Abstract
The investigation regarding the clinical significance of quantitative hepatitis B core antibody (anti-HBc) in HCC patients after transarterial chemoembolization (TACE) is rare. The aim of this study was to determine the prognostic significance of anti-HBc in HCC patients after TACE. A total of 66 HCC patients with complete data and treated with TACE in Nanfang Hospital, Southern Medical University were retrospectively analyzed. Univariate analyses were performed for 20 pretreatment variables and those with a P value less than 0.05 by univariate analysis were subjected to Cox proportional hazards model. Among the 66 HCC patients, the 0.5-, 1-, and 2-year overall survival rates were 92.42%, 50.00%, and 21.21%, respectively. We defined 11.88 S/CO, with a maximum sum of sensitivity and specificity, as the optimal cut-off value of baseline anti-HBc level to predict the OS rate in HCC patients after TACE. Univariate and multivariate analyses revealed that baseline anti-HBc level, AFP level and vascular invasion were the only three independent significant prognostic factors of overall survival (P = 0.001, 0.020 and 0.010, respectively). We demonstrated that baseline anti-HBc level, combining AFP level and vascular invasion, might be a novel biomarker for predicting the survival of HCC patients after TACE.
Keywords: Hepatocellular carcinoma, transarterial chemoembolization, hepatitis B core antibody
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third leading cause of cancer-related death in the world, resulting in more than 500,000 deaths annually [1]. Due to rapid proliferation, increased intrahepatic spreading, and metastasis, the survival rate for unresectable HCC patients is typically poor [2]. In developing countries such as China, transarterial chemoembolization (TACE) remains the primary treatment for unresectable HCC patient [3]. Although the prognostic factors related to the survival of HCC patients after the treatment of TACE have been analyzed in many previous studies and review articles [4-10], there have been no studies for assessing the performance of quantitative anti-HBc level in patients with HCC undergoing TACE.
Anti-HBc, as one of the novel biomarkers of HBV infection, has been ignored in real-life clinical practice for a long time. It has been demonstrated that anti-HBc plays an important role in HBV infection, which is helpful for judging the prognosis of chronic hepatitis B (CHB) patients [11]. Moreover, anti-HBc is closely correlated with hepatic inflammatory activities and may serve as a surrogate marker for the host anti-HBV immune response [12]. Considering the above evidences, we hypothesized that anti-HBc levels may be correlated with the outcome of TACE. The aim of this study was to determine the prognostic significance of anti-HBc in HCC patients after TACE.
Patients and methods
Patients
This study enrolled patients who underwent TACE since 2005 in Nanfang Hospital, Southern Medical University. A diagnosis of HCC was made on the basis of pathology or the diagnostic criteria of the American Association of the Study of Liver Disease [13]. The crucial inclusion criteria of the current retrospective study were hepatitis B patients with cirrhosis. The exclusion criteria were as follows: (1) patients who underwent previous treatment before the first TACE, (2) World Health Organization (WHO) performance status > 2, (3) patients with significantly impaired liver function (Child-Pugh score class C), (4) patients who received only intra-arterial chemotherapy, or without embolization, (5) co-infection with hepatitis A, hepatitis C, hepatitis D, hepatitis E, or human immunodeficiency virus and (6) loss to follow-up within three months after treatment (In addition to the deaths). Finally, 66 HCC patients with complete data were enrolled. All patients provided informed consent before undergoing TACE.
Clinical and laboratory evaluation
Clinical and laboratory data were collected from all patients prior to TACE. Clinical data included age, sex, diabetes, Barcelona Clinic Liver Cancer (BCLC) stage and Child-Pugh score. In addition, imaging data were collected based on abdominal computed tomography (CT) or magnetic resonance imaging (MRI), including tumor size and vascular invasion. Laboratory data included platelet count (PLT), hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg), hepatitis B e antibody (anti-HBe), hepatitis B core antibody (anti-HBc), HBV DNA, α-fetoprotein (AFP), albumin (ALB), total bilirubin (TBil), prothrombin time (PT), blood type, alanine aminotransferase (ALT), and aspartate aminotransferase (AST). The serum HBV DNA level was measured with the Cobas Taqman HBV Kit (Roche Diagnostics; lower limit of detection, 15 IU/mL or 84 copies/mL). HBeAg, Anti-HBe, anti-HBc were detected using an Architect assay (Abbott Laboratories, Abbott Park, IL). Aminotransferases were measured according to standard procedures locally at the time of sampling.
