Abstract
This study investigates the application value of diffusion-weighted magnetic resonance imaging in predicting cervical cancer radiosensitivity. Twenty-five patients who were newly diagnosed as cervical cancer and accepted simple radiotherapy were included in this study. Before external irradiation, 20 GY and at the end of irradiation, routine 1.5 T MRI and diffusion-weighted magnetic resonance imaging scanning were carried. Apparent diffusion coefficient (ADC) value of primary tumor was measured. Its correlation with tumor regression rate was analyzed. ADC values of before irradiation, 20 GY and at the end of irradiation was (0.93 ± 0.14) × 10-3 mm2/s, (1.25 ± 0.17) × 10-3 mm2/s and (1.55 ± 0.13) × 10-3 mm2/s, respectively. There were statistical significant differences (P< 0.01). D-value of ADC values between before and 20 GY external irradiation was (0.33 ± 0.16) mm2/s. The tumor volume before and at the end of external irradiation were (37.48 ± 26.83) cm3 and (4.41 ± 3.72) cm3 respectively, with tumor regression rate of before and after external irradiation of (0.86 ± 0.11). ADC values of before irradiation, 20 GY and at the end of irradiation did not correlate with tumor regression rate. D-value of ADC values between before and 20 GY external irradiation positively correlated with tumor regression rate (r = 0.423, P = 0.035). ADC value of cervical cancer increased after radiotherapy and early changes of ADC value was positively correlated with tumor regression rate, thus, ADC value could be used as a potential prediction factor for cervical cancer radiosensitivity.
Keywords: Cervical cancer, radiosensitivity, diffusion-weighted magnetic resonance imaging, apparent diffusion coefficient
Introduction
Cervical cancer (CC) is the fourth most common malignancy in women worldwide [1,2], with 85% of cases occurring in developing countries, where cervical cancer is a leading cause of cancer death in women [3,4]. Radiotherapy is often used in the management of patients with cervical cancer as definitive therapy for those with locally advanced disease (stages IB2 or to IVA) or for those who are poor surgical candidates [5]. In recent years, with the development and improvement of radiotherapy techniques, CC radiotherapy effectiveness is significantly increased. However, recurrence in irradiation field accounts for the majority of the failure treatment cases. Therefore, predicting CC radiosensitivity before or in early stage of radiotherapy and giving individualized comprehensive treatment according to different sensitivity, is an important way to improve the efficacy of radiotherapy.
It is found that part of the malignant tumors begins to retreat after a period of radiation ending in clinical and it is difficult to predict radiotherapy sensitivity using anatomical and morphological techniques. Tumor tissue function changes often appear before tumor tissue volume changes, as a result, tissue function is a better predictive index for therapeutic sensitivity [6,7]. The technology of diffusion-weighted magnetic resonance imaging (DWI) is an imaging method using MRI to observe microscopic diffusion motion of water molecules in living tissue. By applying diffusion-weighted gradient pulses to a conventional magnetic resonance sequence, the signal can be made sensitive to the level of localized water diffusibility, which can be quantified as the apparent diffusion coefficient (ADC). It has been proved that DWI can be used as a surrogate biomarker of tumor cellularity by observing the water mobility within tumors [8-10]. And following anti-cancer treatment, significant changes in water diffusion due to alterations in tumor cell density will be detected by DWI too [11,12]. In addition, as a non-invasive technology, DWI can provide physiological function information before morphological change while routine sequential MRI cannot [7,13]. As a result of the aforementioned advantages, DWI might be an ideal predictor of tumor radiosensitivity.
In this study, ADC value of primary tumor was measured and its difference and correlation with tumor regression rate were analyzed in order to investigate the application value of DWI in predicting CC radiosensitivity.
Materials and methods
Patients’ data
A total of 25 cases of patients who were newly diagnosed as CC and admitted to Fujian Cancer Hospital from January 2010 to May 2012 were enrolled in this study. They all were confirmed by pathology and among them 22 cases were squamous cell carcinoma, 3 cases were adenocarcinoma. They aged from 38-70 years old, with an average of 52 years old. According to International Federation of Gynecology and Obstetrics (FIGO) Stage, 2 cases were stage II A, 12 cases were stage II B, 2 cases were stage III A and 9 cases were stage III B. Patients those were pregnant or with radiotherapy contraindications, hysterectomy history, pelvic irradiation history and MRI contraindication were excluded.
Prior written and informed consent were obtained from every patient and the study was approved by the ethics review board of Fujian Cancer Hospital.
