Abstract
Methotrexate is a chemotherapeutic agent used for many cancer treatments. It leads to toxicity with its oxidative injury. The purpose of our study is investigating the medical ozone preconditioning and treatment has any effect on the methotrexate-induced kidneys by activating antioxidant enzymes in rats. Eighteen rats were divided into three equal groups; control, Mtx without and with medical ozone. Nephrotoxicity was performed with a single dose of 20 mg/kg Mtx intraperitoneally at the fifteenth day of experiment on groups 2 and 3. Medical ozone preconditioning was performed at a dose of 25 mcg/ml (5 ml) intraperitoneally everyday in the group 3 and treated with medical ozone for five more days while group 2 was received only 5 ml of saline everyday for twenty days. All rats were sacrificed at the end of third week and the blood and kidney tissue samples were obtained to measure the levels of TNF-α, IL-1β, malondialdehyde, glutathione and myeloperoxidase. Kidney injury score was evaluated histolopatologically. Medical ozone preconditioning and treatment ameliorated the biochemical parameters and kidney injury induced by Mtx. There was significant increase in tissue MDA, MPO activity, TNF-α and IL-1β (P<0.05) and significant decrease in tissue GSH and histopathology (P<0.05) after Mtx administration. The preconditioning and treatment with medical ozone ameliorated the nephrotoxicity induced by Mtx in rats by activating antioxidant enzymes and prevented renal tissue.
Keywords: Kidney, medical ozone, preconditioning, methotrexate, nephrotoxicity
Introduction
Methotrexate (mtx) analogue of folic acid is an effective cytotoxic chemotherapeutic drug and has been widely used in malignancies [1,2]. According to the organ toxicity due to mtx should be the reason for the delay in the treatment or dose reduction. Thus, many antioxidant agents such as alpha lipoic acid [3] and pentoxyfilline [4] were used in studies aiming to prevent Mtx-induced damage.
Medical Ozone is described as three atom molecules of oxygen known as O3. Ozone is applied in medical therapy using a gas mixture composed of oxygen and ozone. Repeated ozone administration also known as preconditioning at nontoxic doses provide an adaptation of tissues to oxidative stress by induction of enzymes or activating the metabolic pathways maintaining an equilibrated redox balance such as increase on GSH levels and decrease on lipid peroxidation [5].
Furthermore the effect of O3 preconditoning and treatment on Mtx induced nephrotoxicity has not been studied and reported elsewhere before. In this present study we aimed to evaluate whether O3 preconditioning and treatment has any effects on Mtx-induced nephrotoxicity in rats.
Material and methods
The experimental procedures were reviewed and approved by Akdeniz University Local Committee on Animal Research Ethics (2015.02.07).
Animals and study groups
Eighteen male Wistar albino rats weighing 250-300 g were used in this study. They were randomly divided into three equal groups: control group (n=6) (rats received 5 ml of saline everyday before and after Mtx administration for twenty days), Mtx administered (n=6) (rats received 5 ml of saline everyday for twenty days) and the Mtx administered and ozone group (n=6) (rats preconditioned and treated with O3/O2 mixture before and after Mtx administration).
Experimental design and groups
The rats maintained at a 22°C constant temperature with a 12-h light/dark cycle and humidity at 60% and food and water available ad libitum. Experiments were conducted in accordance with the ethical guidelines for investigations in laboratory animals and were approved by the Ethical Committee for Animal Experimentation of Akdeniz University. Group 1 was the control group receiving only physiological saline everyday for three weeks. Group 2 was the Methotrexate group receive a single 20 mg/kg dose of Mtx (Onco-Tain; Faulding Pharmaceutics Plc, Leamington Spa, UK) intraperitoneally at the sixteenth day for induce nephroxicity. Group 2 received 5 ml saline intraperitoneally every day for twenty days. Group 3 was the Mtx-medical ozone group preconditioned with 5 ml medical ozone intraperitoneally for fifteen days at dose of 25 mcg/ml and received a single dose of Mtx at the sixteenth day. And the group 3 treated with intraperitoneal ozone administration everyday at the same dose for five days more. Ozone (O3) was generated by an ozone generator (OM-302, Ozone generator, Sedecal, Spain) with a mixture of 97% O2 and 3% O3. The volume of the injected mixture was approximately 5 ml. The ozone flow-rate was kept constant at 3 L/min and tygon polymer tubes and ozone resistant single use silicon-treated polypropylene syringes were used to ensure the containment of O3 and consistency of concentration.
