ratio (OR) with 95% confidence interval (CI) was calculated using fixed or random effects model. Results: 15 studies (7036 cases and 9669 controls) for rs2234693 polymorphism, 14 studies (3904 cases and 6991 controls) for rs9340799 and 3 studies (331 cases and 619 controls) for rs2228480 polymorphism were identified. The final results indicated that the G allele in ESR1 rs9340799 was associated with decreased OA risk (GG+GA vs. AA: OR=0.878, 95% CI=0.792-0.972, P=0.012; G vs. A: OR=0.902, 95% CI=0.836-0.975, P=0.009). The A allele in rs2228480 might be associated with increased OA risk. But no significant association of rs2234693 polymorphism with OA susceptibility was observed. Conclusions: This meta-analysis indicates rs9340799 and rs2228480 rather than rs2234693 polymorphisms are associated with the incidence of OA. Some stable associations should be further confirmed in future.
Keywords: Estrogen receptor alpha, osteoarthritis, polymorphism, meta-analysis
Introduction
Osteoarthritis (OA) is one of the most common disabling skeletal disease, particularly in the elderly [1]. The prevalence rate for OA was 11.3% with approximately 85 million OA patients in 2009 in the world, which might increase to 122 million in 2017. Hence, the burden of OA on the national economy and healthcare system is enormous, both in the developed and developing countries [2,3]. OA has a multifactor etiology resulted from the interactions of environmental factors and genes [4]. Previous familial and twin studies have shown that 39%-70% of OA incidence is attributable to genetic factors [5-7]. To date, many genes from candidate and genome wide association studies have been reported to be related to the development of OA [8-11].
Epidemiological researches show sex-specific differences in the prevalence and incidence of OA, with females generally at a higher risk [12]. Moreover, the prevalence of OA in females increases rapidly among the postmenopausal women [1,4]. These evidences suggest that the influence of estrogen may affect the development of OA [13]. In the estrogen endocrine system, estrogen receptor alpha (ESR1) is an essential mediator in the signal transduction pathway. Previous studies both in vitro and in vivo have reported that ESR1 is expressed in particular chondrocytes and osteoblasts, and influences on the pathological process of OA [14]. The mutations in ESR1 gene may be associated with OA susceptibility. Several studies have investigated the association between single nucleotide polymorphisms (SNPs) in the ESR1 gene and OA risk. Among those SNPs, three were most commonly studied: rs2234693 (Pvu II T>C intron 1), rs9340799 (XbaI A>G in intron 1), and rs2228480 (BtgI G>A in exon 8). However, the results linking ESR1 gene mutations with the risk of OA have been inconsistent [15-22].
Meta-analysis is a useful method to overcome the disadvantages of individual studies, analyze the reasons of inconsistent results, and increase the statistical power and the precision of effect estimates. In this study, we performed a meta-analysis to clarify the associations of the three polymorphisms in the ESR1 gene with OA susceptibility.
Methods
Search strategy
A comprehensive search, restricted to human studies, English and Chinese language papers, was performed using the following databases (PubMed; Web of Science; EMBASE; Wiley Online Library; China National Knowledge Infrastructure; Wanfang database; Database of Chinese Scientific and Technical Periodicals) and the search terms (“osteoarthritis” or “OA” or “osteoarthrosis” or “degenerative arthritis” or “degenerative joint disease”) and (“estrogen receptor” or “ER” or “ESR”) for relevant articles without time restriction. The last search was performed on February 25, 2015. In addition, we searched and identified studies not captured by our database through reviewing reference lists in retrieved articles.
Inclusion criteria
All identified studies were carefully reviewed to determine whether an individual study was eligible for inclusion in this meta-analysis. The inclusion criteria were as follows: (1) case-control or cohort studies focusing on the associations of polymorphisms in the ESR1 gene with OA; (2) studies categorized by the ESR1 genotype; (3) information and results of specific loci (rs2234693, rs9340799 and rs2228480) with OA risk were provided; (4) there was no restriction in publication date, publication language and publication status; (5) animal studies, reviews, comments, and editorials were excluded.
Data extraction and quality assessment
A data extraction sheet was developed that including first authors, publication year, country where the study was conducted, gene information, gender, source of controls, specific classification of OA, genotype distribution in OA cases and controls, P value for Hardy-Weinberg equilibrium (HWE) test in controls. The 9-star Newcastle-Ottawa Scale was used to estimate the quality of included study [23]. Study inclusion, data extraction and quality assessment were performed by 2 independent investigators. Any disagreement was settled by discussion all of the authors.
