Abstract
Objective: The purpose of our study was to observe the influence of dexmedetomidine on complications caused by hemabate in patients undergoing caesarean section. Methods: A total of 120 females (age range, 20-40 years) at 35-40 weeks gestation who delivered by cesarean between September, 2014 and December, 2014 were enrolled in our study. Patients were randomly allocated into three groups that received intravenously physiological saline 20 mL (placebo group), lower dose (0.5 μg kg-1) of dexmedetomidine (low-dex gruop) and higher dose (1 μg kg-1) of dexmedetomidine (high-dex group) during cesarean section, following the delivery of the infant and intramuscular hemabate injection. Results: Nausea, vomiting, chest congestion and elevated blood pressure were the most common adverse events of placebo group. Compared with placebo group, the above mentioned adverse reactions decreased significantly in both low-dex group and high-dex group (P<0.05), whereas there were no significant difference between low-dex group and high-dex group (P>0.05). As to patient satisfaction score, low-dex group and high-dex group were all higher than placebo group (P<0.05). Furthermore, there were more patients satisfied with high-dex group than low-dex group (P<0.05). Conclusion: Dexmedetomidine (0.5 μg kg-1 and 1 μg kg-1) were all effective in preventing adverse reactions introduced by hemabate and improve parturients’ satisfaction in patients undergoing cesarean delivery. And higher dose (1 μg kg-1) of dexmedetomidine is superior to lower dose (0.5 μg kg-1) in patient satisfaction.
Keywords: Dexmedetomidine, caesarean section, hemabate, complications
Introduction
Hemabate is the synthetic 15-methyl analogue of prostaglandin F2α, and has been accounted for to be 84-96% successful in the treatment of persistent hemorrhage because of uterine atony [1]. Recently, Hemabate was discovered more powerful than oxytocin in avoiding postpartum hemorrhage (PPH) in high-risk patients experiencing cesarean delivery [2]. However, Hemabate may cause prostaglandin-like complications, such as nausea, vomiting, diarrhea, headaches, high blood pressure and bronchial asthma etc [2]. Lamont et al even discontinued their study because of unsatisfactory gastrointestinal adverse reactions of hemabate [3].
Dexmedetomidine is a highly selective a2 adrenergic agonist that has narcotic, pain relieving and anxiolytic effects, but does not result in Inhibition of respiration [4]. Nie et al discovered utilization of dexmedetomidine can diminish the perioperative utilization of opioids in parturients, and the latter is connected with nausea and vomiting in perioperative period [5]. There are reports of dexmedetomidine decreases postoperative [6] or chronic and recurrent [7] nausea and vomiting.
The aim of our study was to observe the influence of dexmedetomidine on complications caused by hemabate in patients undergoing caesarean section.
Patients and methods
Study population
This study design (2012-172) was authorized by the Ethics Committee of Guangzhou Women and Children’s Medical Center The protocol was registered with www.chictr.org.cn [ChiCTR-IOR-14005316]. Written informed consent was obtained from every parturient who participated in this trial. Pregnant females who were arranged to undergo scheduled cesarean section under combined spinal-epidural anesthesia between September 2014 and December 2014 were included in this study.
The inclusion criteria were 20-40 years of age, height 155-170 cm and capable of comprehending oral and written Chinese. Exclusion criteria contained long-term opioid pain relieving utilization or NSAIDs, sedative utilization, mental illnesses, preoperative heart rate (HR) lower than 50 bpm with atrial-ventricular conduction abnormalities or rhythm irregularities, neuromuscular and endocrine sicknesses or hypersensitive reactions to a2-adrenergic receptor agonists, or any past abdominal operation containing earlier caesarean delivery. Patients with coagulation issues or contraindications for receiving prostaglandin medications were additionally excluded from the study. Computer-generated randomization sequence was utilized to allocate patients into three groups (40 per group).
