Abstract
Recent evidence indicates the potential role of vitamin D in the prevention of Metabolic syndrome (MetSyn). This is an analytical cross sectional study. A total of 3275 subjects were investigated. 25-hydroxyvitamin D(25[OH]D) was detected by electrochemiluminescence immunoassay (ECLIA) technology. Metabolic syndrome was defined according to the definition of International Diabetes Federation (IDF). Among the participants, the prevalence of the MetSyn was 6.0%. The prevalence of vitamin D deficiency and insufficiency was 50.1% and 25.0% respectively. Subjects with MetSyn presented with significantly lower 25(OH)Vit D serum levels compared with non-MetSyn group. The results shows that vitamin D deficiency is common in Chinese adults, and subjects with lower serum 25(OH)D have a higher risk of the MetSyn. The cut-off value of serum 25(OH)D that reflected MetSyn in Chinese adluts was 15.655 ng/mL.
Keywords: Vitamin D deficiency, metabolic syndrome, cardiovascular risk
Introduction
Metabolic syndrome (MetSyn) is a constellation of CVD risk factors, i.e. abdominal obesity, atherogenic dyslipidemia (high triglycerides and reduced high density lipoprotein cholesterol [HDL-c]), disturbed carbohydrate metabolism, elevated blood pressure, along with a prothrombotic and proinflammatory profile [1]. In recent years, there have been marked advances in our understanding of the potential role ofvitamin D in the prevention of MetSyn and cardiovascular disease (CVD).
Vitamin D, obtained largely from sunlight exposure and to a lesser extent from dietary and supplemental sources, increases the efficiency of intestinal calcium absorption. Hypovitaminosis D is associated with increased bone turnover, osteoporosis, osteomalacia, and an increased risk of fracture [2-4]. More recent evidence from several lines of research has suggested nontraditional roles for vitamin D in conditions which are frequently observed with MetSyn, including reduced insulin secretion and sensitivity [5,6], obesity [7], diabetes [8-11], and hypertension [12]. Data from laboratory studies [13], ecologic studies [14], epidemiologic investigations [15-18] and secondary analyses of small randomized trials [4] suggest a protective effect for vitamin D against CVD. Systematic reviews [19,10,21] suggest a possible inverse association between Vitamin D and cardiovascular risks. In a meta-analysis by Parkera et al [22], individuals with the highest levels of serum vitamin D were associated with a 43% reduction in cardio-metabolic disorders (OR 0.57, 95% CI: 0.48-0.68).
The purpose of our study was to examine the vitamin D status and the cross-sectional associations of vitamin D status with MetSyn in a large population-based cohort of Chinese adults. In this study, we also try to find the appropriate predictive cut-off value for 25-hydroxyvitamin D(25[OH]D) reflecting MetSyn in Chinese adults.
Material and methods
Ethics statement
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the ethics committee of Chinese PLA General Hospital (S2014-076-01). Written informed consent was obtained from all subjects.
Subjects
A cross-sectional study was conducted of 3330 adults aged 21-97 years who were arranged for an annual regular check-up from March to May 2012 in Chinese PLA general hospital in Beijing, which is located at the latitude of 39°9”N. Participants were excluded if they received glucocorticoid, antivulsant within one year, had severe liver or kidney insufficiency, had overt hyperparathyroidism. Totally there were 3275 adults included in the present study.
Assessments
A standard questionnaire was used by trained interviewers to collect information on age, sex, smoking, alcohol drinking and self-reported diabetes, hypertension, and dyslipidemia. Resting blood pressure was measured. Anthropometric measurements such as weight, height and waist circumference were also taken. Body height was measured to the nearest 0.5 cm and body weight to the nearest 0.1 kg. The waist and hip circumferences were measured with a circumference measurement tape. The waist was defined as the point midway between the iliac crest and the costal margin (lower rib) [23]. All measurements were conducted by trained staff.