Statistical analysis
The associations of polymorphisms in the ESR1 gene with OA were estimated by calculating pooled OR and 95% CI under dominant, recessive and codominant genetic models, respectively. In brief, the odds ratio (OR) with 95% confidence intervals (CI) of each study was calculated on a fourfold table that included the sample size distributed by genotype and OA. Pearson’s chi-square with exact probability was used to test departure from HWE for ESR1 genotype distribution in control samples. Pooled measure was calculated as the inverse variance-weighted mean of the logarithm of OR with 95% CI to assess the strength of association between ESR1 polymorphisms and OA. The Q test and I2 were used to assess heterogeneity among included studies [24]. In the presence of substantial heterogeneity (I2>50%) [25], the DerSimonian and Laird random effect model (REM) was adopted as the pooling method; otherwise, the fixed effect model (FEM) was used as the pooling method. Meta-regression with restricted maximum likelihood estimation was performed to assess the potentially important covariates exerting substantial impact on between-study heterogeneity. The ‘leave one out’ sensitivity analysis was carried out using I2>50% as the criteria to evaluate the key studies with substantial impact on between-study heterogeneity [26]. An analysis of influence was conducted [27], which describes how robust the pooled estimator is to removal of individual studies. Publication bias was estimated using modified Egger’s linear regression test, as proposed by Harbord et al. [28]. All statistical analyses were performed with STATA version 13.1 (Stata Corporation, College Station, TX, USA). All reported probabilities (P-value) were two-sided, and that less than 0.05 was considered statistically significant.
Results
Study characteristics
The detailed selection process of our study was illustrated in Figure 1 and 17 eligible articles [15-22,29-37] were included in this meta-analysis. Among them, four articles were published in Chinese [33,34,36,37]. Six studies were conducted in Caucasians, ten studies were performed in Asians and one study was conducted in Mexican mestizo. 15 studies reported the association between rs2234693 and OA risk, 14 studies for rs9340799 and OA risk, and 3 studies for rs2228480 and OA risk. General characteristics and data in the studies included in this meta-analysis were shown in Table 1. The quality score of studies ranged from 5 stars to 8 stars according to the 9-star Newcastle-Ottawa Scale.
Figure 1.
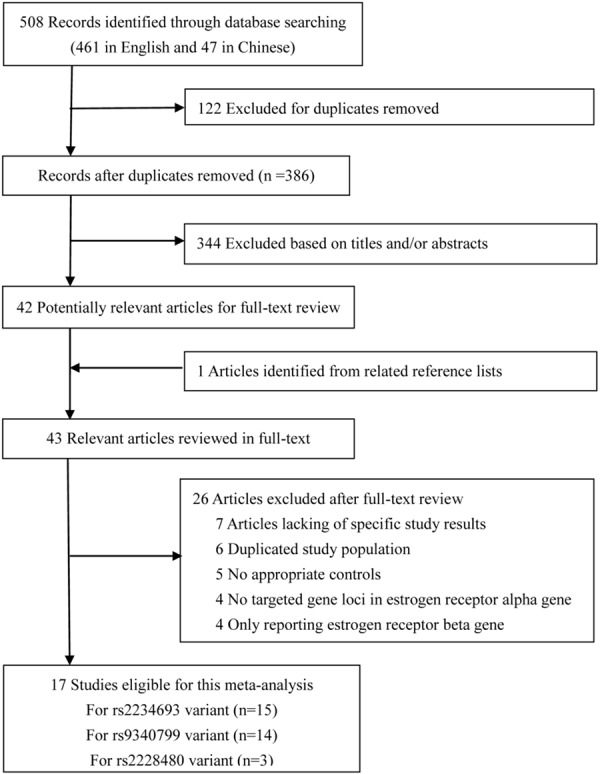
Flow diagram of studies selection in meta-analysis.
Table 1.