Anesthesia and study design
Parturients were seen before surgery. Premedication was not given. After entering into the operating theatre, a peripheral vein of the right arm was intravenous catheterized with an 18-gauge intravenous cannula. Routine monitoring consisting of Electrocardiograph, pulse oximetry and non-invasive blood pressure (NIBP) was started. NIBP was monitored every 5 min amid the surgery and every 15 min at the postanesthesia care unit (PACU). Volume preload with hydroxyethylstarch 6% (HES) 500 ml was performed to all patients in a rate of 20 ml min-1 preceding anesthesia induction. We ordinarily utilized a combined spinal-epidural anesthesia (CSE) technique for cesarean section procedures. The patient was situated in the right arm recumbent position and the epidural space was distinguished at the L3-L4 interspace. Lumbar puncture was performed with a pencil-point spinal needle (26-gauge size) and 0.5% isobaric bupivacaine 1.5 ml (7.5 mg) was infused. After the spinal needle was drawn back, the epidural catheter was put. Thereafter, patients were promptly situated at left-elevating supine position. Operation was started when the sensory nerve block reached at T4-T6 (assessed by sensation lose to pinprick). The epidural catheter was reserved when the spinal anesthesia accomplished a satisfactory effect or for extended operation. Pain amid operation and preceding delivery was dealing with 1% lidocaine 5 ml administrated by the epidural catheter. In the event that the pain was not relieved after 15 min, an alternate salvage dose was utilized. Intravenous sufentanil 5 μg was given if pain was complained following delivery. Patients who received epidural lidocaine or intravenous sufentanil were eliminated from the study. No other pain relieving drug or narcotic was utilized amid operation aside from dexmedetomidine. Oxygen was given to parturients at 5 l min-1 by a face-mask.
Patients received an intramuscular injection of 250 μg hemabate (Pharmacia & Upjohn Company, Kalamazoo, MI, USA) at deltoid muscle and continuous intravenous infusion of 20 units oxytocin diluted in 1,000 ml saline or Ringer’s solution during the cesarean section, immediately following the delivery of the infant. At the same time, patients in placebo group received 20 ml intravenous physiological saline. Patients in low-dex group received 0.5 μg kg-1 intravenous dexmedetomidine (Jiang Su Heng Rui Medicine Co. Ltd, Jiangsu Province, China) diluted to 20 ml with physiological saline. Patients in high-dex group received 1 μg kg-1 intravenous dexmedetomidine. The infusion of above mentioned three group liquids were completed in 30 minutes. The randomization sequence was reserved in our hospital drug store where the trial medicine was ready. The research personnel who registered patients and gathered study results were blinded to group allocation.
Following surgery, the patient was transported to the PACU, and 1 h after the study medicine infusion, the parturient transported to the ward. Patients got epidural analgesia with 0.1% ropivacaine + 0.1 μg/mL sufentanil (continuous, 8 mL/h; bolus, 4 mL; lockout interval, 15 min; 1-h limitation, 16 mL) in a patient-controlled analgesia device for the postoperative 48 h. Instantly after the peritoneum closed, the PCA apparatus was connected to the epidural catheter and the 5-mL loading dose was given.
Data recording
Heart rate (HR), systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded at the following time points: 1 min prior to hemabate administration (T0); 5 min (T1); 20 min (T2); 40 min (T3) and 60 min (T4) following hemabate injection.
All patients had standard follow-up by an anesthetist on the day after operation and an evaluation by a study nurse 24 h postoperatively, with directions to record the incidence of complications. Adverse reactions such as nausea, vomiting, fever, diarrhea, headache, elevated blood pressure and chest congestion were recorded.
The grade of satisfaction (0, very satisfied; 1, satisfied; 2, not satisfied) was assessed at 24 h after operation.
Statistical analysis
The primary outcome was the reduction of occurrence of nausea and vomiting. In the study by Bai et al., the nausea and vomiting was 16.7% and 22.2%, respectively. In our study, the hypothesis was that compared with placebo group, high-dex group would achieve a 30% reduction of nausea and vomiting, while low-dex group would achieve a 15% reduction. A power analysis suggested that with 32 individuals per treatment arm, we would have 80% power to detect differences among the means with a 0.05 significance level (two-tailed). Taking exclusions into account, we aimed to recruit 40 patients in each group.