Sample collection
At the end of the twenty first day, sixth day after Mtx administration the rats were sacrified with decapitation after ether anesthesia and the blood samples and kidney tissues immediately obtained and one portion was stored at -70°C until assayed for tissue antioxidant enzymes. The levels of tumour necrosis factor-alpha (TNF-α) and interleukin-1-beta (IL-1β) from blood and the levels of malondialdehyde (MDA), glutathione (GSH) and myeloperoxidase (MPO) from kidney tissue samples were analysed. The rest of the portions were placed in 10% buffered formaldehyde solution for histopathological analysis and the kidney tissue injury score was performed by an expert pathologist under a light microscope.
Analysis of TNF-α and IL-1β
By the enzyme-linked immunosorbent assay (ELISA) kits (Biosource International, Nivelles, Belgium) the TNF-α and the IL-1β from plasma were analysed.
Measurement of MDA and GSH levels
The MDA levels (nmol MDA/g tissue) were assayed for the products of lipid peroxidation using the method reported by Buege [6]. The GSH levels (nmol GSH/g tissue) were measured using the method of Elman [7]. GSH is reacted with 5,5-ithiobis-2-nitrobenzoic acid resulting in the formation of a product which has a maximal absorbance at 410 nm.
Measurement of MPO activity
According to the Hillegas et al. [8] procedure tissue-associated MPO (U MPO/g tissue) activity was measured from the kidney tissue samples homogenized in 50 mm potassium phosphate buffer (PB, pH 6.0) which homogenates were centrifuged at 41 400 g for 10 min; pellets were suspended in 50 mm PB containing 0.5% hexadecyltrimethylammonium bromide. After three cycles of freezing and thawing, with the sonication between the cycles, kidney samples were centrifuged at 41 400 g for 10 min. Volumes of 0.3 ml were added to 2.3 ml of reaction mixture containing 50 mm PB, o-dianisidine, and 20 mm H2O2 solution. One unit of enzyme activity was defined as the amount of MPO that caused a change in the absorbance measured at 460 nm for 3 min.
Histopathological analysis of kidney tissue
Kidney specimens taken from the rats at the end of the experiment were fixed in 10% buffered formaldehyde. One horizontal section from each kidney was embedded in paraffin, cut at 3 micrometers and stained with hematoxylin-eosin (H&E) and Periodic Acid-Schiff (PAS). Histopathological analysis was performed by an expert pathologist who was blinded to the experiment. For each tissue, semiquantitative scales used in the kidney were as follows; degeneration of glomerular structures, glomerular congestion, dilatation of Bowman’s space, degeneration of proximal tubuli, degeneration of distal tubuli, tubular basal membrane wrinkling, vascular congestion, interstitial edema, inflammation and cell infiltration. The whole microscopic area was examined to score each specimen. Tissue injury was scored using a scoring system. Scores were given as 0 = none, 1 = mild, 2 = moderate and 3 = severe [9].
Statistical analysis
All statistical analysis was performed by GraphPad Prism 3.0 (GraphPad Software, San Diego, USA). The data were expressed as mean ± standard deviation. Group comparisons were performed with the analysis of variance (ANOVA) followed by Tukey’s tests. The P<0.05 was considered as statistically significant.
Results
No rats were died during the experimental period. After sacrification of the rat kidney were normal in appearence in the groups 1 and 3 macroscopically. However, significant differences have seen in oxidative stress parameters and histopathology. The MDA, GSH, MPO, TNF-α and IL-1β levels in kidneys are shown in Table 1.
Table 1.
The effects of medical ozone on MDA and GSH levels as well as MPO activities in kidney tissue, plasma TNF-α and IL-1β levels that are exposed to Mtx induced nephrotoxicity. Each group was consisting of 6 rats. Groups of data were compared with an analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests
| Control | Mtx | Mtx-Ozone | |
|---|---|---|---|
| MDA (nmol/g tissue) | 26.34±2.78 | 62.46±4.19** | 32.57±3.26++ |
| GSH (nmol/g tissue) | 1675.24±89.24 | 987.62±73.69** | 1852.47±153.64++ |
| MPO (U/g tissue) | 17.58±3.62 | 38.72±2.92*** | 23.45±1.97*,+++ |
| TNF-α (pg/ml) | 8.7±0.9 | 27.2±3.8*** | 10.1±2.2+++ |
| IL-1β (pg/ml) | 9.5±1.2 | 23.4±1.8** | 11.4±1.7++ |
Data are mean ± sd.