Characteristics of the studies included in the meta-analysis
| Study (year) | Region | Ethnicity | Source of controls | Disease | Gender (Female, %) | Genotype distributions in cases/controlsa | Sample size | P HWE b | MAF | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||
| Cases | Controls | 11 | 12 | 22 | Cases | Controls | |||||||
| rs2234693 (Pvu II T>C intron 1) | |||||||||||||
| Ushiyama (1998) | Japan | Asian | PB | GOA | 100 | 100 | 19/115 | 35/146 | 11/57 | 65 | 318 | 0.416 | 0.414 |
| Loughlin (2000) | Britian | Caucasian | PB | GOA | 58.2 | 40.1 | 114/110 | 183/187 | 74/72 | 371 | 369 | 0.675 | 0.447 |
| Bergink (2003) | Netherlands | Caucasian | PB | KOA | 58.8 | 67.8 | 434/225 | 737/333 | 312/129 | 1483 | 687 | 0.756 | 0.450 |
| Jin (2004) | Korea | Asian | PB | KOA | 64.9 | 52.1 | 61/152 | 68/183 | 22/62 | 151 | 397 | 0.597 | 0.382 |
| Xue (2004) | China | Asian | PB | KOA | 100 | 100 | 17/57 | 23/87 | 15/32 | 55 | 176 | 0.905 | 0.442 |
| Kang (2007) | Korea | Asian | PB | TOA | 100 | 100 | 40/29 | 46/32 | 14/13 | 100 | 74 | 0.466 | 0.379 |
| Lian (2007) | USA | Caucasian | PB | HIOA | 100 | 100 | 188/1162 | 277/2067 | 102/884 | 567 | 4113 | 0.552 | 0.461 |
| Tian (2009) | China | Asian | PB | KOA | 100 | 100 | 16/15 | 15/16 | 7/9 | 38 | 40 | 0.330 | 0.404 |
| Wise (2009) | USA | Caucasian | PB | HAOA | 51.2 | 51.2 | 101/65 | 145/100 | 58/46 | 304 | 211 | 0.578 | 0.440 |
| Yang (2009) | China | Asian | PB | KOA | 61.7 | 61.7 | 14/12 | 17/23 | 10/5 | 41 | 40 | 0.336 | 0.432 |
| Riancho (2010) | Europe | Caucasian | PB | GOA | 61.4 | 51.6 | 986/732 | 1547/1174 | 614/475 | 3147 | 2381 | 0.934 | 0.443 |
| Borgonio-Cuadra (2012) | Mecixo | Mexican | PB | KOA | 80.9 | 82.9 | 52/51 | 49/50 | 14/16 | 115 | 117 | 0.543 | 0.343 |
| Dai (2014) | China | Asian | PB | KOA | 71.2 | 23.7 | 167/198 | 217/242 | 85/74 | 469 | 514 | 0.997 | 0.395 |
| Liu (2014) | China | Asian | PB | KOA | 100 | 100 | 30/63 | 41/97 | 27/36 | 98 | 196 | 0.900 | 0.449 |
| Stemig (2014) | USA | Caucasian | HB | TOA | 92.9 | 45.9 | 12/7 | 13/19 | 7/10 | 32 | 36 | 0.706 | 0.485 |
| rs9340799 (XbaI -351 A>G in intron 1) | |||||||||||||
| Ushiyama (1998) | Japan | Asian | PB | GOA | 100 | 100 | 36/211 | 28/98 | 1/9 | 65 | 318 | 0.707 | 0.191 |
| Loughlin (2000) | Britian | Caucasian | PB | GOA | 58.2 | 40.1 | 164/161 | 158/165 | 49/43 | 371 | 369 | 0.942 | 0.343 |
| Bergink (2003) | Netherlands | Caucasian | PB | KOA | 58.8 | 67.8 | 643/372 | 682/263 | 158/52 | 1483 | 369 | 0.559 | 0.337 |
| Jin (2004) | Korea | Asian | PB | KOA | 64.9 | 52.1 | 98/256 | 49/126 | 4/15 | 151 | 397 | 0.918 | 0.194 |
| Xue (2004) | China | Asian | PB | KOA | 100 | 100 | 21/40 | 24/82 | 10/54 | 55 | 176 | 0.448 | 0.506 |
| Kang (2007) | Korea | Asian | PB | TOA | 100 | 100 | 65/46 | 31/28 | 4/0 | 100 | 74 | 0.059 | 0.193 |
| Lian (2007) | USA | Caucasian | PB | HIOA | 100 | 100 | 257/1700 | 250/1932 | 62/491 | 569 | 4123 | 0.109 | 0.350 |
| Tian (2009) | China | Asian | PB | KOA | 100 | 100 | 18/6 | 16/21 | 4/13 | 38 | 40 | 0.749 | 0.455 |
| Wise (2009) | USA | Caucasian | PB | HAOA | 51.2 | 51.2 | 148/85 | 116/99 | 43/30 | 307 | 214 | 0.884 | 0.346 |
| Yang (2009) | China | Asian | PB | KOA | 61.7 | 61.7 | 28/24 | 11/13 | 2/3 | 41 | 40 | 0.659 | 0.210 |
| Borgonio-Cuadra (2012) | Mexico | Mexican | PB | KOA | 80.9 | 82.9 | 70/62 | 41/47 | 4/8 | 115 | 117 | 0.821 | 0.241 |
| Dai (2014) | China | Asian | PB | KOA | 71.3 | 23.7 | 288/348 | 152/155 | 29/19 | 469 | 522 | 0.771 | 0.203 |
| Liu (2014) | China | Asian | PB | KOA | 100 | 100 | 36/49 | 43/92 | 19/55 | 98 | 196 | 0.394 | 0.481 |
| Stemig (2014) | USA | Caucasian | HB | TOA | 92.9 | 45.9 | 17/13 | 21/17 | 4/6 | 42 | 36 | 0.912 | 0.372 |
| rs2228480 (BtgI G>A in exon 8) | |||||||||||||
| Jin (2004) | Korea | Asian | PB | KOA | 64.9 | 52.1 | 84/257 | 57/122 | 10/18 | 151 | 397 | 0.436 | 0.214 |
| Jiao (2007) | China | Asian | PB | KOA | 85.5 | 70.3 | 5/16 | 56/97 | 15/5 | 76 | 118 | <0.001 | 0.497 |
| Tawonsawatruk (2009) | Thailand | Asian | HB | KOA | 87.5 | 88.5 | 62/63 | 37/37 | 5/4 | 104 | 104 | 0.776 | 0.221 |
HWE, Hardy-Weinberg equilibrium; MAF, minor allele frequency; HB, hospital based; PB, population based; GOA, generalized osteoarthritis; KOA, knee osteoarthritis; HIOA, hip osteoarthritis; TOA, temporomandibular joint osteoarthritis; HAOA, hand osteoarthritis.