Biochemistry
All biochemical analysis was conducted by the Clinical Diagnostic Laboratory of the Chinese PLA general hospital. Vitamin D adequacy was evaluated by measuring serum 25(OH)D concentration. This serum concentration of 25(OH)D is a good reflection of cumulative exposure to sunlight and dietary intake of vitamin D, and is widely regarded as a robust “gold standard” indicator of vitamin D status [24]. The biochemical test used was the Roche 25 OH Vitamin D TOTAL Assay. This assay used electrochemiluminescence immunoassay (ECLIA) technology for the quantitative determination of 25(OH)D. Cut-off values for serum 25(OH)Vit D levels in adults include: >30 ng/ml for vitamin D sufficiency, 20-30 ng/ml for insufficiency and <20 ng/ml for deficiency. This definition was based on the fact that the serum iPTH will rise significantly once the serum 25(OH)D level drops to less than 30 ng/ml [25]. Analyses for cholesterol, HDL-C and triglycerides were performed using commercially available enzymatic methods. Fasting plasma glucose (FPG) was measured using standard hexokinase enzymatic assays. All of the intra- and inter-assay coefficients of variation were <10%.
MetSyn
MetSyn was defined according to the definition of International Diabetes Federation (IDF) [26]. Participants were classified as having MetSyn if they had central obesity (waist circumference ≥90 cm for South Asian Men or ≥80 cm for South Asian women) and had two or more of the following four components: ≥ triglycerides ≥1.7 mmol/l or receiving drug treatment; 2) HDL-C <1.03 mmol/l in men, or <1.29 mmol/l in women; 3) systolic blood pressure (SBP) ≥130 mmHg or diastolic blood pressure (DBP) ≥85 mmHg or use of antihypertensive medication; 4) FPG ≥5.6 mmol/l or receiving drug treatment.
Statistical analysis
Data was entered and analyzed using SPSS for Windows version 19.0 (Somers, NY, USA). Categorical variables were presented as frequencies and percentages while quantitative variables were presented as mean ± standard deviation (SD) or 95% CI where appropriate. Differences between groups were analyzed using one way analysis of variance or independent samples t-test for continuous data and Chi square test for categorical data. A logistic regression model was used to evaluate the odds ratios (ORs) and 95% CIs of having MetSyn for each group of 25(OH)D compared with the highest group with adjustment for potential confounders, such as age, sex, BMI, waist circumference, FPG, triglyceride, HDL-c, LDL cholesterol (LDL-c), SBP and DBP. A Receiver operating characteristic (ROC) curve was designed to identify the best cut-off value of serum 25(OH)D for indicating MetSyn. The sensitivity and specificity for prediction of MetSyn were calculated. The ROC curve was plotted using the sensitivity as the Y-axis and false-positive rate (1-specificity) as the X-axis. The best possible cut-off point was defined as the highest Youden index (specificity+sensitivity-1). All P values were 2-tailed, and p-value <0.05 was considered to indicate statistical significance.
Results
The study population consisted of 85.53% males and 14.47% females. The mean age of the sample was 60.2 y. Of the 3275 subjects, the prevalence of MetSyn was 6.0%, and the prevalence of vitamin D deficiency and insufficiency was 50.1% and 25.0% respectively.
Characteristics of the study population by MetSyn status
The clinical and laboratory characteristics of study participants are shown in Table 1. As anticipated, subjects with MetSyn exhibited significantly elevated weight, BMI, waist circumference, SBP, DBP, triglycerides, total cholesterol, LDL-C and FPG, but lower HDL-C compared with non-MetSyn group. Importantly, the prevalence of Vitamin D deficiency was significantly higher in those with MetSyn compared with those without MetSyn (82.07% compared with 60.5%, P<0.001). Subjects with MetSyn presented with significantly lower 25(OH)Vit D serum levels compared with non-MetSyn group (15.13±6.23 ng/ml vs. 19.48±8.62 ng/ml, P<0.0001) (Table 1).
Table 1.