P<0.01 compared to control group;
P<0.001 compared to control group;
P<0.0001 compared to control group.
+ P<0.01 compared to Mtx group;
P<0.001 compared to Mtx group;
P<0.0001 compared to Mtx group.
In kidney tissues MDA, MPO, TNF-α and IL-1β levels were found significantly increased in the Mtx group while GSH levels dereased as compared to both control and Mtx + O3 groups.
After Mtx administration major degradation product of lipid peroxidation; MDA levels is a seen as increased when compared with the control group (P<0.001), while preconditioning and treatment with O3 to the Mtx group came back to control MDA levels (P<0.001).
The major cellular antioxidant GSH levels of kidney samples in Mtx group were found lower significantly (P<0.001). On the other hand, preconditioning and treatment with O3 to Mtx group restored the GSH levels to control levels in all kidney samples (P<0.001).
Indicator of neutrophil infiltration Myeloperoxidase (MPO) has higher activity in the kidney samples of the Mtx group when compared to control (P<0.0001). On the other hand, preconditioning and treatment with O3 in Mtx group significantly decreased all MPO activity level (P<0.001).
When compared to control group, proinflammatory cytokine TNF-α levels were found significantly increased (P<0.0001) in the Mtx group, while this Mtx-induced rise in serum TNF-α level was abolished (P<0.0001) with preconditioning and treatment with O3. Similar results were achieved for IL-1β, IL-1β was also found as increased in the Mtx group when compared to control group (P<0.001). After preconditioning and treatment with O3 following Mtx IL-1β was also decreased significantly back to control levels (P<0.001).
Histopathological evaluation and injury scores of the groups were shown in Table 2. Kidney sections from the control group were normal in histological appearance (Figure 1A). The kidney sections from the Mtx group showed some histopathological changes such as interstitial mononuclear inflammation, shrunken glomerules, athropic tubules, glomerular and tubular basal membrane wrincling (Figure 1B). In this group, kidney sections showed tubuler cell loss and apoptotic cell necrosis (Figure 1C). And ozone preconditioning and treatment have shown moderate histopathological changes (Figure 1D). The histopathological damage score in the Mtx + O3 group was found significantly reduced in comparison to the control and Mtx groups (P<0.05).
Table 2.
The degree of histopathologic renal damage was expressed as medians within each section of groups were shown
| Control | Mtx | Mtx-Ozone | |
|---|---|---|---|
| Degeneration of glomerular structures | 0 | 1 (1-2) | 0 (0-1) |
| Glomerular congestion | 0 | 2 (1-2) | 1 (0-1) |
| Dilatation of Bowman’s space | 0 | 2 (1-2) | 1 (0-1) |
| Degeneration of proximal tubuli | 0 | 2 (1-3) | 1 (0-2) |
| Degeneration of distal tubuli | 0 | 1 (1-2) | 1 (0-1) |
| Tubular basal membrane wrinkling | 0 | 2 (0-2) | 1 (0-1) |
| Vascular congestion | 0 | 1 (0-1) | 1 (0-1) |
| Interstitial edema | 0 | 1 (0-1) | 1 (0-1) |
| Inflammation and cell infiltration | 0 | 2 (1-2) | 1 (0-1) |
| Total score | 0 | 14 | 8 |
Figure 1.
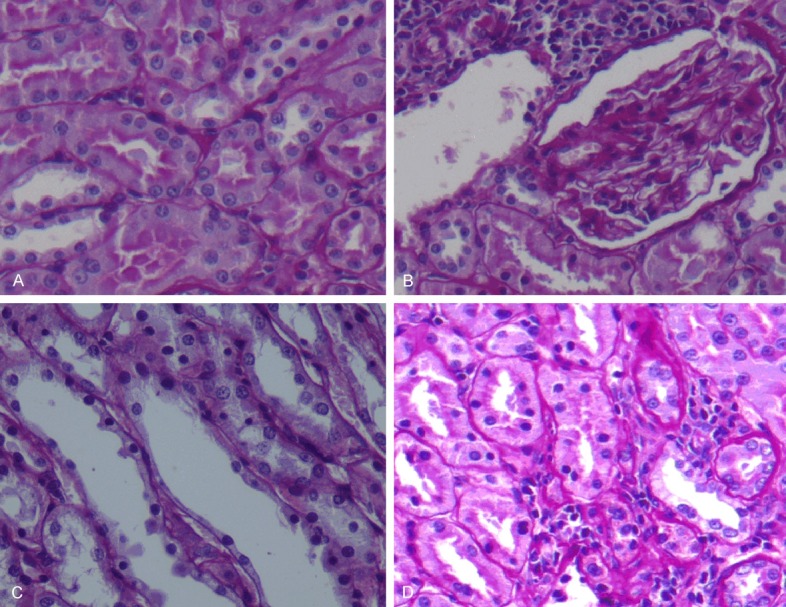
(A) Control group, (B, C) Mtx group, (D) Mtx-O3 group. (A) Control group shows normal kidney architecture. (B) In Mtx group interstitial mononuclear inflammation was seen also note that glomerules are shrunken and tubules are athropic. Glomerular and tubular basal membrane wrincling is seen. (X20, PAS). (C) Tubular cell loss; several tubular cell nuclei are not seen along this tubule. Also not picnotic nucleus reflects apoptotic cell necrosis. (X40, PAS). (D) Moderate degeneration on glomerulus and tubules was seen.