For rs2234693 variant, 11, 12 and 13 represent TT, CT and CC, respectively; for rs9340799 variant, 11, 12 and 13 represent AA, GA and GG, respectively; for rs2228480 variant, 11, 12 and 13 represent GG, AG and AA, respectively.
P value for Hardy–Weinberg equilibrium test in controls.
Quantitative synthesis
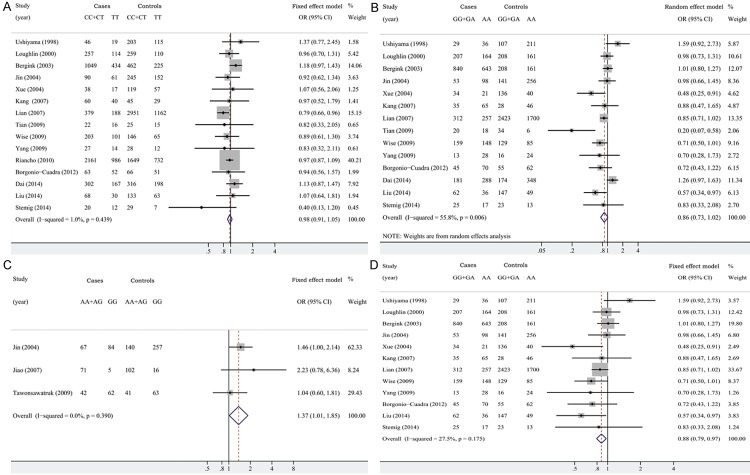
For rs2234693 polymorphism, a total of 7036 cases and 9669 controls were identified. The results suggested there was no significant association between this polymorphism and OA under three genetic models (CC+CT vs. TT: OR=0.976, 95% CI=0.908-1.050, P=0.520, Figure 2A; CC vs. CT+TT: OR=1.002, 95% CI=0.919-1.093, P=0.963; C vs. T: OR=0.990, 95% CI=0.943-1.039, P=0.688) (Table 2).
Figure 2.
Forest plots for the three common ESR1 polymorphisms and OA risk. A: For rs2234693 in dominant model (CC+CT vs. TT); B: For rs9340799 in dominant model (GG+GA vs. AA) among all included studies; C: For rs2228480 in dominant model (AA+AG vs. GG); D: For rs9340799 in dominant model (GG+GA vs. AA) after sensitivity analysis.
Table 2.
