Abstract
We have demonstrated that regulation of Toll-like receptor 4 (TLR4) surface expression levels on blood CD14+ monocytes by simvastatin treatment in patient with sepsis is different from that in patients with severe sepsis. In patients with sepsis simvastatin treatment statistically significantly decreased TLR4 surface expression level on blood CD14+ monocytes, while in patients with severe sepsis simvastatin treatment had no significant influence on TLR4 surface expression level on blood CD14+ monocytes. The changes of plasma interleukin-6 (IL-6) induced by simvastatin in patients with sepsis and severe sepsis were similar with that of TLR4. Our results indicated simvastatin treatment differently influenced inflammation process in patients with sepsis and severe sepsis, which might partially explain the discrepancy, presented by previous trials, about the therapeutic effects of simvastatin treatment in patients with sepsis and severe sepsis.
Keywords: Sepsis, severe sepsis, Toll-like receptor 4, interleukin-6, simvastatin
Introduction
An increasing amount of evidence have indicated the potential benefits of statin in treating sepsis [1]. Recently, results of two randomized placebo-controlled trials (RCT) about atorvastatin use in sepsis were reported on the journal of “American Journal of Respiratory Critical Care Medicine” [2] and “Critical Care” [3] respectively. One trial was performed in patients with sepsis [3]. In this trial, researchers found that de novo administration of atorvastatin in patients with sepsis could prevent sepsis progression to severe sepsis [3]. The other trial was performed in patients with severe sepsis [2]. However, in this trial, researchers found that de novo administration of atorvastatin in patients with severe sepsis showed no any protective effects [2]. The discrepancy of atorvastatin’s effect in septic patients and severe septic patients showed in those two trials prompts us to conclude that maybe it’s the disease severity influencing the therapeutic effects of atorvastatin. A hypothesis might exists: in the patients with sepsis, atorvastatin could obviously and significantly influence the disease condition and the therapeutic effects could be monitored by researchers, while in patients with severe sepsis, because of unknown reason, atorvastatin could not function normally and completely, or significantly influence disease condition, and the therapeutic effects could not be exerted and monitored clearly, or the therapeutic effects were too small for the disease severity and could not reverse the disease progression, were masked by disease severity. In order to test our hypothesis, we performed this prospective study to determine the effects of simvastatin treatment on Toll-like receptor 4 surface expression level on monocytes in patients with sepsis or severe sepsis, which would illustrated an important effect of statin drug: anti-inflammation, a process important for treating sepsis and severe sepsis. The difference of simvastatin’s effects on TLR4 surface expression on monocytes between patients with sepsis and severe sepsis were also compared.
Materials and methods
Study population
Patients with sepsis or severe sepsis admitted to the Intensive Care Unit of Henan Provincial People’s Hospital between August 2013 and August 2014 were enrolled in this prospective study. Written informed consent to participate in this study was obtained from the participants or their relatives. The study protocol was approved by the Ethics Committee of Henan Provincial People’s Hospital before implementation, and conducted in accordance with Declaration of Helsinki and “Good Clinical Practice” (http://www.goodclinicalpractice.com/).
Inclusion criteria of patients with sepsis [4]: > 18 years old; new proven or suspected infection; presence of any two of the signs and symptoms of infection (WCC > 11 or < 4 × 109/L, temperature > 38°C or < 36°C, heart rate > 90/bpm, or respiratory rate > 20/minute) for less than 24 hours.
Inclusion criteria of patients with severe sepsis [5,6]: > 18 years old; fulfilling the diagnosis criteria of sepsis [4]; with one organ dysfunction of less than 24 hours duration.
Patients were excluded from the study if they were moribund or not expected to survive 15 days because of an underlying irreversible medical condition; were pregnant or breast feeding; were intolerant to statins or were unable to have enteral administration of study drug; had severe liver disease (acute liver failure, chronic liver disease Child-Pugh classification C); had statin therapy before hospital admission; or if informed consent could not be obtained.
Twenty healthy volunteers were recruited from hospital workers and relatives of the study investigators.