Continuous variables are displayed as the mean ± standard deviation or median with range, determining by the normality of the data distribution. Categorical variables are expressed by frequencies and percentages. The differences among the three groups were detected using analysis of variance (ANOVA) or a Kruskal-Wallis test for continuous variables, and using a Fisher’s exact test for categorical variables, as appropriate. In addition, the differences between placebo group, low-dex group and high-dex group were examined using the paired Student’s t-test or Wilcoxon signed ranks test. For all analyses, a two-sided P<0.05 was considered to suggest a statistically significant difference. Statistical analyses were performed using SPSS 15.0 statistical software (SPSS Inc., Chicago, IL, USA).
Results
Of the 120 parturients, four withdrew from the study. Three patients received epidural lidocaine one in placebo group, two in low-dex group. The other one was in high-dex group: received intravenous sufentanil because of a post-partum pain. Thus, 116 parturients completed the study (Figure 1). The patients were between 20 and 40 years of age and 35 and 40 weeks gestation. No significant differences were identified in maternal age, gravidity and parity, gestational age at delivery and reason for cesarean delivery among the three groups (all P>0.05, Table 1).
Figure 1.
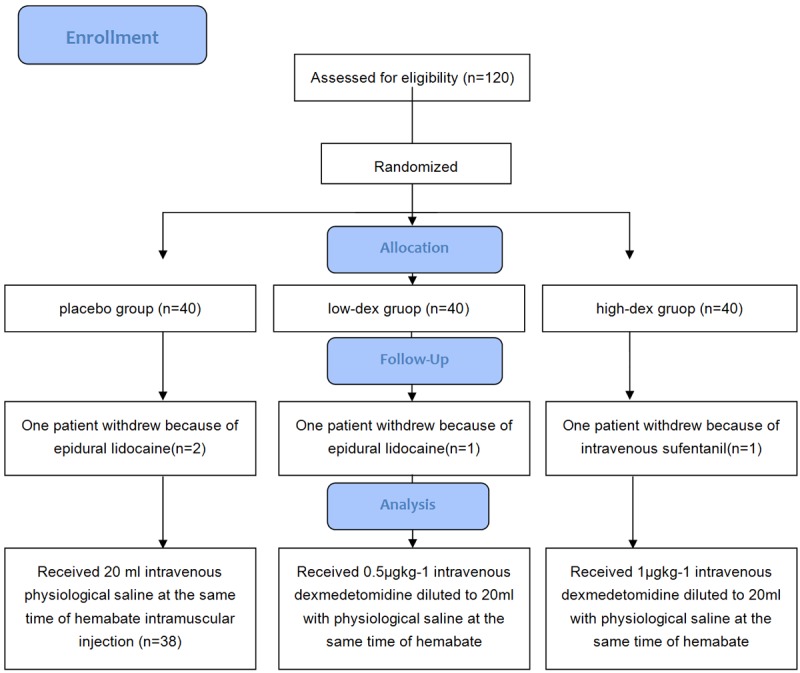
CONSORT flow diagram.
Table 1.
Patient characteristics in the three group
| placebo group | low-dex gruop | high-dex gruop | |
|---|---|---|---|
| Age (years) | 31±5 | 30±6 | 29±3 |
| Height (cm) | 163±6 | 162±3 | 164±4 |
| Weight (kg) | 75±6 | 73±8 | 72±7 |
| Gestational period (weeks) | 39.1±0.7 | 38.6±0.9 | 38.7±1.2 |
| Duration of surgery (min) | 39.1±6.3 | 40.6±7.2 | 39.5±6.9 |
Data are expressed as mean ± SD.
HR and SBP of placebo group increased significantly (both P<0.05) whereas HR of High-dex group decreased significantly (P<0.05) at 20 min after hemabate injection, and all went back to basic values without intervention at 40 min after hemabate use (Table 2).
Table 2.