Pooled ORs and 95% CIs of the associations between three polymorphisms in ESR1 gene and OA risk
| Contrasts | Data | No. (case/control) | I2 (%) | P Q a | Method | OR | 95% CI | P | P bias b |
|---|---|---|---|---|---|---|---|---|---|
| rs2234693 (Pvu II -397 T>C intron 1) | |||||||||
| CC+CT vs. TT | All studies | 7036/9669 | 1.0 | 0.439 | FEM | 0.976 | 0.908-1.050 | 0.520 | 0.704 |
| CC vs. CT+TT | All studies | 7036/9669 | 16.5 | 0.269 | FEM | 1.002 | 0.919-1.093 | 0.963 | 0.446 |
| C vs. T | All studies | 7036/9669 | 26.9 | 0.159 | FEM | 0.990 | 0.943-1.039 | 0.688 | 0.836 |
| rs9340799 (XbaI -351 A>G in intron 1) | |||||||||
| GG+GA vs. AA | All studies | 3904/6991 | 55.8 | 0.006 | REM | 0.865 | 0.733-1.021 | 0.051 | 0.135 |
| Sensitivity analysisc | 3397/6429 | 27.5 | 0.175 | FEM | 0.878 | 0.792-0.972 | 0.012 | 0.416 | |
| GG vs. GA+AA | All studies | 3904/6991 | 29.2 | 0.144 | FEM | 0.896 | 0.766-1.049 | 0.172 | 0.419 |
| Sensitivity analysisc | 3397/6429 | 0.0 | 0.612 | FEM | 0.872 | 0.739-1.027 | 0.101 | 0.507 | |
| G vs. A | All studies | 3904/6991 | 60.7 | 0.002 | REM | 0.882 | 0.771-1.008 | 0.066 | 0.180 |
| Sensitivity analysisc | 3397/6429 | 25.7 | 0.191 | FEM | 0.902 | 0.836-0.975 | 0.009 | 0.381 | |
| rs2228480 (BtgI +594 G>A in exon 8) | |||||||||
| AA+AG vs. GG | All studies | 331/619 | 0.0 | 0.390 | FEM | 1.371 | 1.014-1.853 | 0.040 | 0.833 |
| Sensitivity analysisd | 255/501 | 0.0 | 0.321 | FEM | 1.312 | 0.958-1.797 | 0.090 | NON | |
| AA vs. AG+GG | All studies | 331/619 | 55.5 | 0.106 | REM | 2.207 | 0.891-5.469 | 0.087 | 0.850 |
| Sensitivity analysisd | 255/501 | 0.0 | 0.833 | FEM | 1.430 | 0.720-2.837 | 0.307 | NON | |
| A vs. G | All studies | 331/619 | 0.0 | 0.448 | FEM | 1.348 | 1.083-1.678 | 0.008 | 0.654 |
| Sensitivity analysisd | 255/501 | 0.0 | 0.353 | FEM | 1.268 | 0.979-1.643 | 0.072 | NON |
ESR1, estrogen receptor alpha; OA, osteoarthritis; No, number of included samples size; FEM, fixed effect model; REM, random effect model.
P value for between-study heterogeneity based on Q test.
P value for publication bias based on Egger’s linear regression test proposed by Harbord.
Sensitivity analysis excluded Tian’s and Dai’s studies.
Sensitivity analysis excluded Jiao’s study.
For rs9340799 polymorphism, a total of 3904 cases and 6991 controls were identified. The results suggested there was no significant association between this polymorphism and OA under three genetic models (GG+GA vs. AA: OR=0.865, 95% CI=0.733-1.021, P=0.051, Figure 2B; GG vs. GA+AA: OR=0.896, 95% CI=0.766-1.049, P=0.172; G vs. A: OR=0.882, 95% CI=0.771-1.008, P=0.066) (Table 2).
For rs2228480 polymorphism, a total of 331 cases and 619 controls were identified. The meta-analysis indicated that the polymorphism A allele was associated with OA risk under dominant genetic model (AA+AG vs. GG: OR=1.371, 95 % CI 1.014-1.853, P=0.040, Figure 2C) and codominant genetic model (A vs. G: OR=1.348, 95% CI=1.083-1.678, P=0.008), but non-significant association was observed under recessive genetic model (AA vs. AG+GG: OR=2.207, 95% CI=0.891-5.469, P=0.087) (Table 2).
Meta-regression
The between-study heterogeneity (I2>50%) could be found among some groups in Table 2, especially in rs9340799 and rs2228480 with OA risk. The univariate meta-regression, with the covariates of published year, ethnicity (Caucasian, Asian and Mexican), gender (male and female), source of controls, disease (keen OA and others), mean age, sample size, MAF and quality, was applied to all meta-analyses. Except for MAF to rs9340799, no aforementioned covariates had a significant impact on between-study heterogeneity. After adjusting the influence of MAF on rs9340799, the pooled results did not change substantially.
Sensitivity analysis
To explore the heterogeneity among researches of ESR1 polymorphisms and OA, sensitivity analysis was performed for the groups with an I2>50%. The ESR1 allelic frequencies deviated significantly from HWE among one studies by Jiao et al. [34] for rs2228480 polymorphism. After we excluded the study, the corresponding pooled ORs were materially altered in the codominant and additive model. For rs9340799 polymorphism, the sensitivity analysis found that two studies conducted by Tian et al. [36] and Dai et al. [17] were found to be the key contributors to between-study heterogeneity. After excluding these two studies, the meta-analysis showed a significant association of G allele with decreased risk of OA (GG+GA vs. AA: OR=0.878, 95% CI=0.792-0.972, P=0.012, Figure 2D; G vs. A: OR=0.902, 95% CI=0.836-0.975, P=0.009) (Table 2).