TACE procedures
Superior mesenteric and celiac arteriography was initially performed to assess anatomy, tumor burden, and the tumor-feeding artery. Two or three kinds of chemotherapeutic agents including epirubicin, platinum, mitomycin C were infused. Next, an emulsion of chemotherapeutic agents and 5-20 mL lipiodol was administered (the amount of lipiodol was selected depending on the tumor size and vasculature). Chemotherapeutic agents and doses were adjusted based on liver function and white blood cell counts. The catheter was selectively inserted into the tumor-feeding artery as close as possible to the tumor. Then gelatin sponge particles were used for further embolization. For patients with difficult intubation due to hepatic artery distortion, micro catheters were adopted.
Statistical analysis
All statistical analyses were conducted using SPSS 18.0 software (SPSS Inc., Chicago, IL). Data were expressed as counts and percentages for categorical variables and as median (minimum, maximum) for continuous variables (Because none of them are subject to normal distribution). Qualitative differences between subgroups were analyzed using χ2 tests for categorical parameters. For analyze of performance of quantitative anti-HBc level in predicting treatment outcome, area under the receiver operator characteristic curve (AUROC) was calculated. The AUROCs were compared by Delong test. Sensitivity, specificity, positive likelihood ratio (LR+) and negative likelihood ratio (LR-) of several cut-off values of anti-HBc levels were calculated to explore the best cut-off value in predicting the survival of HCC patients after TACE.
The observed points in this study were 0.5-, 1-, 2-year overall survival (OS) rates. OS was calculated from the time of the first chemoembolization to the date of death regardless of cause. Univariate and multivariate Cox regression analyses were performed to assess factors related to patient survival. All statistical analyses were based on two-tailed hypothesis tests with a significance level of P < 0.05.
Results
Clinical characteristics
This study included 66 patients with HCC who had undergone TACE during the study period, for whom complete data were available. The demographic, virological and clinical characteristics of the patients are summarized in Table 1. The median age was 52.00 (23.00, 80.00) years, predominantly men (92.42%) and 27.27% of patients were HBeAg positive. The median of baseline HBV DNA, HBsAg, HBeAg, anti-HBe, anti-HBc levels was 3.04 (0, 8.72) log10 copies/mL, 205.63 (2.72, 3715.30) IU/mL, 0.10 (0.04, 28.61) PEIU/ml, 0.01 (0, 3.02) PEIU/ml, and 11.50 (9.09, 14.99) S/CO, respectively.
Table 1.
Baseline characteristics of 66 HCC patients underwent TACE
| Characteristics | Number/median (minimum, maximum) | Percentage (%) |
|---|---|---|
| Gender | ||
| M/F | 61/5 | 92.42/7.58 |
| Age (year) | 52.0 (23.0, 80.0) | |
| HBsAg (IU/mL) | 205.63 (2.72, 3715.30) | |
| HBeAg (PEIU/ml) | 0.10 (0.04, 28.61) | |
| Anti-HBe (PEIU/ml) | 0.01 (0, 3.02) | |
| Anti-HBc (S/CO) | 11.50 (9.09, 14.99) | |
| HBV DNA level, lg copies/mL | 3.04 (0, 8.72) | |
| AFP (ng/mL) | ||
| ≤ 200/> 200 | 17/49 | 25.76/74.24 |
| ALT (U/L) | ||
| ≤ 40/> 40 | 35/31 | 53.03/46.97 |
| AST (U/L) | ||
| ≤ 40/> 40 | 19/47 | 28.79/71.21 |
| PLT (109/L) | ||
| < 125/≥ 125 | 29/37 | 43.94/56.06 |
| ALB (g/L) | ||
| < 40/40-55 | 49/17 | 74.24/25.76 |
| TBIL (µmol/L) | ||
| 3.42-20.5/> 20.5 | 41/25 | 62.12/37.88 |
| PT (S) | ||
| ≤ 13/> 13 | 16/50 | 24.24/75.76 |
| Blood type | ||
| A/B/AB/O | 23/9/5/29 | 32.85/13.63/7.58/43.94 |
| BCLC stage | ||
| A/B/C | 2/19/45 | 3.03/28.79/68.18 |
| Child-Pugh | ||
| A/B | 25/41 | 37.88/62.12 |
| Diabetes | ||
| Yes/No | 23/43 | 34.85/65.15 |
| Tumor size (cm) | ||
| < 3/3-5/> 5 | 12/10/44 | 18.18/15.15/66.67 |
| Vascular invasion | ||
| Yes/No | 50/16 | 75.76/24.24 |
TACE transarterial chemoembolization, HBsAg hepatitis B surface antigen, HBeAg hepatitis B e antigen, Anti-HBe hepatitis B e antibody, Anti-HBc hepatitis B core antibody, AFP α-fetoprotein, ALT alanine aminotransferase, AST aspartate aminotransferase, PLT platelet count, ALB albumin, TBIL total bilirubin, PT prothrombin time, BCLC stage Barcelona Clinic Liver Cancer stage.