Characteristics of the study population by Metsyn status
| ALL | Metsyn | Non-Metsyn | P value | |
|---|---|---|---|---|
| N | 3275 | 184 | 3091 | |
| Sex n (%) | ||||
| Men | 2801 (85.53) | 154 (83.70) | 2647 (85.64) | 0.4674 |
| Women | 474 (14.47) | 30 (16.30) | 444 (14.36) | |
| Age (years), mean (SD) | 60.17 (19.68) | 47.00 (12.58) | 60.96 (19.75) | <.0001 |
| BMI (kg/m2), mean (SD) | 24.77 (3.10) | 28.24 (2.85) | 24.49 (2.95) | <.0001 |
| Waist circumference (cm), mean (SD) | 83.65 (8.97) | 93.70 (5.71) | 81.72 (8.16) | <.0001 |
| SBP (mmHg), mean (SD) | 129.23 (16.44) | 140.51 (17.67) | 128.20 (15.94) | <.0001 |
| DBP (mmHg), mean (SD) | 76.93 (10.72) | 86.85 (9.64) | 76.02 (10.35) | <.0001 |
| Triglyceride (mmol/L), mean (SD) | 1.45 (1.01) | 2.47 (1.79) | 1.39 (0.91) | <.0001 |
| Total Cholesterol (mmol/L), mean (SD) | 4.75 (0.96) | 5.14 (0.98) | 4.72 (0.95) | <.0001 |
| LDL-c (mmol/L), mean (SD) | 2.92 (0.82) | 3.13 (0.79) | 2.91 (0.82) | <.0001 |
| HDL-c (mmol/L), mean (SD) | 1.34 (0.36) | 1.11 (0.28) | 1.35 (0.35) | <.0001 |
| FPG (mmol/L), mean (SD) | 5.51 (1.11) | 5.80 (1.57) | 5.49 (1.07) | 0.0006 |
| 25(OH)D (ng/ml), mean (SD) | 19.24 (8.56) | 15.13 (6.23) | 19.48 (8.62) | <.0001 |
| Vitamin D deficiency n (%) | 2021 (61.71) | 151 (82.07) | 1870 (60.50) | <.0001 |
| Vitamin D insufficiency n (%) | 893 (27.27) | 27 (14.67) | 866 (28.02) | |
| Vitamin D sufficiency n (%) | 361 (11.02) | 6 (3.26) | 355 (11.48) |
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL-c, low density lipoprotein cholesterol; HDL-c, high density lipoprotein cholesterol; FPG, fasting plasma glucose.
MetSyn status of the study population according to 25(OH)D level
We categorized the subjects using the commonly used cut-off points of serum 25(OH)D (as described in methods) into four groups [25(OH)D <10 ng/ml, 10 ng/ml ≤25(OH)D <20 ng/ml, 20 ng/ml ≤25(OH)D <30 ng/ml, 25(OH)D >30 ng/ml] (Table 2). The proportion of subjects in the lowest 25(OH)D group was significantly higher for females than for males (P<0.001). The proportion of subjects with MetSyn decreased significantly with the increase of 25(OH)D (P<0.001 for chi-squared statistic).
Table 2.
MetSyn status of the study population according to 25(OH)D groups
| 25(OH)D (ng/ml) | P value | ||||
|---|---|---|---|---|---|
|
|
|||||
| <10 (n, %) | 10 to 20 (n, %) | 20 to 30 (n, %) | ≥30 (n, %) | ||
| Metsyn (n=182) | 30 (16.5) | 121 (66.5) | 25 (13.7) | 6 (3.2) | <.0001 |
| Non-Metsyn (n=3083) | 275 (8.9) | 1595 (51.7) | 858 (27.8) | 355 (11.5) | |
Risk of MetSyn according to 25(OH)D level
The risk of having MetSyn increased progressively across the highest to the lowest groups of 25(OH)D. As shown in Table 3, after adjustment for potential factors (age, sex, BMI, waist circumference, FPG, triglyceride, HDL-c, LDL-c, SBP and DBP), the multivariable adjusted OR for MetSyn for the lowest 25(OH)D group (<10 ng/ml) compared with the highest 25(OH)D group (>30 ng/ml) was 3.14 (95% CI: 1.17-8.43, P = 0.023).