Discussion
Because of their larger perfusion and excretion capacity of compounds at increased concentrations kidneys are easily susceptible to damage from drugs that occur in renal tubular cells during absorption and secretion. Methotrexate (mtx) analogue of folic acid is an effective cytotoxic chemotherapeutic drug and has been widely used in many malignancies [1,2].
Mtx is an antimetabolite that competitively inhibits the dihydrofolate reductase on tetrahydrofolate synthesis of folic acid resulted with the impairment of synthesis of purine end pyrimidine thus DNA [10]. In patients receiving Mtx treatment, the risk of kidney toxicity is 2% [11]. Kidney toxicity due to Mtx treatment can occur both at low or high doses. High doses of Mtx make kidney damage with two ways tubular injury with the precipitation of Mtx in kidney tubules and decrease on the glomerular filtration rate [10]. It can mostly be ameliorated with hydration and make the urine alkaline.
In this study we aim to investigate the protective effect of medical ozone on kidney tissues from their oxidative damage induced by Mtx. Antioxidant enzymes activated by medical ozone prevented lipid peroxidation and neutrophil infiltration of the kidney tissues of Mtx-induced nephrotoxicity in rats. Furthermore, ozone preconditioning and treatment reduced the plasma cytokines and improved the kidney histopathological changes caused by Mtx.
Ozone-therapy has beneficial effects in various diseases, such as caustic esophageal burns, diabetic foot and radiation induced cystitis [12-14]. It can be used by topical, rectal, peritoneal (in animal studies) or oral (ozonated water or oil) routes. The term “O3 preconditioning” refers to the repeated application of ozone in order to increase antioxidant enzymes and keep the organism alert, as in vaccination. Ozone preconditioning acts to protect against cellular damage by free radicals [5].
Thus, O3 used in our study protected kidneys against Mtx-induced nephrotoxicity.
Ozone preconditioning can produce protection against renal ischemia reperfusion injury [15]. A controlled number and dose of ozone treatments conferred protection against different physiopathological processes mediated by ROS was reported [5].
In a previous study it was reported that ozone exposure altered the levels of inflammatory cytokines, such as tumor necrosis factor [16], transforming growth factor [17], interferon [18] and interleukin [19]. In our study systemic inflammatory response indicators; plasma TNF-α and IL-1β levels were also found increased due to Mtx administration but decreased by ozone preconditioning and treatment.
Lipid peroxidation by free oxygen radicals is an important causes of destruction and oxidative damage to cell membranes these containing unsaturated fatty acids, nucleic acids and proteins. It can resulted with methotrexate induced tissue damage [6,20]. The decreasing on tissue MDA levels revealed that ozone preconditioning and treatment prevented kidney tissue from oxidative damage.
At previous studies reported that stimulation of antioxidant enzyme activities such as glutathione by ozone prepared the tissue against the effects of reactive oxygen species [21,22]. GSH plays a particularly important role in the maintenance and regulation of the thiol-redox status of the cell [23]. Tissue GSH depletion is one of the primary factors permitting kidney tissue damage is associated with oxidative stress caused by Mtx in our study. O3 was previously reported to increase GSH levels in erythrocytes and it may show the same action in hepatocytes, thus increasing the detoxification capacity of these cells [24]. In our study preconditioning and treatment with ozone reversed the levels of GSH into normal levels.