Radiation schedule
All the 25 cases of CC patients all underwent simple pelvic radiotherapy. Detailed radiation therapy schedule was as follows. The patients were firstly treated with electron linear accelerator 6-18 MVX ray (Elekta Oncology Systems Ltd, Crawley, UK) conventional lead block external irradiation for all aspects of pelvic. The four-field radiotherapy prescribed dose (DT) was 30 GY/15 F-40 GY/20 F. Then changed to anteroposterior field plus central lead block external irradiation and the DT was 30 GY/15 F-40 GY/20 F. High dose rate 192Ir intracavitary brachytherapy was carried out during the same period of central lead block and the frequency of brachytherapy was once per week and the A point DT was 35-42 GY/5-6 F.
MRI scanning
Conventional MRI and DWI scanning were carried out before, 20 GY and at the end of external irradiation. Signa 1.5 T Excite III HD superconducting magnetic resonance imaging (GE, Fairfield City, USA) was used for MRI and DWI scanning. For conventional MR scanning, 8 channel phased-array surface coil pairs were adopted and external-body surface coil was applied for DWI scanning. Conventional MRI sequence scanning included axial T1WI FSE, axial T2WI FSE, sagittal FSE T2WI and axial DWI. MRI. Spin echo planar imaging (SE-EPI) sequence was taken for DWI scanning. Cross-section, sagittal and coronal 3D-LAVA scanning was taken after injection of contrast agent GD-DTPA (1.5 ml/s, 0.1 mmol/kg). The scan range was 2 cm from the lower edge of pubic symphysis up to abdominal aortic bifurcation level. The data were transferred to ADW 4.2 workstation (GE, Fairfield City, USA) for image post-processing and ADC value measurement.
MRI data processing
Primary CC ADC value was measured on the ADC fusion maps these with b values of 0 and 800 s/mm2. Region of interest (ROI) that sele-cted was as close as possible to 3/4 the maximum cross-sectional area of parenchyma tumor and the necrosis area was avoided. In the process of external irradiation and at the end of external irradiation, if the primary tumor has retreated completely while cervical prototype was visible, normal cervical tissue ADC value was measured in the same position. Data were processed by an physician-in-charge or above MRI diagnostician and a radiotherapeutic physicians with double blind method, and the average value was taken. The value that with great difference was determined after discussion. Primary CC size and signal changes on cross-section and sagittal T2WI-FSE images were observed. Primary CC was outlined and the tumor volume was calculated quantitatively. Tumor regression rate = tumor volume before external irradiation-tumor volume after external irradiation)/tumor volume before external irradiation.
Statistical analysis
All statistical analyses were performed using SPSS version 16.0 (SPSS Inc, Chicago, IL, USA) for Windows. The D-value of ADC value between external irradiation (20 GY) and before radiotherapy was set as R value. Statistical analysis was performed on ADC value of before, 20 GY and at the end of irradiation, R value and tumor regression rate. T test was used for difference analysis and Spearman’s test was adopted for correlation analysis. Two sided test with P value less than 0.05 was considered as statistically significant.
Results
ADC value variation
To make sure of the change of ADC value, ADC values were recorded and compared. Scanning time of external irradiation for 20 GY was 12-18 days after the start of irradiation, with the median time of 14 days. The scanning time at the end of external irradiation was 45-60 days after the start of irradiation, with the median time of 49 days. The ADC values of before, 20 GY and at the end of external irradiation were (0.93 ± 0.14) × 10-3 mm2/s, (1.25 ± 0.17) × 10-3 mm2/s and (1.55 ± 0.13) × 10-3 mm2/s, respectively (Figure 1). ADC value showed a progressive increase tendency with the increase of external irradiation dose. T test showed that there were significant differences while mutual compared. The comparative values of before and 20 GY irradiation, before and the end of external irradiation, 20 GY irradiation and the end of external irradiation were-10.508, -19.257 and -12.540, respectively, all with P value less than 0.01. R value was (0.33 ± 0.16) × 10-3 mm2/s. To sum up, these results showed that ADC value changed significantly at different times of the radiotherapy.
Figure 1.