Table 3.
Unadjusted and adjusted ORs and 95% CI from logistic regression predicting the likelihood of having MetSyn for each groups of plasma 25(OH)D concentration
| 25(OH)D (ng/ml) | Number of patients | Number of Metsyn | Incidence of Metsyn (%) | Unadjusted-OR (95% CI) | Adjusted OR (95% CI)* | P-value |
|---|---|---|---|---|---|---|
| <10 | 305 | 30 | 9.84 | 6.454 (2.649-15.726) | 3.137 (1.168-8.424) | 0.0233 |
| 10 to 20 | 1716 | 121 | 7.05 | 4.488 (1.962-10.271) | 2.317 (0.946-5.673) | 0.0659 |
| 20 to 30 | 883 | 25 | 2.83 | 1.724 (0.701-4.238) | 1.178 (0.452-3.074) | 0.7373 |
| ≥30 | 361 | 6 | 1.66 | 1 | 1 |
Adjusted ORs: adjusted for age, sex, BMI, waist circumference, FPG, triglyceride, HDL-C, LDL-C, SBP and DBP.
The ROC curve reflecting MetSyn from serum 25(OH)D is shown in Figure 1. The AUC of serum 25(OH)D for the prediction of MetSyn was 0.662 (95% CI, 0.624-0.700; P<0.001). The best cut-off value of serum 25(OH)D for indicating MetSyn, according to the maximum of the Youden index, was 15.655 ng/mL. Sensitivity and specificity were 67.0% and 39.0%, respectively.
Figure 1.
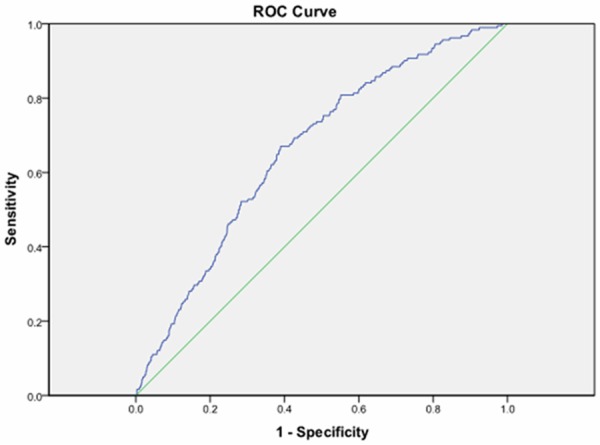
Reciever operating characteristic curve of serum 25(OH)D reflecting MetSyn. The cut-off value of serum 25(OH)D was 15.655 ng/ml (area under the curve = 0.662; 95% CI,0.624-0.700; P<0.001, sensitivity 67.0%, specificity 39.0%).
Discussion
There are alarming rates of low serum 25(OH)D levels among seemingly healthy populations. In Asia, generally low serum 25(OH)D concentrations have been reported. Our results showed that there were 61.71% subjects with vitamin D deficiency in this population, and the mean serum 25(OH)D was 19.24±8.56 ng/ml. These results are comparable with a population-based cross-sectional study on middle-aged and elderly Chinese population, which showed that their average 25(OH)D level was 16.19 ng/mL with deficiency proportion of 69.2% [27]. In Beijing, China, mean serum 25(OH)D in adolescent girls was 12.0-14.4 ng/ml [28]. In Japan, a low mean serum 25(OH)D of 13.6 ng/ml was observed in women younger than 30 years and 12 ng/ml in immobile older persons [29]. In the Korea National Health and Nutrition Examination Survey (KNHANES), Vitamin D insufficiency (<20 ng/ml) was found in 47.3% males and 64.5% females [30]. Modern life styles reduce UVB exposure, due to clothing, working and exercising indoors. Air pollution blocks UVB radiation and we increasingly avoid sunshine and use sunscreens to avoid skin aging and skin cancers. People live longer and skin synthesis and gut absorption of vitamin D fall with age. This has raised several health concerns with regard to the relationship of the low vitamin D levels and the disease consequences of serum 25(OH)D level alterations in these populations. Recently, vitamin D receptors are found in most tissues and cells in the body, which opened a whole new area of research. Since many of the factors leading to increased MetSyn risk also affect vitamin D status, the study on the role of vitamin D in determining MetSyn risks became quite popular.
