Abstract
Background: To study the lung protective effects of heme oxygenase-1 (HO-1) expression and sevoflurane preconditioning in patients with lobectomy. Methods: 30 patients receiving lobectomy were divided into two groups: propofol intravenous anesthesia group (Pro group) and sevoflurane preconditioning group (Sev group). In Pro group, propofol was used for intravenous anesthetic. In Sev group, 1%-2% sevoflurane was used during anesthesia induction to one lung ventilation (OLV). Venous blood was taken before OLV (T1), at the end of OLV (T2) and at 30 min after lung ventilation (T3) to measure the concentration of serum malondialdehyde (MDA) in two groups. HO-1 protein and mRNA expression in resected lung tissue were measured with PT-PCR and Western blot technique. Oxygenation index was detected at 2 hours after operation. Results: HO-1 protein (2.88±0.23 ng/ml) and mRNA expression in Sev group were significantly higher compared to protein (1.89±0.12 ng/ml)and mRNA expression in Pro group (P<0.05). Difference was not found in MDA concentration at T1 compared to T2 (P>0.05), however, at T3, MDA concentration was higher in Pro group than that in Sev group (P<0.05). oxygenation index in Sev group was 380±67 mmHg, which was significantly different from that in Pro group (290±56 mmHg) (P<0.05). Conclusion: Sevoflurane preconditioning can reduce oxidative stress injury induced by OLV and protect lung tissue by increasing HO-1 expression in lung tissue.
Keywords: Sevoflurane, heme oxygenase-1, lung function, lobectomy
Introduction
Acute respiratory distress syndrome is the major cause of death for patients after lobectomy. Effective lung protection during operation plays an important role in reducing postoperative pulmonary complications and postoperative mortality [1]. OLV can lead to hypoxemia through pulmonary shunt, and acute hypoxic vasoconstriction leads to increase of pulmonary circulation pressure in operative side lung, followed by reduced blood filling and oxygen supply in non-ventilated lung tissue. When the non-ventilated lung ventilates, rapid increase of blood perfusion and concentration of oxygen result in lung tissue oxidative stress. Studies have showed that lung oxidative stress injury caused by OLV is one of the important mechanisms during perioperative period.
Animal experiments have shown that sevoflurane preconditioning could upregulate HO-1 expression in lung tissue, thereby reducing lung oxidative stress [1,2]. In this study, In order to simulate lung protective effect of sevoflurane preconditioning, we gave patients sevoflurane at 30 min before OLV and observed its effects on HO-1 expression in operative side lung tissue and lung protection.
In this study, we investigated the lung protective effect of sevoflurane preconditioning and its effects on HO-1 expression in operative side lung tissue by administering sevoflurane at 30 min before OLV.
Materials and methods
Patients
30 patients underwent elective lobectomy, aged 40 to 60, weighting 50-75 kg and ASA grade I~II, were included. Preoperative examination was normal and history of diabetes or hypertension was not found. The patients were randomized into two groups (n=15). In Pro group, propofol was used for intravenous anesthetic, in Sev group; 1%-2% sevoflurane was used from anesthesia induction to OLV. The operations were performed by the same group of doctors.
Anesthesia
0.02 mg/kg penehyclidine and 0.05 mg/kg midazolam were given through intramuscular injection at 30 min before anesthesia. Intravenous access was established with routine monitoring of electrocardiogram, blood oxygen saturation, invasive radial artery blood pressure and bispectral value. Anesthetic induction: Intravenous agents were added in order etomidate 0.3-0.5 mg/kg, atracurium 0.6-1.0 mg/kg and sufentanil 0.3-0.5 μg/kg. Right or left double lumen endobronchial tube (Mallinekrodt company, Ireland) was inserted and followed by mechanical ventilation. Respiratory ventilation parameter settings in two lung ventilation: Tidal volume 8-10 ml/kg, respiratory ratio 1:2, frequency 10-12/min, oxygen concentration 100%, end-tidal carbon dioxide partial pressure 35-45 mmHg. Parameter settings in OLV: Tidal volume 6-8 ml/kg, respiratory frequency 14-16/min, other parameters unchanged. Maintenance of anesthesia: In Pro group, continuous intravenous propofol (6-8 mmg/kg•h) was given. In Sev group, 1.0%-3.0% sevoflurane was inhaled continuously at 30 min before OLV, and then intravenous propofol was used till the end of operation. Intravenous sufentanil and atracurium were used intermittently in two groups. Sevoflurane and propofol concentration were adjusted during intraoperative time to maintain bispectral value 40 to 60. All anesthetics discontinued 10 min after the end of operation.