Study protocol
After admission, all the patients with sepsis or severe sepsis received treatments according to the guideline suggested by American College of Chest Physicians/Society of Critical Care Medicine or Surviving Sepsis Campaign Guidelines Committee [4,5]. For the purpose of study, enrolled patients were randomly assigned to two group: simvastatin group and control group using a computer-generated random number list with the treatment methods allocation concealed. Patients in simvastatin group were treated with simvastatin of 40 mg/day while patients in control group were not. Simvastatin treatment was continued daily until Day 15 after admission or until discharge from the ICU, whichever of these occurred first.
Baseline data for diagnosis of sepsis or severe sepsis from medical notes and laboratory reports were obtained and Acute Physiology and Chronic Health Evaluation II (APACHE II) scores were calculated to evaluate the severity of patients’ conditions at baseline. Sequential Organ Failure Assessment (SOFA) [7] scores were also collected in patients with severe sepsis.
Laboratory investigations
In the healthy control (HC), venous blood was drawn at study entry. In the septic patients, blood samples were prospectively collected on day 1, 5, 10 and 15 after admission. The blood samples were immediately placed into ethylenediamine tetra-acetic acid (EDTA) test tube. The peripheral blood mononuclear cells (PBMCs) were isolated by density-gradient centrifugation using Ficoll-Paque Premium (GE healthcare, USA) according to manufacturer’s manual. Flow cytometry was employed to identify TLR4/CD14 double positive PBMCs by TLR4 and CD14 specific antibody (TLR4 antibody: Cat. No. 551964; CD14 antibody: Cat. No. 555397; BD Biosciences, USA); and expression level of TLR4 on TLR4/CD14 double positive PBMCs was presented as mean fluorescent intensity (MFI).
Plasma samples were prepared immediately by centrifugation (2000×g, 4°C for 10 min) of peripheral venous blood. Aliquots were stored at -80°C before analysis. Interleukin-6 level were measured by commercially available enzyme-linked immunosorbent assays (ELISA) kit according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA).
Statistical analysis
Statistical analysis was performed using SPSS 21.0 (SPSS Inc., Chicago, Illinois, USA). All the data were presented as mean ± standard deviation or count (percentage) or median (interquartile range, IQR) unless otherwise stated. TLR4 levels and IL-6 concentration between different groups were compared by Wilcoxon rank sum test. Categorical data were compared by Chi-square test or Fisher exact test. P < 0.05 was considered as statistically significant.
Results
Baseline characteristics
As shown in Table 1, 54 patients diagnosed with sepsis and 47 patients diagnosed with severe sepsis were involved in this prospective study. No significant difference in baseline characteristics was observed between patients in simvastatin group and control group. 28 patients with sepsis received de novo simvastatin treatment and 26 patients with sepsis were used as control. 23 patients with severe sepsis received de novo simvastatin treatment and 24 patients with severe sepsis were used as control.
Table 1.