Selection of anti-HBc cutoff value
To evaluate the correlation between the OS and the quantitative baseline HBsAg, HBeAg, anti-HBe, anti-HBc, HBV DNA, respectively, areas under the receiver operator characteristic curve (AUROC) were calculated in Figure 1. The AUROC of anti-HBc level was the highest (0.755, P = 0.011) while other 4 values showed no statistical significance.
Figure 1.
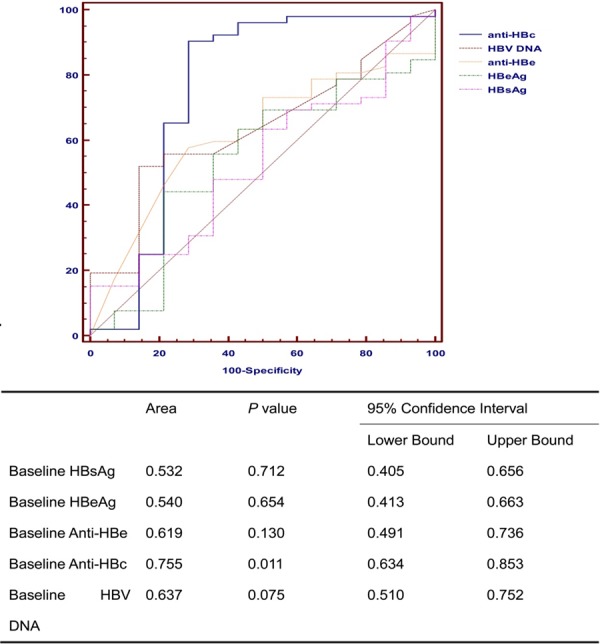
Receiver operating characteristics curves for overall survival in HCC patients underwent TACE.
Table 2 shows the sensitivity and specificity of baseline anti-HBc level in predicting the OS. Obviously, the sum of sensitivity and specificity would achieve the highest when the cut-off value is 11.88 S/CO. Therefore, we adopted 11.88 S/CO as the optimal cut-off value of baseline anti-HBc level in the following analyses. In this study, 15 patients had baseline anti-HBc > 11.88 S/CO and 51 patients had baseline anti-HBc ≤ 11.88 S/CO, among which 66.67% (10/15) and 7.84% (4/51) remained alive at the end of observation (χ2 = 20.607, P < 0.001).
Table 2.
Performance of baseline anti-hbc level in predicting the os after TACE
| Cut-off values | Sensitivity (%) | 95% CI | Specificity (%) | 95% CI | +LR | -LR |
|---|---|---|---|---|---|---|
| < 9.09 | 0 | 0.0-6.8 | 100 | 76.8-100.0 | 1 | |
| ≤ 9.09 | 1.92 | 0.05-10.3 | 100 | 76.8-100.0 | 0.98 | |
| ≤ 9.57 | 1.92 | 0.05-10.3 | 85.71 | 57.2-98.2 | 0.13 | 1.14 |
| ≤ 10.63 | 25 | 14.0-38.9 | 85.71 | 57.2-98.2 | 1.75 | 0.88 |
| ≤ 10.67 | 25 | 14.0-38.9 | 78.57 | 49.2-95.3 | 1.17 | 0.95 |
| ≤ 11.56 | 65.38 | 50.9-78.0 | 78.57 | 49.2-95.3 | 3.05 | 0.44 |
| ≤ 11.63 | 65.38 | 50.9-78.0 | 71.43 | 41.9-91.6 | 2.29 | 0.48 |
| ≤ 11.88* | 90.38 | 79.0-96.8 | 71.43 | 41.9-91.6 | 3.16 | 0.13 |
| ≤ 11.98 | 90.38 | 79.0-96.8 | 64.29 | 35.1-87.2 | 2.53 | 0.15 |
| ≤ 12.04 | 92.31 | 81.5-97.9 | 64.29 | 35.1-87.2 | 2.58 | 0.12 |
| ≤ 12.06 | 92.31 | 81.5-97.9 | 57.14 | 28.9-82.3 | 2.15 | 0.13 |
| ≤ 12.15 | 96.15 | 86.8-99.5 | 57.14 | 28.9-82.3 | 2.24 | 0.067 |
| ≤ 12.57 | 96.15 | 86.8-99.5 | 42.86 | 17.7-71.1 | 1.68 | 0.09 |
| ≤ 12.7 | 98.08 | 89.7-100.0 | 42.86 | 17.7-71.1 | 1.72 | 0.045 |
| ≤ 13.77 | 98.08 | 89.7-100.0 | 0 | 0.0-23.2 | 0.98 | |
| ≤ 14.99 | 100 | 93.2-100.0 | 0 | 0.0-23.2 | 1 |
The cut-off value has the highest sum of sensitivity and specificity;
OS Overall Survival, TACE transarterial chemoembolization, CI confidence Interval, +LR positive likelihood ratio, -LR negative likelihood ratio.