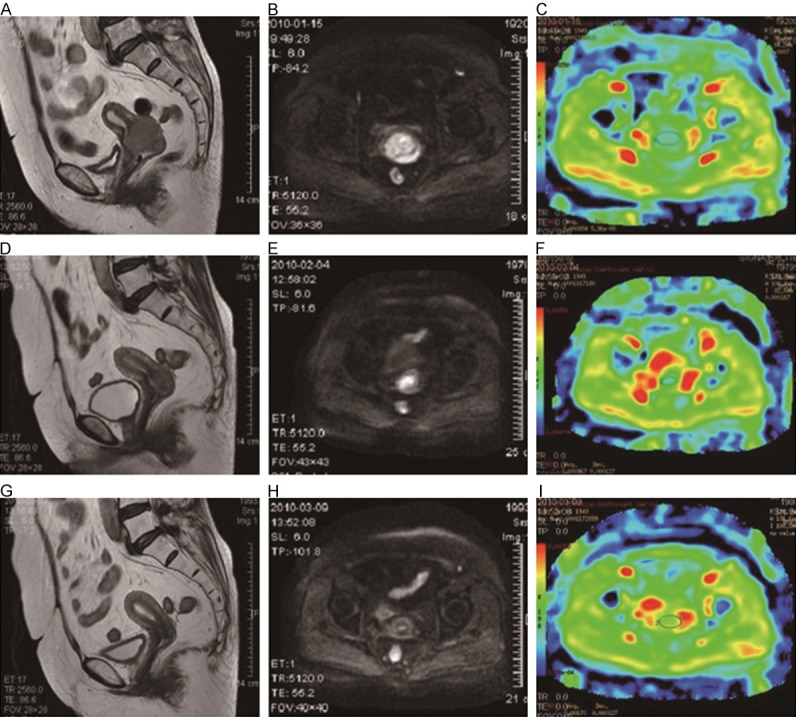
T2WI, DWI and ADC images of cervical tumor at different times of radiosensitivity. T2WI, DWI and ADC images before treatment (A-C) indicated that the cervical mass with the volume of 54.60 cm3 showing a high signal on T2WI-FSE, a high signal on DWI and the ADC value was 0.894 × 10-3 mm2/s. T2WI, DWI and ADC images at 20 GY of therapy (D-F) argued that high signal area on T2WI-FSE of the cervical mass shrunk greatly (23.7 cm3) and DWI high signal had shrunk, additionally, the ADC value was 0.967 × 10-3 mm2/s. T2WI, DWI and ADC images after radiotherapy (G-I) showed that the cervical shape was normal and the high signal on T2WI-FSE almost disappeared with the volume of 2.00 cm3. DWI high signal area regressed and the ADC value was 1.700 × 10-3 mm2/s.
Tumor regression condition
In order to calculate the cervical tumor anatomic form shrinkage, tumor volume at different times was calculated. According to axial and sagittal T2WI-FSE images results, primary CC lump of mid and at the end of external irradiation had shrunk than that of before irradiation. High intensity signal on T2WI-FSE decreased gradually after irradiation and T2WI-FSE showed isointensity and low intensity signals. By sketching CC primary tumor contour, CC tumor volume was calculated. Average tumor volume before irradiation was (37.48 ± 26.83) cm3 and the mean tumor volume after irradiation was (4.41 ± 3.72) cm3. Ratio of reduced primary CC volume (D-value of tumor volume before and after irradiation) and CC volume before irradiation, i.e. the final tumor regression rate was 0.86 ± 0.11. The D-value of ADC value between mid external irradiation (20 GY) and before radiotherapy, namely R value, had a positive correlation to tumor regression rate (r = 0.423, P = 0.035). In conclusion, we considered that in early stage of the radiotherapy, the more significantly ADC values increased in DWI images, the more regression the tumor will be at the end of radiotherapy.
Discussion
Research of DWI technology in diagnosis of malignant tumor is very mature. According to Yang et al. and Naganawa et al., DWI can identify CC and normal cervical perfectly [8,10]. Lei Mo et al. show that, quantitative ADC value analysis also helps to assess CC pathological type and grading [14]. Our previous study showed that, ADC value of CC metastatic lymphadenopathy is lower than these of normal lymph node and reactive hyperplasia lymph node [15]. However, studies of DWI in tumor radiosensitivity prediction are rare. Early studies in animal and cell models suggest that DWI has potential prediction in tumor curative effect [16,17]. Theilmann et al. had studied on chemotherapy reaction in patients with breast cancer liver metastases. Their result showed that ADC value changed little in patients with poor treatment response, while ADC value changed obviously in patients with good treatment response, indicating that DWI can predict chemotherapy response [18]. Kun Cao et al. show that average ADC value variation range in CC residual after radiochemotherapy group is lower than that of non residue group. They argue that ADC value variation can be the judgment index of whether a CC lesion is easy to have residual after radiochemotherapy [19].