Analysis of concentration of MDA
Venous blood sample 4 ml was drawn at T1, T2 and T3, centrifuged for 5 min for serum, and preserved at -80°C. MDA concentration was analyzed using TBA method according to the instruction (Kit purchased from Nanjing Jiancheng Bioengineering Institute).
HO-1 protein expression analysis in resected lung tissue
Cytoplasmic protein was extracted from 40 mg lung tissue, and the protein concentration was determined using the Bradford method. The protein (100 μg) was added to each well for sodium dodecyl sulfate polyacrylamide gel electrophoresis, and then transferred to a polyvinylidene difluoride (PVDF) membrane for Ponceau red staining. The effect of transfer and the position of the target protein were determined according to the marker protein. The PVDF membrane was incubated with 5% bovine serum albumin at 37°C for 1 h, followed by incubation with rabbit anti-rat monoclonal antibody (1:250) and then with horseradish peroxidase-labeled goat anti-rabbit IgG antibody (1:15000) at 37°C for 1 h, respectively. The signals were detected with an ECL chemiluminescence kin. The experiment in each group was repeated three times.
HO-1 mRNA expression analysis
Total RNA was extracted from 50-100 mg lung tissue with TRlzol reagent according to the manufacturers recommended steps. Its purity and quantitative analysis were studied with UV spectrophotometer (from Bio-Rad Corporation), and RNA integrity was verified on 1% formaldehyde denaturing gel electrophoresis. HO-1 mRNA expression was detected by Semi-quantitative RT-PCR according to instructions in one-step kit (from Katara Company). Primers were synthesized by Beijing Oak biotechnology company. HO-1 upstream: 5’-ATGGAGCGCCCACAGCTCGA-3’, downstream: 5’-CTCCAGAGTGTTCATGCGAG-3’; β-actin upstream: 5-TCTGGATCACCTTCTGCTGG-3’, downstream: 5’-GGGGATGATGAGGTGATCGAGGACG-3’. The amplification reaction involved 25 cycles of reverse transcriptase at 94°C for 3 min, denaturation at 94°C for 30 seconds, annealing at 58°C for 30 seconds, and extension at 72°C for 1 min. PCR products were scanned and photographed with the Fluor-S multi-functional imaging system after 1.5% agarose gel electrophoresis.
Determination of oxygenation index
Blood sample was drawn from radial artery at 2 h after operation and oxygenation index (PaO2/FiO2) was measured.
Statistics
The statistical package SPSS Version 12.0 was applied to complete data processing, values of P<0.05 were considered statistically significant. Measurement data were expressed as mean ± standard deviation, and repeated measures design data using repeated measures analysis of variance, count data were compared using chi-square test.
Results
There was no statistical difference between two groups in gender, age, weight, intraoperative fluid volume and OLV time (P>0.05) (Table 1). Difference was not found in MDA concentration at T1 compared to T2 (P>0.05), however, at T3, MDA concentration was higher in Pro group than that in Sev group (P<0.05) (Table 2). In Sev group, HO-1 protein (2.88±0.23 ng/ml) and mRNA expression were significantly higher compared to protein (1.89±0.12 ng/ml) and mRNA expression in Pro group (P<0.05) (Figures 1, 2). oxygenation index in Sev group was 380±67 mmHg, which was different statistically from that in Pro group (290±56 mmHg) (P<0.05).
Table 1.
General information in two groups (n=15)
| Sev group | Pro group | |
|---|---|---|
| Gender composition ratio (M/F) | 10/5 | 9/6 |
| Age (year) | 54±12 | 56±10 |
| Weight (kg) | 63±8 | 65±10 |
| Total infusion volume (ml) | 1530±435 | 1480±387 |
| Time of OLV (min) | 95±26 | 110±22 |
Table 2.
Concentration of MDA in two groups (nmol/ml)
| T1 | T2 | T3 | |
|---|---|---|---|
| Sev group | 4.8±0.6 | 5.1±0.4 | 8.6±0.7 |
| Pro group | 5.0±0.4 | 5.3±0.3 | 10.7±0.8 |
Figure 1.
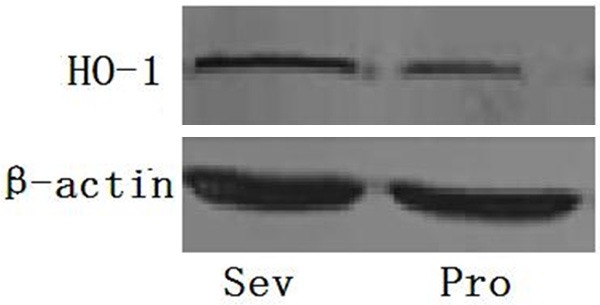
HO-1 protein expression in two groups after OLV.