The leukocytes accumulation in tissue triggered by free oxygen radicals and the enzymes (myeloperoxidase, protease and elastase) secretion activated from neutrophils thus leads to further tissue damage. In production of oxidants by neutrophils MPO plays a great role [25,26]. Oxidative stress and infiltration with polimorphonuclear neutrophils may also play a role in Mtx-induced kidney injury [27]. In our study an index of polimorphonuclear leukocyte infiltration MPO level is seen to be increased. This indicates that contribution of leucocytes induced oxidative injury in kidney tissues. Preconditioning and treatment with O3 decreased the MPO activity
According to literature search, this is the first study investigating the effect of medical ozone preconditioning and treatment on experimental study of Mtx-induced nephrotoxicity in rats. Findings from our study revealed that kidney injury ameliorated by intraperitoneal medical ozone administration, while with antioxidant effect the ozone prevented the lipid peroxidation and neutrophil infiltration of the rat kidney tissues. The decreasing on tissue MDA levels and MPO activities with an increasing on levels of GSH revealed that ozone preconditioning prevented kidney tissue from oxidative stress.
In kidney, where a cumulative and prolonged effect of Mtx is expected, O3 preserved the kidneys by antioxidant enzymes and O3 preconditioning and treatment also showed protective effects on histopathology. Further studies with O3 in humans are necessary for clinical use in the prevention of Mtx-induced kidney injury.
Conclusion
In conclusion, intraperitoneal preconditioning and treatment with O3 ameliorated the kidney injury induced by methotrexate in rats by activating antioxidant enzymes.
Acknowledgements
We acknowledge to Dr. Başar ŞENYÜZ from Ozonklinik Antalya Turkey for supplying O3.
Disclosure of conflict of interest
None.
References
- 1.Bleyer WA. Methotrexate: clinical pharmacology, current status and therapeutic guidelines. Cancer Treat Rev. 1977;4:87–101. doi: 10.1016/s0305-7372(77)80007-8. [DOI] [PubMed] [Google Scholar]
- 2.Widemann BC, Balis FM, Kempf-Bielack B, Bielack S, Pratt CB, Ferrari S, Bacci G, Craft AW, Adamson PC. High-dose methotrexate-induced nephrotoxicity in patients with osteosarcoma. Cancer. 2004;100:2222–32. doi: 10.1002/cncr.20255. [DOI] [PubMed] [Google Scholar]
- 3.Cakır T, Polat C, Basturk A, Gul M, Aslaner A, Durgut H, Sehirli AO, Aykac A, Bahar L, Sabuncuoglu MZ. The effect of alpha lipoic acid on rat kidneys in methotrexate induced oxidative injury. Eur Rev Med Pharmacol Sci. 2015;11:2132–9. [PubMed] [Google Scholar]
- 4.Asvadi I, Hajipour B, Asvadi A, Asl NA, Roshangar L, Khodadadi A. Protective effect of pentoxyfilline in renal toxicity after methotrexate administration. Eur Rev Med Pharmacol Sci. 2011;15:1003–9. [PubMed] [Google Scholar]
- 5.Leon OS, Menendez S, Merino N, Castillo R, Sam S, Perez L, Cruz E, Bocci V. Ozone oxidative preconditioning: a protection against cellular damage by free radicals. Mediators Inflamm. 1998;7:289–94. doi: 10.1080/09629359890983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–10. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 7.Beutler E. A Manual of Biochemical Methods. New York: Grune & Stratton; 1975. Glutathione in Red Blood Cell Metabolism; pp. 112–4. [Google Scholar]
- 8.Hillegass LM, Griswold DE, Brickson B, Albrightson-Winslow C. Assessment of myeloperoxidase activity in whole rat kidney. J Pharmacol Methods. 1990;24:285–95. doi: 10.1016/0160-5402(90)90013-b. [DOI] [PubMed] [Google Scholar]
- 9.Cetiner M, Sener G, Sehirli AO, Eksioglu-Demiralp E, Ercan F, Sirvanci S, Gedik N, Akpulat S, Tecimer T, Yeğen BC. Taurine protects against methotrexate-induced toxicity and inhibits leukocyte death. Toxicol Appl Pharmacol. 2005;209:39–50. doi: 10.1016/j.taap.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Tian H, Cronstein BN. Understanding the mechanisms of action of methotrexate: implications for the treatment of rheumatoid arthritis. Bull NYU Hosp Jt Dis. 2007;65:168–73. [PubMed] [Google Scholar]