Overall survival
At the median follow-up of 13 months (range, 1-66 months), 26 patients (39.10%) remained alive. The 0.5-, 1-, and 2-year OS rates for all patients were 94.42%, 50.00%, and 21.21%, respectively (Figure 2).
Figure 2.
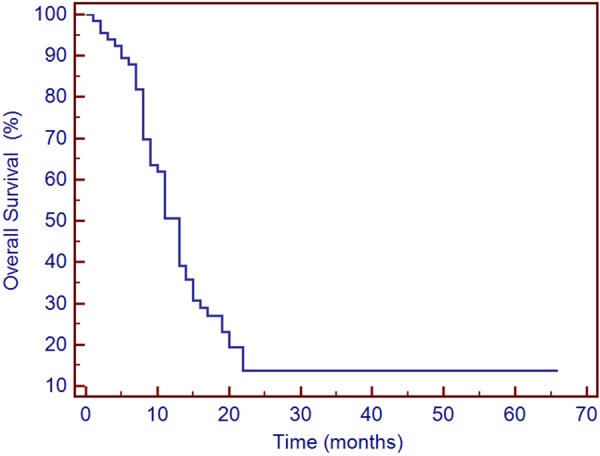
Survival curve of patients treated with TACE.
Correlation between baseline variables and OS
Univariate analysis identified the following factors as affecting OS: low anti-HBc level (≤ 11.88 S/CO, P = 0.001), elevated AFP level (> 200 ng/mL, P < 0.001), large tumor (3-5 cm or > 5 cm, P < 0.001), BCLC stage (B or C, P = 0.009), Child-Pugh stage (B, P = 0.013) , diabetes (P = 0.013), and vascular invasion (P < 0.001). We then entered these significant factors in multivariate analysis by using Cox’s proportional hazard model, and we found that along with AFP and vascular invasion, baseline anti-HBc was an independent predictive factor for OS (hazard ratio [HR] = 0.066, 95% confidence interval [CI] = 0.013-0.340, P = 0.010) (Table 3) .
Table 3.