The results in this study showed that CC primary tumor volume reduced gradually with radiotherapy dose accumulation, and instead, its ADC value increased while radiotherapy dose accumulated. This result was similar to those of Naganawa et al. and Yun Zhang et al. [10,20], reflecting reduction in tumor cell density and water molecules moving obstacle after radiotherapy. D-value of ADC between before irradiation and 20 GY external irradiation had a positive correlation to CC primary tumor regression rate, which, was consistent with Harry’s results [21]. This result indicated that at early radiotherapy period (median time of this study was 14th days after the stat of radiotherapy); the difference in ADC value might be used to predict radiosensitivity of CC. In practice, if we used this technology and had predicted that some CC was not sensitive to radiotherapy, we can timely use radiosensitizers; combine with chemotherapy or biologic therapy. Meanwhile, we can switch with intensity modulated radiotherapy by increasing single dose, or even create clinical intervention conditions such as surgery.
Mardor et al. show that ADC value of brain tumor before radiotherapy is related to tumor volume shrink, indicating DWI technology may used as non invasive tool for tumor curing efficacy prediction before the start of therapy [22]. However, our study showed that CC ADC value before treatment was not related to tumor regression rate. This contradiction might be due to the lack of observing tumor regression time or the sample size enrolled in our study was not big enough. Kim et al. indicate that ADC value decrease again during prostate cancer recurrence, thus, ADC value can be sued for recurrence prediction [23]. In the present study, all the 25 cases of CC patients were followed up, and MRI scanning was performed in 2 patients with primary lesion recurrence. The scanning time were 7 and 19 months after radiotherapy respectively and ADC value decreased again. The ADC values of before, at the end of radiotherapy and tumor recurrence in the two patients were (0.96 × 10-3, 1.26 × 10-3 and 0.98 × 10-3 mm2/s) and (0.93 × 10-3, 1.65 × 10-3, and 1.12 × 10-3 mm2/s), respectively. Therefore, we considered that DWI technology might have certain application value in monitoring and diagnosis of tumor recurrence.
In short, DWI scan as a conventional imaging diagnosis for malignant tumor, is hopeful to be widely used in predicting early tumor radiotherapy sensitivity without invasion, both in economics and practical aspects of considerable research value.
Disclosure of conflict of interest
None.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining pirorites to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 3.International Agency for Research on cancer. Cervical Cancer Estimated Incidence, Mortality and Prevalence Worldwide in 2012. World Health Organization; 2012, Availableat http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed May 4, 2015.
- 4.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 5.Gaffney DK, Erickson-Wittmann BA, Jhingran A, Mayr NA, Puthawala AA, Moore D, Rao GG, Small W Jr, Varia MA, Wolfson AH, Yashar CM, Yuh W, Cardenes HR. ACR Appropriateness Criteria(R) on Advanced Cervical Cancer Expert Panel on Radiation Oncology-Gynecology. Int J Radiat Oncol Biol Phys. 2011;81:609–614. doi: 10.1016/j.ijrobp.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Padhani AR. Functional MRI for anticancer therapy assessment. Eur J Cancer. 2002;38:2116–2127. doi: 10.1016/s0959-8049(02)00388-x. [DOI] [PubMed] [Google Scholar]
- 7.Pickles MD, Gibbs P, Lowry M, Turnbull LW. Diffusion changes precede size reduction in neoadjuvant treatment of breast cancer. Magn Reson Imaging. 2006;24:843–847. doi: 10.1016/j.mri.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Yang Duan, Xue Dong, Yahong Luo. Study on the apparent diffusion coefficient of cervical carcinoma in magnetic resonance diffusion imaging. Chinese Journal of CT and MRI. 2013;9:49–51. [Google Scholar]