Influence analysis and publication bias
Influence analysis, after removing one study at a time, was performed to evaluate the stability of the results. After excluding Tian’s and Dai’s studies, the significant associations for rs9340799 polymorphisms under dominant and codominant genetic models were robust by influence analysis. No publication bias was observed in any above-mentioned analyses (Table 2).
Discussions
To the best of our knowledge, this is the first meta-analysis which comprehensively assessed the associations between three common polymorphisms in the ESR1 and OA risk. The final results indicated that the G allele in ESR1 rs9340799 was associated with low OA risk, and the A allele in ESR1 rs2228480 might be associated with increased OA risk. But no significant association of ESR1 rs2234693 polymorphisms with OA susceptibility was observed in our study.
Between-study heterogeneity is common in meta-analysis for genetic association studies [38]. Our meta-analysis showed significant between-study heterogeneity in ESR1 rs9340799 and rs2228480 with OA. An indeterminate number of characteristics that vary among studies could be the sources of between-study heterogeneity, e.g. published year, ethnicity, gender, source of controls, disease type, age, sample size, MAF and quality, etc. Therefore we used meta-regression and sensitivity analysis, which aim to reduce between-study heterogeneity and explore the potential important causes of heterogeneity for both covariates and studies. Except for MAF to rs9340799, no aforementioned covariates had a significant impact on between-study heterogeneity. After adjusting the influence of MAF on rs9340799, the pooled results did not change substantially. Thus the corresponding subgroup analyses were omitted. In addition, the sensitivity analysis identified three studies as being key contributors to between-study heterogeneity. After carefully estimating the three studies, we thought that these studies had a low quality, such as allelic frequencies in Jiao’s study [34] deviated significantly from HWE, and Dai’s results and analyses [17] were significantly incorrect. Hence, it is reasonable to exclude the three studies for weak heterogeneity and robust results in final analyses. No significant influence and publication bias were observed in all above-mentioned analyses.
The association of ESR1 rs9340799 polymorphisms with OA risk has been investigated by a recent meta-analysis, which finds that G allele in rs9340799 polymorphism contributed significantly to an increased risk of OA [39]. After carefully reading the article, we could find the authors obviously reversed the exposure and non-exposure (genotype) and summarized an incorrect conclusion. Moreover, the publication bias and strong heterogeneity were detected in the article. In principle, the conclusion in our study was consistent with the article, and we further updated and corrected the association between ESR1 rs9340799 polymorphisms with OA risk with updated data and elaborate analyses. In addition, our results are consistent with a previous meta-analysis about ESR1 gene with bone mineral density and fracture risk in women [40]. They reported shows GG homozygotes in rs9340799 may have higher bone mineral density and also a decreased risk of fractures, whereas the rs2234693 polymorphism is not associated with either bone mineral density or fracture risk [40].
Some limitations of the present meta-analysis should be taken into consideration when interpreting the results. First, the present meta-analysis was based on original data and the potential confounding factors were not adjusted. Second, the effect of gene-gene and gene-environment interactions may influence the biological effects of the polymorphisms of the ESR1 gene. Third, because it was very difficult to get the full papers published in various languages, we only included the studies published in English and Chinese. Four, although we do our best to apply meta-regression and sensitivity analysis to explore the between-study heterogeneity, the heterogeneity in the present meta-analysis should be noted, which may affect the results. Finally, the included samples for ESR1 rs2228480 and OA risk were not adequate to deduce a robust conclusion and the association should be further confirmed in future.
Conclusions
This meta-analysis indicates rs9340799 and rs2228480 rather than rs2234693 polymorphisms are associated with the incidence of OA. Future large-scale randomized, controlled researches are recommended to assess a stable outcome for some small sample groups. In addition, future research should focus on an integrated approach that not only incorporates psychological, physical, behavioral, and genetic effects but also investigates the interactions between these factors.
Disclosure of conflict of interest
None.