Baseline characteristics of patients
| Patients with sepsis | |||
|
| |||
| Simvastatin (n = 28) | control (n = 26) | P | |
|
| |||
| Age, years, mean (SD) | 48.9 (18.3) | 51.4 (21.3) | 0.826 |
| Male gender, % | 50 | 56 | 0.777 |
| APACHE II, mean (SD) | 12.4 (4.3) | 11.8 (5.8) | 0.902 |
| Ch, mmol/L, median (IQR) | 3.8 (2.9, 4.7) | 4.1 (3.4, 5.3) | 0.538 |
| WBC, × 109/L, median (IQR) | 12.3 (10.3, 18.9) | 13.6 (12.2, 19.2) | 0.472 |
| CRP, mg/L, mean (SD) | 214.4 (121.8) | 197.3 (112.8) | 0.613 |
| Source of infection, number of cases | |||
| Pneumonia | 18 | 19 | 0.487 |
| Cellulitis | 3 | 2 | 1.000 |
| Urinary tract | 1 | 2 | 0.604 |
| Gastrointestinal | 6 | 3 | 0.470 |
|
| |||
| Patients with severe sepsis | |||
|
| |||
| Simvastatin (n = 23) | control (n = 24) | P | |
|
| |||
| Age, mean (SD) | 56.8 (21.3) | 58 (19.7) | 0.817 |
| Male gender, % | 52 | 50 | 0.882 |
| APACHE II, mean (SD) | 21.3 (8.2) | 22.7 (7.8) | 0.663 |
| SOFA score, mean (SD) | 8.2 (3.0) | 8.5 (3.1) | 0.516 |
| Ch, mmol/L, median (IQR) | 4.3 (3.1, 5.7) | 4.6 (3.3, 4.9) | 0.318 |
| WBC, × 109/L, median (IQR) | 16.8 (12.3, 23.1) | 17.2 (13.3, 21.8) | 0.524 |
| Source of infection, number of cases | |||
| Pneumonia | 11 | 12 | 0.882 |
| Cellulitis | 3 | 1 | 0.348 |
| Urinary tract | 4 | 4 | 1.000 |
| astrointestinal | 5 | 7 | 0.559 |
Effects of simvastatin treatment
As shown in Figure 1A, in patients with sepsis simvastatin treatment statistically significantly and time-dependently reduced TLR4 surface expression level on monocytes; in parallel with the change of TLR4 expression, plasma IL-6 concentration was also significantly decreased concomitantly in patients treated with simvastatin as compared with control (Figure 1B).
Figure 1.
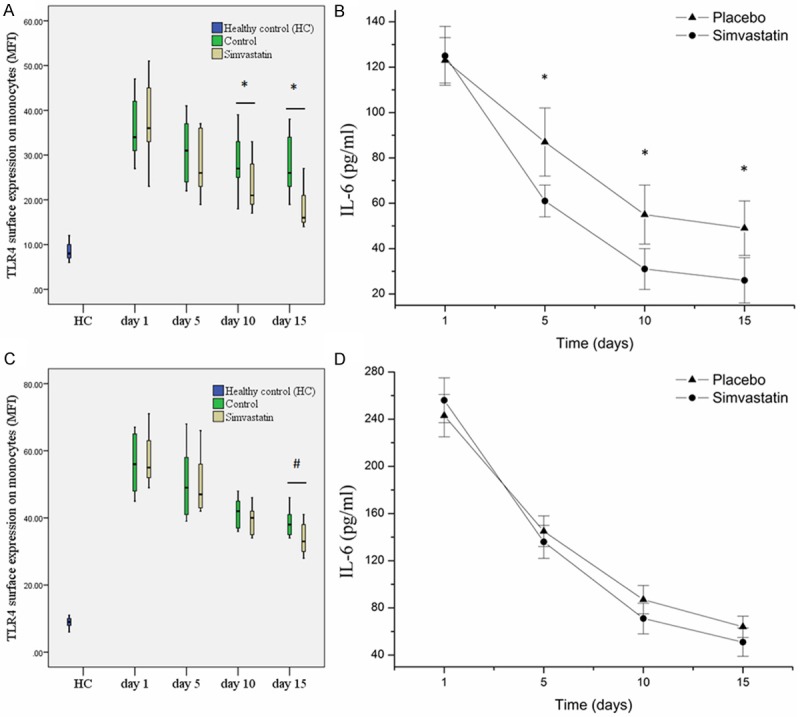
Regulation of Toll-like receptor 4 (TLR4) surface expression levels and plasma interleukin-6 (IL-6) concentration by simvastatin treatment in patients with sepsis (A, B) and severe sepsis (C, D). (A, C) TLR4 surface expression levels. (B, D) Plasma IL-6 concentration. MFI: mean fluorescent intensity. *P < 0.05; #P > 0.05.
In patients with severe sepsis, although TLR4 surface expression levels on monocytes showed a little difference between simvastatin treated patients and control, the difference didn’t reach significance (Figure 1C), but we still could observe the lowering effect of simvastatin treatment on TLR4 expression. Meanwhile, plasma IL-6 concentration showed no significant difference between simvastatin group and control group (Figure 1D), as previously reported [2].