- 11.Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11:694–703. doi: 10.1634/theoncologist.11-6-694. [DOI] [PubMed] [Google Scholar]
- 12.Guven A, Gundogdu G, Sadir S, Topal T, Erdogan E, Korkmaz A, Surer I, Ozturk H. The efficacy of ozone therapy in experimental caustic esophageal burn. J Pediatr Surg. 2008;43:1679–84. doi: 10.1016/j.jpedsurg.2008.01.064. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Sanchez G, Al-Dalain SM, Menendez S, Re L, Giuliani A, Candelario-Jalil E, Alvarez H, Fernández-Montequín JI, León OS. Therapeutic efficacy of ozone in patients with diabetic foot. Eur J Pharmacol. 2005;523:151–61. doi: 10.1016/j.ejphar.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Clavo B, Gutierrez D, Martin D, Suarez G, Hernandez MA, Robaina F. Intravesical ozone therapy for progressive radiation-induced hematuria. J Altern Complement Med. 2005;11:539–41. doi: 10.1089/acm.2005.11.539. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Xing B, Liu X, Zhan B, Zhou J, Zhu H, Chen Z. Similarities between ozone oxidative preconditioning and ischemic preconditioning in renal ischemia/reperfusion injury. Arch Med Res. 2008;39:169–78. doi: 10.1016/j.arcmed.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Paulesu L, Luzzi E, Bocci V. Studies on the biological effects of ozone: 2. Induction of tumor necrosis factor (TNF-alpha) on human leucocytes. Lymphokine Cytokine Res. 1991;10:409–12. [PubMed] [Google Scholar]
- 17.Bocci V. A reasonable Approach for the Treatment of HIV Infection in the Early Phase with Ozonetherapy (Autohaemotherapy). How ‘Inflammatory’ Cytokines may have A therapeutic Role. Mediators Inflamm. 1994;3:315–21. doi: 10.1155/S0962935194000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bocci V, Paulesu L. Studies on the biological effects of ozone 1. Induction of interferon gamma on human leucocytes. Haematologica. 1990;75:510–5. [PubMed] [Google Scholar]
- 19.Bocci V, Valacchi G, Corradeschi F, Fanetti G. Studies on the biological effects of ozone: 8. Effects on the total antioxidant status and on interleukin-8 production. Mediators Inflamm. 1998;7:313–7. doi: 10.1080/09629359890820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uz E, Oktem F, Yilmaz HR, Uzar E, Ozgüner F. The activities of purine-catabolizing enzymes and the level of nitric oxide in rat kidneys subjected to methotrexate: protective effect of caffeic acid phenethyl ester. Mol Cell Biochem. 2005;277:165–70. doi: 10.1007/s11010-005-5875-x. [DOI] [PubMed] [Google Scholar]
- 21.Bocci V. Is it true that ozone is always toxic? The end of a dogma. Toxicol Appl Pharmacol. 2006;216:493–504. doi: 10.1016/j.taap.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Bocci V. Does ozone therapy normalize the cellular redox balance? Implications for therapy of human immunodeficiency virus infection and several other diseases. Med Hypotheses. 1996;46:150–4. doi: 10.1016/s0306-9877(96)90016-x. [DOI] [PubMed] [Google Scholar]
- 23.Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. 2009;390:191–214. doi: 10.1515/BC.2009.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bocci V, Luzzi E, Corradeschi F, Paulesu L, Rossi R, Cardaioli E, Di Simplicio P. Studies on the biological effects of ozone: 4. Cytokine production and glutathione levels in human erythrocytes. J Biol Regul Homeost Agents. 1993;7:133–8. [PubMed] [Google Scholar]
- 25.Winterbourn CC, Vissers MC, Kettle AJ. Myeloperoxidase. Curr Opin Hematol. 2000;7:53–8. doi: 10.1097/00062752-200001000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Donnahoo KK, Meng X, Ayala A, Cain MP, Harken AH, Meldrum DR. Early kidney TNF-alpha expression mediates neutrophil infiltration and injury after renal ischemia-reperfusion. Am J Physiol. 1999;277:922–9. doi: 10.1152/ajpregu.1999.277.3.R922. [DOI] [PubMed] [Google Scholar]
- 27.Kolli VK, Abraham P, Isaac B, Selvakumar D. Neutrophil infiltration and oxidative stress may play a critical role in methotrexate-induced renal damage. Chemotherapy. 2009;55:83–90. doi: 10.1159/000192391. [DOI] [PubMed] [Google Scholar]