References
- 1.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26:355–69. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185–99. doi: 10.1093/bmb/lds038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, Laslett LL, Jones G, Cicuttini F, Osborne R, Vos T, Buchbinder R, Woolf A, March L. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323–30. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 4.Johnson VL, Hunter DJ. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol. 2014;28:5–15. doi: 10.1016/j.berh.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Spector TD, MacGregor AJ. Risk factors for osteoarthritis: genetics. Osteoarthritis Cartilage. 2004;12:S39–44. doi: 10.1016/j.joca.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Valdes AM, Spector TD. The contribution of genes to osteoarthritis. Med Clin North Am. 2009;93:45–66. doi: 10.1016/j.mcna.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 7.MacGregor A, Spector T. Twins and the genetic architecture of osteoarthritis. Rheumatology. 1999;38:583–8. doi: 10.1093/rheumatology/38.7.583. [DOI] [PubMed] [Google Scholar]
- 8.M Valdes A. Genetic markers of osteoarthritis. Curr Rheumatol Rev. 2010;6:257–67. doi: 10.2174/157339710793205639. [DOI] [PubMed] [Google Scholar]
- 9.Pan F, Tian J, Winzenberg T, Ding C, Jones G. Association between GDF5 rs143383 polymorphism and knee osteoarthritis: an updated meta-analysis based on 23,995 subjects. BMC Musculoskelet Disord. 2014;15:404. doi: 10.1186/1471-2474-15-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramos YF, Metrustry S, Arden N, Bay-Jensen AC, Beekman M, de Craen AJ, Cupples LA, Esko T, Evangelou E, Felson DT, Hart DJ, Ioannidis JP, Karsdal M, Kloppenburg M, Lafeber F, Metspalu A, Panoutsopoulou K, Slagboom PE, Spector TD, van Spil EW, Uitterlinden AG, Zhu Y arcOGEN Consortium; TreatOA Collaborators. Valdes AM, van Meurs JB, Meulenbelt I. Meta-analysis identifies loci affecting levels of the potential osteoarthritis biomarkers sCOMP and uCTX-II with genome wide significance. J Med Genet. 2014;51:596–604. doi: 10.1136/jmedgenet-2014-102478. [DOI] [PubMed] [Google Scholar]
- 11.Valdes AM, Spector TD. Genetic epidemiology of hip and knee osteoarthritis. Nat Rev Rheumatol. 2011;7:23–32. doi: 10.1038/nrrheum.2010.191. [DOI] [PubMed] [Google Scholar]
- 12.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769–81. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Tanamas SK, Wijethilake P, Wluka AE, Davies-Tuck ML, Urquhart DM, Wang Y, Cicuttini FM. Sex hormones and structural changes in osteoarthritis: a systematic review. Maturitas. 2011;69:141–56. doi: 10.1016/j.maturitas.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Roman-Blas JA, Castaneda S, Largo R, Herrero-Beaumont G. Osteoarthritis associated with estrogen deficiency. Arthritis Res Ther. 2009;11:241. doi: 10.1186/ar2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stemig M, Myers SL, Kaimal S, Islam MS. Estrogen receptor-alpha polymorphism in patients with and without degenerative disease of the temporomandibular joint. Cranio. 2015;33:129–33. doi: 10.1179/2151090314Y.0000000013. [DOI] [PubMed] [Google Scholar]
- 16.Liu W, Shao FM, Yan L, Cao HX, Qiu D. Polymorphisms in the gene encoding estrogen receptor alpha are associated with osteoarthritis in Han Chinese women. Int J Clin Exp Med. 2014;7:5772–7. [PMC free article] [PubMed] [Google Scholar]
- 17.Dai X, Wang C, Dai J, Shi D, Xu Z, Chen D, Teng H, Jiang Q. Association of single nucleotide polymorphisms in estrogen receptor alpha gene with susceptibility to knee osteoarthritis: a case-control study in a Chinese Han population. Biomed Res Int. 2014;2014:151457. doi: 10.1155/2014/151457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borgonio-Cuadra VM, Gonzalez-Huerta C, Duarte-Salazar C, de Los Angeles Soria-Bastida M, Cortes-Gonzalez S, Miranda-Duarte A. Analysis of estrogen receptor alpha gene haplotype in Mexican mestizo patients with primary osteoarthritis of the knee. Rheumatol Int. 2012;32:1425–30. doi: 10.1007/s00296-011-1890-2. [DOI] [PubMed] [Google Scholar]