Adverse events
Serial blood samples were taken to monitor any serious adverse events due to statin therapy. There were no cases of raised transaminase levels or CK levels in either group.
Discussion
Sepsis is a complex syndrome caused by uncontrolled systemic inflammatory response to infection. The augmentation of sepsis are multifactorial and ultimately lead to multiple organ dysfunction [6]. In critically ill patients sepsis is the leading cause of death [8]. Anti-inflammation is an important method to treat sepsis [5].
HMG-CoA-reductase inhibitors (statins) such as simvastatin have been shown that they could not only reduced the incidence of cardiovascular events [9] but also improve the outcomes in patients with sepsis [10,11]. Besides their cholesterol lowering effect, statins have anti-inflammatory and immunomodulatory benefits. They can inhibit lipopolysaccharide (LPS)-mediated activation of human peripheral mononuclear cells and endothelial cells [12] and reduce the level of the proinflammatory cytokines tumor necrosis factor α (TNF-α) and interleukin (IL)-6 [13]. In the past decade a number of experimental and clinical investigations have been performed to study statin use in sepsis [1-3,10,14-20]. However, whether statin should be used in septic patients is still in debate. Recently there are two reports of randomized placebo-controlled trials about statin use in septic patients [2,3]. They stated that de novo use of statin in septic patients could prevent the progression to severe sepsis, while in severe septic patients de novo use of statin didn’t provide any protective effects. Their findings prompt us conclude a hypothesis: in patients with sepsis statin could normally exert its anti-inflammation ability while in severe septic patients statin could not. In order to test our hypothesis, this prospective study was performed in patients with sepsis and severe sepsis to monitor the influence of simvastatin treatment on TLR4 surface expression on blood monocytes, which was a pivotal player in response to infection. Our results showed that in septic patients’ simvastatin could down-regulate TLR4 surface expression on monocytes and plasma IL-6 concentration, which indicated the anti-inflammation effect of simvastatin. However, in patients with severe sepsis simvastatin treatment have no significant effect on TLR4 surface expression and plasma IL-6 concentration, although a little difference between the two groups existed. Our findings seemingly support our hypothesis. Although at present we don’t know why the anti-inflammation ability is inhibited in severe septic patients, we still can imagine that in severe septic patients organs dysfunction exist, and organs status would influence the therapeutic effects of statin, that’s, the disease severity influence the therapeutic effects of statin. As regard to the mechanisms about how organs dysfunction influence the therapeutic effects of statin, further experimental and clinical researches should be performed.
Acknowledgements
This work was supported by the Department of Science and Technology of Henan Province, People’s Republic of China (No. 132102310220).
Disclosure of conflict of interest
None.
References
- 1.Wan YD, Sun TW, Kan QC, Guan FX, Zhang SG. Effect of statin therapy on mortality from infection and sepsis: a meta-analysis of randomized and observational studies. Crit Care. 2014;18:R71. doi: 10.1186/cc13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kruger P, Bailey M, Bellomo R, Cooper DJ, Harward M, Higgins A, Howe B, Jones D, Joyce C, Kostner K, McNeil J, Nichol A, Roberts MS, Syres G, Venkatesh B. A multicenter randomized trial of atorvastatin therapy in intensive care patients with severe sepsis. Am J Respir Crit Care Med. 2013;187:743–750. doi: 10.1164/rccm.201209-1718OC. [DOI] [PubMed] [Google Scholar]