Figure 2.
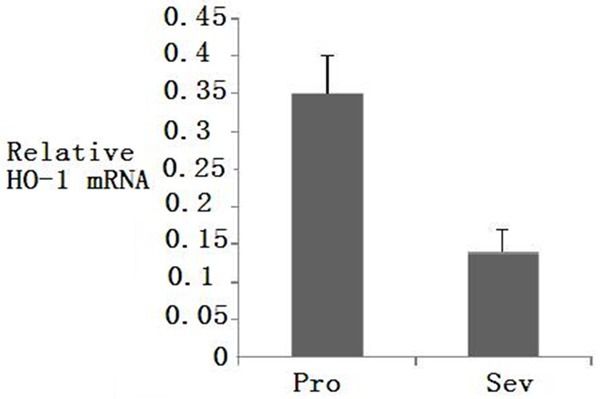
HO-1 mRNA expression in two groups after OLV.
Discussion
Perioperative acute lung injury has long been considered as a potential postoperative complication with complex pathophysiological process and high fatality which seriously affect patients` recovery [1-6].
OLV is now widely used in cardiothoracic surgery, which not only can fully expose the surgical field and create a good operating condition, but also avoid secretions or exudates into contralateral lung to reduce lung complications. However, OLV process often causes increased pulmonary shunt, leading to hypoxemia, hypoxic pulmonary vasoconstriction and excessive stretch during ventilation. Ipsilateral lung injury followed at the end of OLV and after lung recruitment [7]. Oxidative stress injury is an important cause of perioperative lung injury [8]. (After Ipsilateral lung recover ventilation and, but a large number of oxygen molecules into the tissues release large amounts of oxygen free radicals and cause tissue damage). Once ipsilateral lung ventilation recovers and low blood perfusion lung/hypoxia improves, excessive oxygen molecules in the tissues will release large amounts of oxygen free radicals and cause tissue damage. Prolonged inhalation of high concentrations of oxygen also can cause “oxidative stress” which leads to lung damage. HO-1 is a kind of initial enzyme and the rate-limiting enzyme which degrade heme into carbon monoxide, iron and bilirubin. Recent studies have found that HO-1 also has a variety of physiological regulation such as anti-oxidation, anti-inflammatory, anti-apoptotic and cell protection function [9], and is the most important endogenous protective proteins in body. HO-1 and its metabolites have a strong anti-oxidative stress effect against ischemia-reperfusion injury and oxidative injury [10-12]. In our study, HO-1 expression is statistically higher in Sev group compared to that in Pro group. It indicated that sevoflurane preconditioning can increase HO-1 expression in lung tissue and protect lung tissue against oxidative stress injury. These results are similar to the previous reports that have been done in animal experiments [13-15].
MDA is a decomposition product of lipid peroxide concentration of in serum, MDA concentration may indirectly reflect the cell damaged by oxygen free radical [16]. The results showed that serum MDA concentrations were significantly increased after lung recruitment, indicating that oxidative stress injury occurred mainly in lung recruitment process. Oxygenation index is an important indicator of postoperative lung function. In the study, oxygenation index was higher at 2 h after operation in Sev group than that in Pro group, suggesting that sevoflurane preconditioning is conducive to the recovery of postoperative pulmonary function.
In summary, sevoflurane preconditioning could protect lung tissue by upregulating HO-1 expression and reducing oxidative stress injury. Our study was designed from clinical point, and was combined with previous studies and theories. Protective effects of sevoflurane preconditioning on lung tissue were observed. However, specific mechanism involved needs further study.
Acknowledgements
This research was supported by the Science and Technology research funded projects of the Development Projects of Science and Technology of Shandong Province, China (2014gsf118160).
Disclosure of conflict of interest
None.