Univariate and multivariate analysis of baseline variables affecting OS in patients receiving TACE
| Factors | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Gender: M/F | 1.098 | 0.395-3.053 | 0.858 | |||
| Age: ≥ 40 | 0.557 | 0.258-1.201 | 0.135 | |||
| Baseline HBsAg (IU/mL) | 1 | 1-1 | 0.977 | |||
| Baseline HBeAg (PEIU/ml) | 1.003 | 0.991-1.014 | 0.665 | |||
| Baseline Anti-HBe (PEIU/ml) | 1.032 | 0.927-1.147 | 0.567 | |||
| Baseline HBV DNA level, lg copies/mL | 1.053 | 0.904-1.226 | 0.506 | |||
| Anti-HBc: > 11.88 (S/CO) | 0.183 | 0.074-0.484 | 0.001 | 0.066 | 0.013-0.340 | 0.010 |
| AFP (ng/mL): > 200 | 16.674 | 5.021-55.38 | 0.000 | 7.763 | 1.367-43.797 | 0.020 |
| ALT (U/L): > 40 | 1.408 | 0.817-2.428 | 0.218 | |||
| AST (U/L): > 40 | 1.519 | 0.780-2.960 | 0.218 | |||
| PLT (109/L): < 125 | 0.788 | 0.452-1.374 | 0.401 | |||
| ALB (g/L): < 40 | 1.250 | 0.654-2.388 | 0.499 | |||
| TBIL (µmol/L): > 20.5 | 1.053 | 0.603-1.837 | 0.856 | |||
| PT (S): > 13 | 0.809 | 0.429-1.525 | 0.512 | |||
| Blood type: A/B/AB/O | - | - | 0.863 | - | - | |
| BCLC stage: B/C | - | - | 0.009 | - | - | 0.706 |
| Child-Pugh: B | 2.217 | 1.181-4.164 | 0.013 | 0.648 | 0.317-1.325 | 0.234 |
| Diabetes | 2.023 | 1.159-3.530 | 0.013 | 0.669 | 0.357-1.257 | 0.212 |
| Tumor Size (cm): 3-5/> 5 | - | - | 0.000 | - | - | 0.055 |
| Vascular invasion | 11.787 | 3.627-38.302 | 0.000 | 7.022 | 1.589-31.032 | 0.010 |
TACE transarterial chemoembolization, HR hazard ratio, CI confidence Interval, HBsAg hepatitis B surface antigen, HBeAg hepatitis B e antigen, Anti-HBe hepatitis B e antibody, Anti-HBc hepatitis B core antibody, AFPα-fetoprotein, ALT alanine aminotransferase, AST aspartate aminotransferase, PLT platelet count, ALB albumin, TBIL total bilirubin, PT prothrombin time, BCLC stage Barcelona Clinic Liver Cancer stage.
Discussion
The prognostic factors related to the survival of HCC patients after the treatment of TACE have been analyzed in many previous studies and review articles [4-10]. However, to the best of our knowledge, there have been no studies for assessing the performance of quantitative anti-HBc level, a novel immunological biomarker, in patients with HCC undergoing TACE. In the present report, we chose chronic hepatitis B patient with cirrhosis, who finally developed HCC and underwent TACE as research object. Our results demonstrated that a baseline anti-HBc level > 11.88 S/CO is associated with higher rates of Overall Survival in patients with HCC undergoing TACE.
In the current study, the 0.5-, 1-, and 2-year overall survival rates were 92.42%, 50.00%, and 21.21%, respectively, which were basically in accordance with other studies [14-16], indicating the survival rate of HCC patients after TACE is still low. For patients with unresectable HCC, the goal of TACE as a palliative treatment is to prolong survival and to enhance quality of life. However, unlike other neoplasms, the survival of HCC depends not only on tumor status, but also on hepatic functional reserve, performance status, and response to treatment [17-19]. Therefore, it would be necessary to find better predicting factors for HCC treatment outcomes before deciding a treatment option.
Anti-HBc, as one of the old biomarkers of HBV infection, has been ignored in real-life clinical practice for a long time. Recently, Fan R et al [15] proved that a baseline anti-HBc level is associated with HBeAg seroconversion in CHB patients treated with both Peg-IFN and NUC in two well-controlled and large cohorts. This study demonstrated that qAnti-HBc levels are closely correlated with hepatic inflammatory activities and can serve as a new treatment response marker. Therefore, in the current study, we evaluated the correlation between the OS of HCC patients and the quantitative baseline HBsAg, HBeAg, anti-HBe, anti-HBc, HBV DNA, respectively, by calculating the areas under the receiver operator characteristic curve (AUROC). The AUROC of anti-HBc level was the highest (0.755, P = 0.011) while other 4 values showed no statistical significance. Indeed, we also defined a unified optimal cut-off value of 11.88 S/CO with a maximum sum of sensitivity and specificity for predicting the OS, which will be convenient for its application in real-life clinical practice.
Although the baseline anti-HBc level has been proved to be independently associated with the OS in patients with HCC undergoing TACE, the mechanism underlying the predictive value of anti-HBc level is still unknown. As we know, anti-HBc plays an important role in HBV infection. It reflects the presence of serum Dane particle and intra-hepatic HBV replication, which is helpful for judging the prognosis of chronic hepatitis B [20]. Besides, many studies have shown that B lymphocytes played a crucial role in mediating immune response against HBV [21,22], and it was speculated that native HBcAg is released from damaged hepatocytes and provide potent antigenic stimulation to B-cells [12,23]. Furthermore, other studies also revealed that high levels of anti-HBc are related to the high serum IL-2R levels, which is mainly released from sensitization of T lymphocytes, and the production of anti-HBc is greatly enhanced by HBcAg-specific Th cell activation [24,25]. The above evidences reveal that anti-HBc is closely correlated with hepatic inflammatory activities and may serve as a surrogate marker for the host anti-HBV immune response. Therefore, the results indicated that HCC patients with higher qAnti-HBc levels may have a higher host immune response, resulting in a higher likelihood of achieving a better outcome after TACE.