The large NHANES III and NHANES 2003-2004 have shown a significant inverse association between serum 25(OH)Vit D concentration and MetSyn [31,32]. Our results are in accordance with these studies showing that subjects with MetSyn have significantly lower 25(OH)Vit D levels compared with non-MetSyn (15.13±6.23 vs. 19.48±8.62, P<0.0001). In the MetSyn group, 82.07% of the people suffered from vitamin D deficiency. A possible explanation for this observation could be that vitamin D is fat soluble and is readily stored in adipose tissue and it could be sequestered in the larger body pool of fat of obese individuals [33], since obesity is quite common in MetSyn subjects. Interestingly, vitamin D may also play a role in the maintenance of a healthy body weight, as women randomized to calcium and vitamin D in the Women’s Health Initiative clinical trial, experienced less weight gain compared to women on placebo [34].
Our results showed that age was positively associated with vitamin D status, where older participants had higher vitamin D levels. However, this finding concurs with that from the study among Malay adults [35] and the national study from Korea [30], which found Vitamin D insufficiency most prevalent in the relatively younger age groups. The reason for this result remains unclear. Possibly, the older subjects in this study was not representative of the general population because they were with high education, better income, and maybe they spend more time outdoors and usually pay more attention to their health, while younger people sit in their cars and offices. Maybe low levels of physical activity is the reason for increased body weight, dyslipidemia, hypertension and high blood glucose, and also for lower 25(OH)D concentrations. Further studies are needed to investigate whether there are some other more logical reasons for this paradoxical finding.
Over 40 studies show inverse correlations of vitamin D status with MetSyn risk or with the incidence or severity of its components [36]. Our results are in accordance with these studies showing that 25(OH)D concentrations were inversely associated with MetSyn risk, and subjects with 25(OH)D levels <10 ng/mL had a more than 3-fold higher prevalence of MetSyn compared to those with 25(OH)D levels ≥30 ng/mL after adjustment. In the Canadian Health Measures Survey [37], OR for MetSyn = 0.5, but 95% CI, 0.24-1.06, for those in the highest quartile of 25(OH)D vs. the rest. Inverse associations of MetSyn risk with 25(OH)D reported in the Netherlands (n = 1286 community dwelling subjects aged 65-88 y [mean 25(OH)D = 53.5 nmol/l and 47.8% subjects <50 nmol/L], gave an adjusted OR for MetSyn for lowest vs. highest strata of 25(OH)D of 1.54 (95% CI 1.23-1.94) [38].
Our study showed that the OR for the MetSyn increased abruptly in subjects with 25(OH)D decreasing. The predictive cut-off value for 25(OH)D reflecting MetSyn was 15.66 ng/ml (sensitivity = 0.670, specificity = 0.390). To our knowledge, this is the first study that has performed a ROC curve in investigating the effect of 25(OH)D levels on METS, and find a cut-off point for 25(OH)D to predict MetSyn, and vitamin D supplementation maybe necessary at this level.
Our study has some limitations, it was just cross-sectional and lack of life style data, further prospective, randomized placebo controlled trials are quite important. We also did not measure the parathyroid hormone and additional markers of islet β cell function or inflammation markers, which may have provided more information of MetSyn. Strengths of this study include the large cohort and the assessment of a cutoff point of 25(OH)D to detect MetSyn, in which level subjects should consider vitamin D supplementation.
Vitamin D deficiency is common in Chinese adults, and subjects with lower serum 25 (OH)D have a higher risk of the MetSyn. The cut-off value of serum 25(OH)D that reflected MetSyn in Chinese adluts was 15.655 ng/mL, and vitamin D supplementation should be considered at this level.