References
- 1.Yu S, Shi M, Liu C, Liu Q, Guo J, Yu S, Jiang T. Time course changes of oxidative stress and inflammation in hyperoxia-induced acute lung injury in rats. Iran J Basic Med Sci. 2015;18:93–103. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.McDonald CI, Fung YL, Shekar K, Diab SD, Dunster KR, Passmore MR, Foley SR, Simonova G, Platts D, Fraser JF. The impact of acute lung injury, ECMO and transfusion on oxidative stress and plasma selenium levels in an ovine model. J Trace Elem Med Biol. 2015;30:4–10. doi: 10.1016/j.jtemb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Mannam P, Srivastava A, Sugunaraj JP, Lee PJ, Sauler M. Oxidants in Acute and Chronic Lung Disease. J Blood Lymph. 2014;4:1000128. doi: 10.4172/2165-7831.1000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pietrofesa RA, Solomides CC, Christofidou-Solomidou M. Flaxseed Mitigates Acute Oxidative Lung Damage in a Mouse Model of Repeated Radiation and Hyperoxia Exposure Associated with Space Exploration. J Pulm Respir Med. 2014;4:1000215. doi: 10.4172/2161-105X.1000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yashiro M, Tsukahara H, Matsukawa A, Yamada M, Fujii Y, Nagaoka Y, Tsuge M, Yamashita N, Ito T, Yamada M, Masutani H, Yodoi J, Morishima T. Redox-active protein thioredoxin-1 administration ameliorates influenza A virus (H1N1)-induced acute lung injury in mice. Crit Care Med. 2013;41:171–81. doi: 10.1097/CCM.0b013e3182676352. [DOI] [PubMed] [Google Scholar]
- 6.Chow CW, Herrera Abreu MT, Suzuki T, Downey GP. Oxidative stress and acute lung injury. Am J Respir Cell Mol Biol. 2003;29:427–31. doi: 10.1165/rcmb.F278. [DOI] [PubMed] [Google Scholar]
- 7.Pinhu L, Whitehead T, Evans T, Griffiths M. Ventilator-associated lung injury. Lancet. 2003;361:332–40. doi: 10.1016/S0140-6736(03)12329-X. [DOI] [PubMed] [Google Scholar]
- 8.Kirkby KA, Adin CA. Products of heme oxygenase and their por-tential therapeutic applications. Am J Physiol Renal Physiol. 2006;290:563–71. doi: 10.1152/ajprenal.00220.2005. [DOI] [PubMed] [Google Scholar]
- 9.Ong S, Shen X, Tang Y, Wang Z, Guo W, Ding G, Wang Q, Fu Z. Sinomenine pretreatment attenuates cold ischemia/reperfusion injury in rats: the role of hemeoxygenase-1. Int Immunopharmacol. 2010;10:679–84. doi: 10.1016/j.intimp.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Arimori Y, Takahashi T, Nishie H, Inoue K, Shimizu H, Omori E, Kawanishi S, Toda Y, Morimatsu H, Morita K. Role of heme oxygenase-1 in protection of the kidney after hemorrhagic shock. Int J Mol Med. 2010;26:27–32. doi: 10.3892/ijmm_00000430. [DOI] [PubMed] [Google Scholar]
- 11.Ge ZJ, Jiang GJ, Zhao YP, Wang GX, Tan YF. Systemic perfluorohexane attenuates lung injury induced by lipopolysacchad ride in rats: the role of heme oxygenase-1. Pharmacol Rep. 2010;62:170–7. doi: 10.1016/s1734-1140(10)70254-1. [DOI] [PubMed] [Google Scholar]
- 12.Larsen M, Webb G, Kennington S, Kelleher N, Sheppard J, Kuo J, Unsworth-White J. Mannitol in cardioplegia as an oxygen free radical scavenger measured by malondialdehyde. Perfusion. 2002;17:51–5. doi: 10.1191/0267659102pf528oa. [DOI] [PubMed] [Google Scholar]
- 13.Lee H, Park YH, Jeon YT, Hwang JW, Lim YJ, Kim E, Park SY, Park HP. Sevoflurane post-conditioning increase nuclear factor erythroid 2-related factor and haemoxygenase-1 expression via protein kinase C pathway in a rat model of transient global cerebral ischaemia. Br J Anaesth. 2015;114:307–18. doi: 10.1093/bja/aeu268. [DOI] [PubMed] [Google Scholar]
- 14.Zhao S, Wu J, Zhang L, Ai Y. Post-conditioning with sevoflurane induces heme oxygenase-1 expression via the PI3K/Akt pathway in lipopolysaccharide-induced acute lung injury. Mol Med Rep. 2014;9:2435–40. doi: 10.3892/mmr.2014.2094. [DOI] [PubMed] [Google Scholar]
- 15.Xiong XQ, Lin LN, Wang LR, Jin LD. Sevoflurane attenuates pulmonary inflammation and ventilator-induced lung injury by upregulation of HO-1 mRNA expression in mice. Int J Nanomedicine. 2013;6:1075–81. doi: 10.2147/IJN.S41625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lases EC, Durkens VA, Gerritsen WB, Haas FJ. Oxidative stress after lung resection therapy: a pilot study. Chest. 2000;117:999–1003. doi: 10.1378/chest.117.4.999. [DOI] [PubMed] [Google Scholar]