In addition, we also demonstrated that elevated AFP level, large tumor, BCLC stage, Child-Pugh stage, diabetes, and vascular invasion affected OS. However, when we entered these factors in multivariate analysis, we found that only baseline anti-HBc, AFP level and vascular invasion were the independent predictive factors for OS. Many studies have strongly supported the feasibility of AFP level and vascular invasion before treatment as useful markers in predicting treatment response and patients’ survival after therapies including TACE, although the AFP cutoff value was different in those studies [4,6,7,26-28]. In the present study, a cutoff level of 200 ng/ml was used for predicting the OS because AFP over 200 ng/mL lasting for more than 8 weeks is a crucial evidence for the diagnosis for HCC in real-life clinical practice [29,30]. The role of AFP in HCC progression has been studied recently and it has been demonstrated that AFP promotes cell proliferation and angiogenesis, and that it increases the resistance of cells toward tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis [31,32]. However, the mechanism of AFP in HCC development and prognosis is still a mystery and further study is needed.
Our study had included a sufficient number of indicators and the results are important for deciding on a treatment option for HCC patients. What’s more, this is the first study to indicate that anti-HBc might be an independent predictor of OS for HCC patients after TACE. Nonetheless, there are some limitations of the current study. First, there are limited numbers of patients. Second, this is a retrospective study, which may cause selective bias. Therefore, better controlled, prospective and larger sample size cohorts would be needed.
In conclusion, baseline anti-HBc level, in addition to AFP level and vascular invasion, might be a novel biomarker for predicting the survival of HCC patients after TACE.
Acknowledgements
This study was partly supported by the grant from the National Natural Science Foundation of China (81470856).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Chen H, Wang M, Zhao Y, Cai G, Qi X, Han G. Combination therapy of sorafenib and TACE for unresectable HCC: A systematic review and meta-analysis. PLoS One. 2014;9:e91124. doi: 10.1371/journal.pone.0091124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi M, Chen JA, Lin XJ, Guo RP, Yuan YF, Chen MS, Zhang YQ, Li JQ. Transarterial chemoembolization as initial treatment for unresectable hepatocellular carcinoma in southern China. World J Gastroenterol. 2010;16:264–269. doi: 10.3748/wjg.v16.i2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Chen Y, Ge N, Zhang L, Xie X, Zhang J, Chen R, Wang Y, Zhang B, Xia J, Gan Y, Ren Z, Ye S. Prognostic significance of alpha-fetoprotein status in the outcome of hepatocellular carcinoma after treatment of transarterial chemoembolization. Ann Surg Oncol. 2012;19:3540–3546. doi: 10.1245/s10434-012-2368-5. [DOI] [PubMed] [Google Scholar]
- 5.O’Suilleabhain CB, Poon RT, Yong JL, Ooi GC, Tso WK, Fan ST. Factors predictive of 5-year survival after transarterial chemoembolization for inoperable hepatocellular carcinoma. Br J Surg. 2003;90:325–331. doi: 10.1002/bjs.4045. [DOI] [PubMed] [Google Scholar]
- 6.Ji SK, Cho YK, Ahn YS, Kim MY, Park YO, Kim JK, Kim WT. Multivariate analysis of the predictors of survival for patients with hepatocellular carcinoma undergoing transarterial chemoembolization: Focusing on superselective chemoembolization. Korean J Radiol. 2008;9:534–540. doi: 10.3348/kjr.2008.9.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Chen Y, Ge N, Zhang L, Xie X, Zhang J, Chen R, Wang Y, Zhang B, Xia J, Gan Y, Ren Z, Ye S. Prognostic significance of alpha-fetoprotein status in the outcome of hepatocellular carcinoma after treatment of transarterial chemoembolization. Ann Surg Oncol. 2012;19:3540–3546. doi: 10.1245/s10434-012-2368-5. [DOI] [PubMed] [Google Scholar]