- 9.Ross BD, Chenevert TL, Kim B, Ben-Yoseph O. Magnetic resonance imaging and spectroscopy: application to experimental neuro-oncology. QMagn Reson Biol Med. 1994;1:89–106. [PMC free article] [PubMed] [Google Scholar]
- 10.Naganawa S, Sato C, Kumada H, Ishigaki T, Miura S, Takizawa O. Apparent diffusion coefficient in cervical cancer of the uterus: comparison with the normal uterine cervix. Eur Radiol. 2005;15:71–78. doi: 10.1007/s00330-004-2529-4. [DOI] [PubMed] [Google Scholar]
- 11.Hamstra DA, Rehemtulla A, Ross BD. Diffusion magnetic resonance imaging: a biomarker for treatment response in oncology. J. Clin. Oncol. 2007;25:4104–4109. doi: 10.1200/JCO.2007.11.9610. [DOI] [PubMed] [Google Scholar]
- 12.Ross BD, Moffat BA, Lawrence TS, Mukherji SK, Gebarski SS, Quint DJ, Johnson TD, Junck L, Robertson PL, Muraszko KM, Dong Q, Meyer CR, Bland PH, McConville P, Geng H, Rehemtulla A, Chenevert TL. Evaluation of cancer therapy using diffusion magnetic resonance imaging. Mol Cancer Ther. 2003;2:581–587. [PubMed] [Google Scholar]
- 13.Lee KC, Moffat BA, Schott AF, Layman R, Ellingworth S, Juliar R, Khan AP, Helvie M, Meyer CR, Chenevert TL, Rehemtulla A, Ross BD. Prospective early response imaging biomarker for neoadjuvant breast cancer chemotherapy. Clin Cancer Res. 2007;13:443–450. doi: 10.1158/1078-0432.CCR-06-1888. [DOI] [PubMed] [Google Scholar]
- 14.Mo L, Jiang XQ, Chen AM. Preliminary study of DWI in the diagnosis of cervical cancer tumor density correlation. Chinese Journal of CT and MRI. 2011;11:44–46. [Google Scholar]
- 15.Chen YB, Liao J, Xie R, Chen GL, Chen G. Discrimination of metastatic from hyperplastic pelvic lymph nodes in patients with cervical cancer by diffusion-weighted magnetic resonance imaging. Abdom Imaging. 2011;36:102–109. doi: 10.1007/s00261-009-9590-z. [DOI] [PubMed] [Google Scholar]
- 16.Jennings D, Hatton BN, Guo J, Galons JP, Trouard TP, Raghunand N, Marshall J, Gillies RJ. Early response of prostate carcinoma xenografts to docetaxel chemotherapy monitored with diffusion MRI. Neoplasia. 2002;4:255–262. doi: 10.1038/sj.neo.7900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee KC, Moffat BA, Schott AF, Layman R, Ellingworth S, Juliar R, Khan AP, Helvie M, Meyer CR, Chenevert TL, Rehemtulla A, Ross BD. Prospective early response imaging biomarker for neoadjuvant breast cancer chemotherapy. Clin Cancer Res. 2007;13:443–450. doi: 10.1158/1078-0432.CCR-06-1888. [DOI] [PubMed] [Google Scholar]
- 18.Theilmann RJ, Borders R, Trouard TP, Xia G, Outwater E, Ranger-Moore J, Gillies RJ, Stopeck A. Changes in water mobility measured by diffusion MRI predict response of metastatic breast cancer to chemotherapy. Neoplasia. 2004;6:831–837. doi: 10.1593/neo.03343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao K, Zhang XP, Wang N. Diffusion-weighted imaging evaluation of the effects of early (chemo-) radiation therapeutic of cervical cancers. Chinese Journal of Medical Technology. 2009;25:1657–1660. [Google Scholar]
- 20.Zhang Y, Liang BL, Gao L, Ye RX, Shen J, Zhong JL. Study on magnetic resonance diffusion weighted characteristics of normal uterus and cervical cancer. Ai Zheng. 2007;26:508–512. [PubMed] [Google Scholar]
- 21.Harry VN, Semple SI, Gilbert FJ, Parkin DE. Diffusion-weighted magnetic resonance imaging in the early detection of response to chemoradiation in cervical cancer. Gynecol Oncol. 2008;111:213–220. doi: 10.1016/j.ygyno.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 22.Mardor Y, Roth Y, Ochershvilli A, Spiegelmann R, Tichler T, Daniels D, Maier SE, Nissim O, Ram Z, Baram J, Orenstein A, Pfeffer R. Pretreatment prediction of brain tumors’ response to radiation therapy using high b-value diffusion-weighted MRI. Neoplasia. 2004;6:136–142. doi: 10.1593/neo.03349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim CK, Park BK, Lee HM. Prediction of locally recurrent prostate cancer after radiation therapy: incremental value of 3T diffusion-weighted MRI. J Magn Reson Imaging. 2009;29:391–397. doi: 10.1002/jmri.21645. [DOI] [PubMed] [Google Scholar]