- 3.Patel JM, Snaith C, Thickett DR, Linhartova L, Melody T, Hawkey P, Barnett AH, Jones A, Hong T, Cooke MW, Perkins GD, Gao F. Randomized double-blind placebo-controlled trial of 40 mg/day of atorvastatin in reducing the severity of sepsis in ward patients (ASEPSIS Trial) Crit Care. 2012;16:R231. doi: 10.1186/cc11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ ACCP/SCCM Consensus Conference Committee. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. 1992. Chest. 2009;136:e28. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 5.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 6.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G Sccm/Esicm/Accp/Ats/Sis. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 7.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 8.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014;5:4–11. doi: 10.4161/viru.27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 10.Stamm J. Simvastatin and survival in sepsis. Circulation. 2004;110:e315. doi: 10.1161/01.CIR.0000142202.91948.D4. author reply e315. [DOI] [PubMed] [Google Scholar]
- 11.Almog Y, Shefer A, Novack V, Maimon N, Barski L, Eizinger M, Friger M, Zeller L, Danon A. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation. 2004;110:880–885. doi: 10.1161/01.CIR.0000138932.17956.F1. [DOI] [PubMed] [Google Scholar]
- 12.Rice JB, Stoll LL, Li WG, Denning GM, Weydert J, Charipar E, Richenbacher WE, Miller FJ Jr, Weintraub NL. Low-level endotoxin induces potent inflammatory activation of human blood vessels: inhibition by statins. Arterioscler Thromb Vasc Biol. 2003;23:1576–1582. doi: 10.1161/01.ATV.0000081741.38087.F9. [DOI] [PubMed] [Google Scholar]
- 13.Weis M, Pehlivanli S, Meiser BM, von Scheidt W. Simvastatin treatment is associated with improvement in coronary endothelial function and decreased cytokine activation in patients after heart transplantation. J Am Coll Cardiol. 2001;38:814–818. doi: 10.1016/s0735-1097(01)01430-9. [DOI] [PubMed] [Google Scholar]
- 14.Pasin L, Landoni G, Castro ML, Cabrini L, Belletti A, Feltracco P, Finco G, Carozzo A, Chiesa R, Zangrillo A. The effect of statins on mortality in septic patients: a meta-analysis of randomized controlled trials. PLoS One. 2013;8:e82775. doi: 10.1371/journal.pone.0082775. United States. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ajrouche R, Al-Hajje A, El-Helou N, Awada S, Rachidi S, Zein S, Salameh P. Statins decrease mortality in Lebanese patients with sepsis: A multicenter study. Pharm Pract (Granada) 2013;11:102–108. doi: 10.4321/s1886-36552013000200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruger PS, Harward ML, Jones MA, Joyce CJ, Kostner KM, Roberts MS, Venkatesh B. Continuation of statin therapy in patients with presumed infection: a randomized controlled trial. Am J Respir Crit Care Med. 2011;183:774–781. doi: 10.1164/rccm.201006-0955OC. [DOI] [PubMed] [Google Scholar]
- 17.Shyamsundar M, McKeown ST, O’Kane CM, Craig TR, Brown V, Thickett DR, Matthay MA, Taggart CC, Backman JT, Elborn JS, McAuley DF. Simvastatin decreases lipopolysaccharide-induced pulmonary inflammation in healthy volunteers. Am J Respir Crit Care Med. 2009;179:1107–1114. doi: 10.1164/rccm.200810-1584OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodgkinson CP, Ye S. Statins inhibit toll-like receptor 4-mediated lipopolysaccharide signaling and cytokine expression. Pharmacogenet Genomics. 2008;18:803–813. doi: 10.1097/FPC.0b013e3283050aff. [DOI] [PubMed] [Google Scholar]
- 19.Merx MW, Liehn EA, Graf J, van de Sandt A, Schaltenbrand M, Schrader J, Hanrath P, Weber C. Statin treatment after onset of sepsis in a murine model improves survival. Circulation. 2005;112:117–124. doi: 10.1161/CIRCULATIONAHA.104.502195. [DOI] [PubMed] [Google Scholar]
- 20.Almog Y, Shefer A, Novack V, Maimon N, Barski L, Eizinger M, Friger M, Zeller L, Danon A. Prior Statin Therapy Is Associated With a Decreased Rate of Severe Sepsis. Circulation. 2004;110:880–885. doi: 10.1161/01.CIR.0000138932.17956.F1. [DOI] [PubMed] [Google Scholar]