HR, SBP, DBP changes in the three groups of patients before and after hemabate use
| Group | T0 | T1 | T2 | T3 | T4 | |
|---|---|---|---|---|---|---|
| HR (beats/min) | placebo group | 79.5±7.5 | 78.1±8.1 | 95.0±9.8* | 90.7±8.6a | 85.9±7.9 |
| low-dex gruop | 80.4±11.0 | 79.5±11.5 | 85.8±9.2 | 83.2±9.7 | 82.5±8.5 | |
| high-dex gruop | 83.5±7.9 | 80.5±8.9 | 72.3±7.2* | 76.8±9.6 | 80.7±9.8 | |
| SBP(mmHg) | placebo group | 114.6±6.7 | 121.7±7.1 | 132.±8.0* | 123.1±8.6 | 119.3±7.2 |
| low-dex gruop | 115.8±7.2 | 118.0±6.8 | 120.8±8.1 | 121.7±7.1 | 116.8±8.0 | |
| high-dex gruop | 115.0±8.0 | 117.1±7.4 | 118.6±7.2 | 121.1±7.7 | 118.5±8.2 | |
| DBP (mmHg) | placebo group | 75.4±6.9 | 75.5±5.7 | 74.3±7.4 | 73.6±8.1 | 73.8±9.6 |
| low-dex gruop | 76.7±9.6 | 76.2±8.7 | 74.5±7.6 | 72.0±9.2 | 73.6±8.9 | |
| high-dex gruop | 74.9±6.2 | 77.5±5.3 | 75.4±7.6 | 73.1±8.5 | 76.6±9.1 |
Data are expressed as mean ± SD;
P<0.05, compared with T0.
The adverse events that occurred in the three groups are summarized in Table 3. Nausea, vomiting, chest congestion and elevated blood pressure were the most common adverse events of placebo group. Compared with placebo group, the above mentioned adverse reactions decreased significantly in both low-dex group and high-dex group (P<0.05), whereas there were no significant difference between low-dex group and high-dex group (P>0.05).
Table 3.
Incidence of adverse reactions after postoperative 24 hours
| Incidence | placebo group (n, %) | low-dex gruop (n, %) | high-dex gruop (n, %) |
|---|---|---|---|
| Nausea | 31 (77.5) | 19 (47.5)* | 11 (27.5)*,Δ |
| Vomiting | 26 (65.0) | 5 (12.5)* | 2 (5.0)* |
| Chest congestion | 15 (37.5) | 5 (12.5)* | 3 (7.5)* |
| Elevated blood pressure | 5 (12.5) | 0 (0.0)* | 0 (0.0)* |
| Fever | 3 (7.5) | 2 (5.0) | 2 (5.0) |
| Diarrhea | 2 (5.0) | 2 (5.0) | 1 (2.5) |
| Headache | 1 (2.5) | 0 (0.0) | 0 (0.0) |
Data are expressed as number of observations and percentages.
P<0.05, compared with placebo group.
P<0.05, compared with low-dex group.
As to patient satisfaction score, low-dex group and high-dex group were all higher than placebo group (P<0.05). Furthermore, there were more patients satisfied with high-dex group than low-dex group (P<0.05) (Table 4).
Table 4.
Comparison of patient satisfaction among the three groups
| placebo group (n, %) | low-dex gruop (n, %) | high-dex gruop (n, %) | |
|---|---|---|---|
| Very satisfied | 3 (7.5) | 12 (30.0) | 22 (55.0)Δ |
| Satisfied | 9 (22.5) | 19 (47.5) | 14 (35.0) |
| Not satisfied | 28 (70.0) | 9 (22.5) | 4 (10.0) |
| Value of Ridit | 0.70 | 0.46* | 0.34* |
Data are expressed as numbers and percentages.
P<0.05, compared with placebo group.
P<0.05, compared with low-dex group.
Discussion
PPH is accounted for to happen in ~5% of all deliveries, and the risk is essentially more prominent with cesarean delivery than vaginal delivery [8]. In China, PPH is the most well-known serious obstetric complication and the heading reason for maternal mortality, representing 49.9% of maternal deaths [2]. Hemabate is the synthetic 15-methyl analogue of prostaglandin F2α. Hemabate may cause prostaglandin-like complications, including nausea, vomiting, diarrhea, headaches, high blood pressure and bronchial asthma brought about by the contraction of smooth muscles [2]. It might additionally act on the thermoregulatory center, raising the basal body temperature [2]. Patients may encounter hot flashes, sweating and increased irritability.
In our trial, nausea, vomiting, chest congestion and elevated blood pressure were the most common adverse events of hemabate, whereas the occurrence of fever, diarrhea and headache is relatively rare.