- 8.Guiu B, Deschamps F, Boulin M, Boige V, Malka D, Ducreux M, Hillon P, de Baere T. Serum gamma-glutamyl-transferase independently predicts outcome after transarterial chemoembolization of hepatocellular carcinoma: External validation. Cardiovasc Intervent Radiol. 2012;35:1102–1108. doi: 10.1007/s00270-011-0293-9. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Chen Y, Ge N, Zhang L, Xie X, Zhang J, Chen R, Wang Y, Zhang B, Xia J, Gan Y, Ren Z, Ye S. Prognostic significance of alpha-fetoprotein status in the outcome of hepatocellular carcinoma after treatment of transarterial chemoembolization. Ann Surg Oncol. 2012;19:3540–3546. doi: 10.1245/s10434-012-2368-5. [DOI] [PubMed] [Google Scholar]
- 10.Lee YK, Kim SU, Kim DY, Ahn SH, Lee KH, Lee DY, Han KH, Chon CY, Park JY. Prognostic value of alpha-fetoprotein and des-gamma-carboxy prothrombin responses in patients with hepatocellular carcinoma treated with transarterial chemoembolization. BMC Cancer. 2013;13:5. doi: 10.1186/1471-2407-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan R, Sun J, Yuan Q, Xie Q, Bai X, Ning Q, Cheng J, Yu Y, Niu J, Shi G, Wang H, Tan D, Wan M, Chen S, Xu M, Chen X, Tang H, Sheng J, Lu F, Jia J, Zhuang H, Xia N, Hou J, Dou X, Shi J, Ren H, Wang M, Ma H, Gao Z, Zhang H, Chen C. Baseline quantitative hepatitis B core antibody titre alone strongly predicts HBeAg seroconversion across chronic hepatitis B patients treated with peginterferon or nucleos (t) ide analogues. Gut. 2015 doi: 10.1136/gutjnl-2014-308546. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou FQ, Song LW, Yuan Q, Fang LL, Ge SX, Zhang J, Sheng JF, Xie DY, Shang J, Wu SH, Sun YT, Wei SF, Wang MR, Wan MB, Jia JD, Luo GH, Tang H, Li SC, Niu JQ, Zhou WD, Sun L, Xia NS, Wang GQ. Quantitative hepatitis B core antibody level is a new predictor for treatment response in HBeAg-positive chronic hepatitis B patients receiving peginterferon. Theranostics. 2015;5:218–226. doi: 10.7150/thno.10636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iozzino M, Spaziani E, Picchio M, Saltarelli A, Di Filippo A, De Angelis F, Narilli F, Stagnitti F, Cianni R. Long-term survival after transarterial chemoembolization for hepatocellular carcinoma. Clin Ter. 2011;162:129–132. [PubMed] [Google Scholar]
- 15.Fan W, Zhang Y, Wang Y, Yao X, Yang J, Li J. Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte ratios as predictors of survival and metastasis for recurrent hepatocellular carcinoma after transarterial chemoembolization. PLoS One. 2015;10:e119312. doi: 10.1371/journal.pone.0119312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xue TC, Jia QA, Ge NL, Zhang BH, Wang YH, Ren ZG, Ye SL. The platelet-to-lymphocyte ratio predicts poor survival in patients with huge hepatocellular carcinoma that received transarterial chemoembolization. Tumour Biol. 2015;36:6045–6051. doi: 10.1007/s13277-015-3281-x. [DOI] [PubMed] [Google Scholar]
- 17.Sotiropoulos GC, Druhe N, Sgourakis G, Molmenti EP, Beckebaum S, Baba HA, Antoch G, Hilgard P, Radtke A, Saner FH, Nadalin S, Paul A, Malago M, Broelsch CE, Lang H. Liver transplantation, liver resection, and transarterial chemoembolization for hepatocellular carcinoma in cirrhosis: Which is the best oncological approach? Dig Dis Sci. 2009;54:2264–2273. doi: 10.1007/s10620-008-0604-4. [DOI] [PubMed] [Google Scholar]