Acknowledgements
This research was supported by Healthcare Scientific Research Foundation of the Army of China to C.L.L. (No: 12BJZ41), Scientific Rese-arch Foundation of Capital Medical Develo-pment of China to Y.P. (No: 2007-3029), Miao Pu Fund of Chinese PLA General Hospital (Y.H.L. grant numbers: 12KMM41), and Scientific Support Fund of Chinese PLA General Hospital (Y.H.L. grant numbers: No: 2014FC-TSYS-1008).
Disclosure of conflict of interest
None.
References
- 1.Liberopoulos EN, Mikhailidis DP, Elisaf MS. Diagnosis and management of the metabolic syndrome in obesity. Obes Rev. 2005;6:283–296. doi: 10.1111/j.1467-789X.2005.00221.x. [DOI] [PubMed] [Google Scholar]
- 2.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 3.Saquib N, von Muhlen D, Garland CF, Barrett-Connor E. Serum 25-hydroxyvitamin D, parathyroid hormone, and bone mineral density in men: the Rancho Bernardo study. Osteoporos Int. 2006;17:1734–1741. doi: 10.1007/s00198-006-0191-1. [DOI] [PubMed] [Google Scholar]
- 4.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326:469–475. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79:820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 6.Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209:823–825. doi: 10.1126/science.6250216. [DOI] [PubMed] [Google Scholar]
- 7.Bell NH, Epstein S, Greene A, Shary J, Oexmann MJ, Shaw S. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest. 1985;76:370–373. doi: 10.1172/JCI111971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 9.Isaia G, Giorgino R, Adami S. High prevalence of hypovitaminosis D in female type 2 diabetic population. Diabetes Care. 2001;24:1496. doi: 10.2337/diacare.24.8.1496. [DOI] [PubMed] [Google Scholar]
- 10.Scragg R, Holdaway I, Singh V, Metcalf P, Baker J, Dryson E. Serum 25-hydroxyvitamin D3 levels decreased in impaired glucose tolerance and diabetes mellitus. Diabetes Res Clin Pract. 1995;27:181–188. doi: 10.1016/0168-8227(95)01040-k. [DOI] [PubMed] [Google Scholar]
- 11.Scragg R, Sowers M, Bell C Third National Health and Nutrition Examination Survey. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 12.Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 13.Manson JE, Bassuk SS. Vitamin D and cardiovascular disease. Menopause Manage. 2009;18:28–31. [Google Scholar]
- 14.Zittermann A, Schleithoff SS, Koerfer R. Putting cardiovascular disease and vitamin D insufficiency into perspective. Br J Nutr. 2005;94:483–492. doi: 10.1079/bjn20051544. [DOI] [PubMed] [Google Scholar]
- 15.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giovannucci E, Liu Y, Hollis BW, Rimm EB. A prospective study of 25-hydroxyvitamin D and risk of myocardial infarction in men. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 18.Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, Lappé DL, Muhlestein JB Intermountain Heart Collaborative (IHC) Study Group. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. 2010;106:963–968. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 19.Swales HH, Wang TJ. Vitamin D and cardiovascular disease risk: emerging evidence. Curr Opin Cardiol. 2010;25:513–517. doi: 10.1097/HCO.0b013e32833cd491. [DOI] [PubMed] [Google Scholar]
- 20.Grandi NC, Breitling LP, Brenner H. Vitamin D and cardiovascular disease: Systematic review and meta-analysis of prospective studies. Prev Med. 2010;51:228–233. doi: 10.1016/j.ypmed.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Manson JE, Song Y, Sesso HD. Systematic review: Vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med. 2010;152:315–323. doi: 10.7326/0003-4819-152-5-201003020-00010. [DOI] [PubMed] [Google Scholar]