- 19.Riancho JA, Garcia-Ibarbia C, Gravani A, Raine EV, Rodriguez-Fontenla C, Soto-Hermida A, Rego-Perez I, Dodd AW, Gómez-Reino JJ, Zarrabeitia MT, Garcés CM, Carr A, Blanco F, González A, Loughlin J. Common variations in estrogen-related genes are associated with severe large-joint osteoarthritis: a multicenter genetic and functional study. Osteoarthritis Cartilage. 2010;18:927–33. doi: 10.1016/j.joca.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Wise BL, Demissie S, Cupples LA, Felson DT, Yang M, Shearman AM, Aliabadi P, Hunter DJ. The relationship of estrogen receptor-alpha and -beta genes with osteoarthritis of the hand. The J rheumatol. 2009;36:2772–9. doi: 10.3899/jrheum.081208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lian K, Lui L, Zmuda JM, Nevitt MC, Hochberg MC, Lee JM, Li J, Lane NE. Estrogen receptor alpha genotype is associated with a reduced prevalence of radiographic hip osteoarthritis in elderly Caucasian women. Osteoarthritis Cartilage. 2007;15:972–8. doi: 10.1016/j.joca.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang SC, Lee DG, Choi JH, Kim ST, Kim YK, Ahn HJ. Association between estrogen receptor polymorphism and pain susceptibility in female temporomandibular joint osteoarthritis patients. Int J Oral Maxillofac Surg. 2007;36:391–4. doi: 10.1016/j.ijom.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Wells G, Shea B, O’connell D. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. 2000. pp. 3–5. [Google Scholar]
- 24.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37:1148–57. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech Bull. 1999;47:15–7. [Google Scholar]
- 28.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443–57. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 29.Ushiyama T, Ueyama H, Inoue K, Nishioka J, Ohkubo I, Hukuda S. Estrogen receptor gene polymorphism and generalized osteoarthritis. J Rheumatol. 1998;25:134–7. [PubMed] [Google Scholar]
- 30.Loughlin J, Sinsheimer JS, Mustafa Z, Carr AJ, Clipsham K, Bloomfield VA, Chitnavis J, Bailey A, Sykes B, Chapman K. Association analysis of the vitamin D receptor gene, the type I collagen gene COL1A1, and the estrogen receptor gene in idiopathic osteoarthritis. J Rheumatol. 2000;27:779–84. [PubMed] [Google Scholar]
- 31.Bergink AP, van Meurs JB, Loughlin J, Arp PP, Fang Y, Hofman A, van Leeuwen JP, van Duijn CM, Uitterlinden AG, Pols HA. Estrogen receptor alpha gene haplotype is associated with radiographic osteoarthritis of the knee in elderly men and women. Arthritis Rheum. 2003;48:1913–22. doi: 10.1002/art.11046. [DOI] [PubMed] [Google Scholar]
- 32.Jin SY, Hong SJ, Yang HI, Park SD, Yoo MC, Lee HJ, Hong MS, Park HJ, Yoon SH, Kim BS, Yim SV, Park HK, Chung JH. Estrogen receptor-alpha gene haplotype is associated with primary knee osteoarthritis in Korean population. Arthritis Res Ther. 2004;6:R415–21. doi: 10.1186/ar1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xue Y, Li D, Yao L, Zhou Y, Xiao D, Guo S. Relationship between estrogen receptor gene polymorphism and osteoarthritis in Han women [In Chinese] Chin J Rheumatol. 2004;8:583–6. [Google Scholar]
- 34.Jiao B. Single nucleotide polymorphisms of osteoarthritis-related susceptibility genes [In Chieses] [master’s thesis] Beijing: Capital Institute of Physical Education; 2007. [Google Scholar]
- 35.Tawonsawatruk T, Trachoo O, Channoom T, Sura T, Eu-ahsunthornwattana J, Woratanarat P, Wajanavisit W. Association of estrogen receptor-alpha single-nucleotide polymorphism (codon 594 G-->A) and Thai patients affected by knee osteoarthritis. J Med Assoc Thai. 2009;92(Suppl 6):S45–50. [PubMed] [Google Scholar]
- 36.Tian Z, Guo X, Zhou F, Huang C, Li M, Long X. Study on relationship between polymorphisms of estrogen alpha receptor gene and primary knee osteoarthritis among women in Hunan [In Chinese] Practical Preventive Medicine. 2009;16:1724–7. [Google Scholar]
- 37.Yang J, Fu S, Xiao F. Case-control study between estrogen receptor gene polymorphisms and osteoarthritis in southern Sichuan high fluoride areas [In Chinese] West China Medical Journal. 2009;24:826–9. [Google Scholar]
- 38.Boccia S, De Feo E, Galli P, Gianfagna F, Amore R, Ricciardi G. A systematic review evaluating the methodological aspects of meta-analyses of genetic association studies in cancer research. Eur J Epidemiol. 2010;25:765–75. doi: 10.1007/s10654-010-9503-z. [DOI] [PubMed] [Google Scholar]
- 39.Yin YW, Sun QQ, Hu AM, Wang Q, Liu HL. Association of rs9340799 polymorphism in estrogen receptor alpha gene with the risk of osteoarthritis: evidence based on 8,792 subjects. Mol Genet Genomics. 2014;290:513–20. doi: 10.1007/s00438-014-0940-3. [DOI] [PubMed] [Google Scholar]
- 40.Ioannidis JP, Stavrou I, Trikalinos TA, Zois C, Brandi ML, Gennari L, Albagha O, Ralston SH, Tsatsoulis A ER-alpha Genetics Meta-Analysis. Association of polymorphisms of the estrogen receptor alpha gene with bone mineral density and fracture risk in women: a meta-analysis. J Bone Miner Res. 2002;17:2048–60. doi: 10.1359/jbmr.2002.17.11.2048. [DOI] [PubMed] [Google Scholar]