- 18.Sawhney S, Montano-Loza AJ, Salat P, McCarthy M, Kneteman N, Meza-Junco J, Owen R. Transarterial chemoembolization in patients with hepatocellular carcinoma: Predictors of survival. Can J Gastroenterol. 2011;25:426–432. doi: 10.1155/2011/864234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hucke F, Pinter M, Graziadei I, Bota S, Vogel W, Muller C, Heinzl H, Waneck F, Trauner M, Peck-Radosavljevic M, Sieghart W. How to STATE suitability and START transarterial chemoembolization in patients with intermediate stage hepatocellular carcinoma. J Hepatol. 2014;61:1287–1296. doi: 10.1016/j.jhep.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Hoofnagle JH, Seeff LB, Bales ZB, Zimmerman HJ. Type B hepatitis after transfusion with blood containing antibody to hepatitis B core antigen. N Engl J Med. 1978;298:1379–1383. doi: 10.1056/NEJM197806222982502. [DOI] [PubMed] [Google Scholar]
- 21.Oliviero B, Cerino A, Varchetta S, Paudice E, Pai S, Ludovisi S, Zaramella M, Michelone G, Pugnale P, Negro F, Barnaba V, Mondelli MU. Enhanced B-cell differentiation and reduced proliferative capacity in chronic hepatitis C and chronic hepatitis B virus infections. J Hepatol. 2011;55:53–60. doi: 10.1016/j.jhep.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Zgair AK, Ghafil JA, Al-Sayidi RH. Direct role of antibody-secreting B cells in the severity of chronic hepatitis B. J Med Virol. 2015;87:407–416. doi: 10.1002/jmv.24067. [DOI] [PubMed] [Google Scholar]
- 23.Yuan Q, Song LW, Liu CJ, Li Z, Liu PG, Huang CH, Yan Y, Ge SX, Wang YB, Peng CY, Zhang J, Kao JH, Chen DS, Chen PJ, Xia NS. Quantitative hepatitis B core antibody level may help predict treatment response in chronic hepatitis B patients. Gut. 2013;62:182–184. doi: 10.1136/gutjnl-2012-302656. [DOI] [PubMed] [Google Scholar]
- 24.Milich DR, McLachlan A. The nucleocapsid of hepatitis B virus is both a T-cell-independent and a T-cell-dependent antigen. Science. 1986;234:1398–1401. doi: 10.1126/science.3491425. [DOI] [PubMed] [Google Scholar]
- 25.Xiao P, Chen QF, Yang YL, Guo ZH, Chen H. Serum soluble interleukin-2 receptor levels in patients with chronic hepatitis B virus infection and its relation with anti-HBc. World J Gastroenterol. 2006;12:482–484. doi: 10.3748/wjg.v12.i3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeeneldin AA, Salem SE, Tabashy RH, Ibrahim AA, Alieldin NH. Transarterial chemoembolization for the treatment of hepatocellular carcinoma: A single center experience including 221 patients. J Egypt Natl Canc Inst. 2013;25:143–150. doi: 10.1016/j.jnci.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Barman PM, Sharma P, Krishnamurthy V, Willatt J, McCurdy H, Moseley RH, Su GL. Predictors of mortality in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Dig Dis Sci. 2014;59:2821–2825. doi: 10.1007/s10620-014-3247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao J, Li G, Lin S, He K, Lai H, Mo X, Chen J, Lin Y. Prognostic factors of hepatocellular carcinoma patients treated by transarterial chemoembolization. Int J Clin Exp Pathol. 2014;7:1114–1123. [PMC free article] [PubMed] [Google Scholar]
- 29.Marrero JA, El-Serag HB. Alpha-fetoprotein should be included in the hepatocellular carcinoma surveillance guidelines of the American Association for the Study of Liver Diseases. Hepatology. 2011;53:1060–1061. doi: 10.1002/hep.24033. author reply 1061-2. [DOI] [PubMed] [Google Scholar]
- 30.Giannini EG, Farinati F, Trevisani F. Alpha-fetoprotein in hepatocellular carcinoma surveillance: Wake not the dead. Hepatology. 2011;54:376–377. doi: 10.1002/hep.24196. [DOI] [PubMed] [Google Scholar]
- 31.Mitsuhashi N, Kobayashi S, Doki T, Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, Kato A, Yoshitomi H, Nozawa S, Furukawa K, Takeuchi D, Suda K, Miura S, Miyazaki M. Clinical significance of alpha-fetoprotein: Involvement in proliferation, angiogenesis, and apoptosis of hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:e189–e197. doi: 10.1111/j.1440-1746.2008.05340.x. [DOI] [PubMed] [Google Scholar]
- 32.Yang X, Zhang Y, Zhang L, Zhang L, Mao J. Silencing alpha-fetoprotein expression induces growth arrest and apoptosis in human hepatocellular cancer cell. Cancer Lett. 2008;271:281–293. doi: 10.1016/j.canlet.2008.06.017. [DOI] [PubMed] [Google Scholar]