- 22.Parkera J, Hashmia O, Mavrodaris A, Stranges S, Kandala NB, Clarke A, Franco OH. Levels of vitamin D and cardiometabolic disorders: Systematic review and meta-analysis. Maturitas. 2010;65:225–236. doi: 10.1016/j.maturitas.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 23.National Institutes of Health. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults-The Evidence Report. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 24.Springbett P, Buglass S, Young AR. Photoprotection and vitamin D status. J Photochem Photobiol B. 2010;101:160–168. doi: 10.1016/j.jphotobiol.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439–443. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 26.Alberti KG, Zimmet P, Shaw J IDF Epidemiology Task Force Consensus Group. The metabolic syndrome-a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 27.Lu L, Yu Z, Pan A, Hu FB, Franco OH, Li H, Li X, Yang X, Chen Y, Lin X. Plasma 25-hydroxyvitamin D concentration and metabolic syndrome among middle-aged and elderly Chinese individuals. Diabetes Care. 2009;32:1278–1283. doi: 10.2337/dc09-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foo LH, Zhang Q, Zhu K, Ma G, Trube A, Greenfield H, Fraser DR. Relationship between vitamin D status, body composition and physical exercise of adolescent girls in Beijing. Osteoporos Int. 2009;20:417–425. doi: 10.1007/s00198-008-0667-2. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura K, Nashimoto M, Matsuyama S, Yamamoto M. Low serum concentrations of 25-hydroxyvitamin D in young adult Japanese women: a cross sectional study. Nutrition. 2001;17:921–925. doi: 10.1016/s0899-9007(01)00662-1. [DOI] [PubMed] [Google Scholar]
- 30.Choi HS, Oh HJ, Choi H, Choi WH, Kim JG, Kim KM, Kim KJ, Rhee Y, Lim SK. Vitamin D Insufficiency in Korea-A Greater Threat to Younger Generation: The Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J Clin Endocrinol Metab. 2011;96:643–651. doi: 10.1210/jc.2010-2133. [DOI] [PubMed] [Google Scholar]
- 31.Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U. S. adults. Diabetes Care. 2005;28:1228–1230. doi: 10.2337/diacare.28.5.1228. [DOI] [PubMed] [Google Scholar]
- 32.Reis JP, von Muhlen D, Miller ER 3rd. Relation of 25-hydroxyvitamin D and parathyroid hormone levels with metabolic syndrome among US adults. Eur J Endocrinol. 2008;159:41–48. doi: 10.1530/EJE-08-0072. [DOI] [PubMed] [Google Scholar]
- 33.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 34.Caan B, Neuhouser M, Aragaki A, Lewis CB, Jackson R, LeBoff MS, Margolis KL, Powell L, Uwaifo G, Whitlock E, Wylie-Rosett J, LaCroix A. Calcium plus vitamin D supplementation and the risk of postmenopausal weight gain. Arch Intern. 2007;167:893–902. doi: 10.1001/archinte.167.9.893. [DOI] [PubMed] [Google Scholar]
- 35.Moy FM, Bulgiba A. High prevalence of vitamin D insufficiency and its association with obesity and metabolic syndrome among Malay adults in Kuala Lumpur, Malaysia. BMC Public Health. 2011;11:735–741. doi: 10.1186/1471-2458-11-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boucher BJ. Is vitamin D status relevant to metabolic syndrome? Dermatoendocrinol. 2012;4:212–224. doi: 10.4161/derm.20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brenner DR, Arora P, Garcia-Bailo B, Wolever TM, Morrison H, El-Sohemy A, Karmali M, Badawi A. Plasma vitamin D levels and risk of metabolic syndrome in Canadians. Clin Invest Med. 2011;34:E377–384. doi: 10.25011/cim.v34i6.15899. [DOI] [PubMed] [Google Scholar]
- 38.Oosterwerff MM, Eekhoff EM, Heymans MW, Lips P, van Schoor NM. Serum 25-hydroxyvitamin D levels and the metabolic syndrome in older persons: a population-based study. Clin Endocrinol (Oxf) 2011;75:608–613. doi: 10.1111/j.1365-2265.2011.04110.x. [DOI] [PubMed] [Google Scholar]