Contrasted with clonidine, dexmedetomidine is an alpha2 adrenergic receptor agonist with an more specificity to the alpha2 versus the alpha1 receptor. A few physiologic impacts have been exhibited with dexmedetomidine, consisting of tranquilization, antianxiety, pain relief, a decline of expired minimum alveolar concentration (MAC) of inhaled anesthetics, and attenuating the sympathetic nervous response to operation [9-12].
The reasons of dexmedetomidine’s blunting hemabate nausea and vomiting adverse reactions may lie in three perspectives. The primary of them is in a few cases, management of vomiting episodes includes the utilization of intense tranquilizers that incite delayed spans of sedation and sleep [7]. It is possibly dexmedetomidine sedation effect that associated with its impacts on nausea and vomiting. Furthermore, dexmedetomidine is an alpha-2 adrenoreceptor agonist. This medication has an action on the locus coeruleus, which are considerable noradrenergic cells gathering found in the cerebellar pontine, and seems to have regulatory impacts on extracellular dopamine [13]. The alpha-2 adrenoreceptor agonist of dexmedetomidine, which resembles to clonidine, abates the noradrenergic action as a consequence of binding to alpha-2 presynaptic inhibitory adrenoreceptors in the locus coeruleus, a restraint that potentially brings about an anti-emetic impact. Thirdly, it is well known that the gastrointestinal distention activates vagal visceral afferent, this in turn stimulates the vomiting center and instigates nausea and vomiting. By expanding sympathetic outflux and diminishing parasympathetic outflux from the central nervous system, dexmedetomidine may exert its impact by accelerating the gastric emptying and the gastrointestinal motility, which conceivably has an imperative impact in diminishing nausea and vomiting. Comparative anti-emetic results were additionally observed when clonidine was utilized. In Rosa-e-Silva et al’s [14] non-placebo controlled study, they reported a considerable decline in nausea and vomiting in 6 diabetic patients with gastroparesis treated with oral clonidine. Park et al [15] likewise demonstrated less frequent nausea and vomiting, and a less necessities of anti-emetics in patients experiencing major knee surgery, who got oral clonidine 1.5 hours before surgery and at 12 and 24 hours after the first dose.
Chest congestion is a side effect of hemabate we found no report before. The reason may be related to airway smooth muscle contraction as bronchial asthma caused by hemabate. The research of effects of dexmedetomidine on airway smooth muscle contraction is rare, so in the future there should be some research work on this aspect to elucidate the mechanism.
Lamont et al [3] found diarrhea occurred in 21% of the patients who received hemabe, compared with only 5% of the patients in our results. The difference may derive from different population hemabate used for.
Although there was no significant difference between low-dex and high-dex group about their action on adverse reactions’ occurrence, patient satisfaction is different. Our results is similar with Nie Y et al. [5], indicating dexmedetomidine can improve patient satisfaction by sedation or analgesia, although in our trial postoperative is effective.
A few drawbacks exist in our trial. Firstly, since hemabate could be used via myometrium injection and intramuscular injection, the influence of administration route on adverse reactions occurrence is unknown. In the future study, we should add a more test group using hemabate myometrium injection. Secondly, the time to start of lactation, success rates of breastfeeding and the level of newborn exposure to dexmedetomidine via the communicated milk were not assessed. The level of dexmedetomidine in the breast milk was not measured because of lacking of detecting technique. The time to start of lactation following surgery was 55-56 h [5]; notwithstanding, dexmedetomidine infusion finished amid surgery. As indicated by dexmedetomidine’s pharmacokinetics (t1/2¼2 h), its level of breast milk was presumably very low in the trial. Our results ought to just be applicable to parturients experiencing scheduled caesarean section after a normal and uneventful pregnancy. In addition, more trails are necessary to explore maternal and newborn’s safety of dexmedetomidine use.
In conclusion, the results of the present study demonstrated that dexmedetomidine (0.5 μg kg-1 and 1 μg kg-1) were all effective in preventing adverse reactions introduced by hemabate and improve parturients’ satisfaction in patients undergoing cesarean delivery. And higher dose (1 μg kg-1) of dexmedetomidine is superior to lower dose (0.5 μg kg-1) in patient satisfaction.
Disclosure of conflict of interest
None.
References
- 1.Buttino L Jr, Garite TJ. The use of 15 methyl F2 alpha prostaglandin (Prostin 15M) for the control of postpartum hemorrhage. Am J Perinatol. 1986;3:241–243. doi: 10.1055/s-2007-999875. [DOI] [PubMed] [Google Scholar]
- 2.Bai J, Sun Q, Zhai H. A comparison of oxytocin and carboprost tromethamine in the prevention of postpartum hemorrhage in high-risk patients undergoing cesarean delivery. Exp Ther Med. 2014;7:46–50. doi: 10.3892/etm.2013.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamont RF, Morgan DJ, Logue M, Gordon H. A prospective randomised trial to compare the efficacy and safety of hemabate and syntometrine for the prevention of primary postpartum haemorrhage. Prostaglandins Other Lipid Mediat. 2001;66:203–210. doi: 10.1016/s0090-6980(01)00154-x. [DOI] [PubMed] [Google Scholar]
- 4.Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–394. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Nie Y, Liu Y, Luo Q, Huang S. Effect of dexmedetomidine combined with sufentanil for post-caesarean section intravenous analgesia: a randomised, placebo-controlled study. Eur J Anaesthesiol. 2014;31:197–203. doi: 10.1097/EJA.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 6.Massad IM, Mohsen WA, Basha AS, Al-Zaben KR, Al-Mustafa MM, Alghanem SM. A balanced anesthesia with dexmedetomidine decreases postoperative nausea and vomiting after laparoscopic surgery. Saudi Med J. 2009;30:1537–1541. [PubMed] [Google Scholar]
- 7.Khasawinah TA, Ramirez A, Berkenbosch JW, Tobias JD. Preliminary experience with dexmedetomidine in the treatment of cyclic vomiting syndrome. Am J Ther. 2003;10:303–307. doi: 10.1097/00045391-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Carroli G, Cuesta C, Abalos E, Gulmezoglu AM. Epidemiology of postpartum haemorrhage: a systematic review. Best Pract Res Clin Obstet Gynaecol. 2008;22:999–1012. doi: 10.1016/j.bpobgyn.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 10.Khan ZP, Munday IT, Jones RM, Thornton C, Mant TG, Amin D. Effects of dexmedetomidine on isoflurane requirements in healthy volunteers. 1: Pharmacodynamic and pharmacokinetic interactions. Br J Anaesth. 1999;83:372–380. doi: 10.1093/bja/83.3.372. [DOI] [PubMed] [Google Scholar]
- 11.Peden CJ, Cloote AH, Stratford N, Prys-Roberts C. The effect of intravenous dexmedetomidine premedication on the dose requirement of propofol to induce loss of consciousness in patients receiving alfentanil. Anaesthesia. 2001;56:408–413. doi: 10.1046/j.1365-2044.2001.01553.x. [DOI] [PubMed] [Google Scholar]
- 12.Talke P, Chen R, Thomas B, Aggarwall A, Gottlieb A, Thorborg P, Heard S, Cheung A, Son SL, Kallio A. The hemodynamic and adrenergic effects of perioperative dexmedetomidine infusion after vascular surgery. Anesth Analg. 2000;90:834–839. doi: 10.1097/00000539-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Whittington RA, Virag L. Dexmedetomidine-induced decreases in accumbal dopamine in the rat are partly mediated via the locus coeruleus. Anesth Analg. 2006;102:448–455. doi: 10.1213/01.ane.0000195234.07413.5a. [DOI] [PubMed] [Google Scholar]
- 14.Rosa-e-Silva L, Troncon LE, Oliveira RB, Iazigi N, Gallo L Jr, Foss MC. Treatment of diabetic gastroparesis with oral clonidine. Aliment Pharmacol Ther. 1995;9:179–183. doi: 10.1111/j.1365-2036.1995.tb00368.x. [DOI] [PubMed] [Google Scholar]
- 15.Park J, Forrest J, Kolesar R, Bhola D, Beattie S, Chu C. Oral clonidine reduces postoperative PCA morphine requirements. Can J Anaesth. 1996;43:900–906. doi: 10.1007/BF03011802. [DOI] [PubMed] [Google Scholar]


