Abstract
The incidence of contrast-induced nephropathy has an increasing trend as a result of increased use of contrast media during coronary interventional procedures. Contrast-induced nephropathy is one of the major causes for hospital acquired renal failure after coronary interventional procedures. In this study, a total of 126 enrolled patients undergoing elective coronary angiography and/or percutaneous coronary intervention were randomly divided into two groups to investigate the efficacy of recombinant human brain natriuretic peptide in preventing contrast-induced nephropathy in patients undergoing elective coronary angiography and/or percutaneous coronary intervention. Our results showed that there was no statistically significant difference in the primary end points, with similar incidence of contrast-induced nephropathy in the two groups (P=0.770). In compared with the hydration group, the elevation of serum creatinine in the recombinant human brain natriuretic peptide group was less, especially at 48 hours (P=0.047) and at 72 hours (P=0.048) after the procedure. The creatinine clearance from baseline to 72 hours after the procedure was higher in the BNP group than in the hydration group. There were significant differences in creatinine clearance at 48 hours (P=0.016) and at 72 hours (P=0.019) between the two groups. In spite of similar incidence of contrast-induced nephropathy, recombinant human brain natriuretic peptide has its advantages for the protection of the renal function associated with better protection of renal function in patients undergoing elective coronary angiography and/or percutaneous coronary intervention, compared with saline hydration.
Keywords: Recombinant human brain natriuretic peptide, contrast-induced nephropathy, coronary angiography, percutaneous coronary intervention
Introduction
With the increasing prevalence of coronary artery disease, the techniques of percutaneous coronary intervention (PCI) have developed rapidly. The incidence of contrast-induced nephropathy (CIN) has also seen an increasing trend as a result of increased use of contrast media during coronary interventional procedures. CIN is one of the major causes for hospital acquired renal failure [1-3], and significantly increases the in-hospital stay and risk of mortality [4,5].
Periprocedural hydration is a simple effective therapy that is highly recommended in the current guidelines for CIN prevention [6,7]. Zhang et al. applied the B-type natriuretic peptide (BNP) on patients with heart failure and ST-segment elevation myocardial infarction who underwent primary PCI, and observed that by using BNP treatment, the incidence of CIN and elevation of serum creatinine (SCr) were reduced, and estimated glomerular filtration rate (eGFR) was improved [8]. However, whether recombinant human brain natriuretic peptide (rhBNP, Tibet Rhodiola Pharmaceutical Holding Co. Ltd, Chengdu, China) can prevent CIN in patients undergoing elective coronary angiograhpy (CAG) and/or PCI is still unknown. In this setting, this pilot study was performed to investigate the efficacy of rhBNP in preventing CIN.
Materials and methods
Patients
From March 2014 to January 2015, 126 consecutive patients who underwent elective CAG and/or PCI at the Fourth Affiliated Hospital of Harbin Medical University were considered for enrollment in the study. Adult patients (>18 years old) who were referred for PCI were eligible for enrollment. The exclusion criteria were: (1) acute myocardial infarction requiring emergency catheterization; (2) chronic heart failure; (3) have underwent prior PCI or coronary artery bypass grafts before the procedure; (4) have received contrast media within 7 days before the index procedure; (5) acute respiratory insufficiency; (6) acute renal failure; (7) end-stage renal disease requiring dialysis; and (8) patients who are on metformin, nonsteroidal anti-inflammatory drugs, diuretics, mannitol, dopamine, theophylline, ascorbic acid and N-acetylcysteine during the study period.
Study protocol
This was a pilot, single-blind, randomized trial to compare the efficacy of rhBNP with hydration in decreasing the incidence of CIN in patients undergoing elective CAG with or without concomitant PCI. All baseline demographic and clinical characteristics were taken before the procedure. Eligible participants were randomly allocated into two groups of intravenous infusions of either rhBNP (1.5 μg/kg bolus followed by an adjusted dose infusion of 0.01 μg/kg/min) or isotonic saline (0.9%) at 6 hours before and 12 hours after the procedure. SCr and creatinine clearance (CCl) were assayed before, at 24, 48 and 72 hours after the procedure respectively. Interventional procedures were performed using the radial artery approach according to standard clinical practice. The CCl was calculated using the Cockcroft-Gault formula. The study protocol was approved by the institutional ethics committee of Harbin Medical University. All patients gave informed written consent for participation in this study.
CIN was defined as an acute decline in renal function characterized by an absolute rise of 0.5 mg/dL (44.2 µmol/L) in SCr or a >25% increase from baseline to 48 hours after angiography. The primary end point of the study was the development of CIN within 72 hours after the procedure. The secondary end points were the changes in SCr and CCl.
Statistical analysis
All data were presented as percentages for categorical variables and mean ± standard deviations for continuous variables. Comparisons of baseline data were performed using the chi-square test and Student t-test or t’-test (continuous variables) as appropriate. Repeated measurement ANOVA were used to test significant differences among four time points of the SCr and CCl between the two groups. Multivariate logistic regression analysis was used to identify the independent predictors for CIN. Results were two-tailed, and a P<0.05 was considered to be statistically significant. Statistical analyses were performed with SPSS software (version 17.0).
Results
There were 153 patients screened for possible enrollment into the study. Of the patients screened, 27 were excluded (12 not meeting the inclusion criteria, 3 were on the basis of heart failure, 6 declined participation, 4 underwent primary angiography and 2 suffered from hypotension). Finally, 126 patients were enrolled in the study. There was no statistically significant difference in the baseline demographics between the two groups, which included age, gender, risk factors and clinical presentation. There was also no difference in the baseline laboratory results and medications used (Table 1), and baseline angiographic and procedural characteristics (Table 2) between the groups. In this study, no patient developed acute renal failure requiring dialysis; and no major adverse cardiac events (MACE) occurred defined as cardiac death, acute myocardial infarction (defined as >2 times upper limit of creatine kinase-MB levels, positive troponin I and appearance of new left bundle branch block and/or Q wave), or revascularization of the target lesion.
Table 1.
Baseline clinical and characteristics of patients
| rhBNP group (n=63) | Hydration group (n=63) | P value | |
|---|---|---|---|
| Age (years) | 59.35±9.01 | 60.37±9.26 | 0.534 |
| Gender | |||
| Male (%) | 38 (60.3) | 39 (61.9) | 0.855 |
| Female (%) | 25 (39.7) | 24 (38.1) | 0.855 |
| Body mass index (kg/m2) | 23.8 ± 3.7 | 24.1± 3.4 | 0.636 |
| Systolic blood pressure (mmHg) | 138.24±18.708 | 139.87±18.764 | 0.625 |
| Diastolic blood pressure (mmHg) | 85.73±11.261 | 87.35±11.597 | 0.428 |
| Hypertention (%) | 38 (60.3) | 41 (65.1) | 0.581 |
| Hyperlipemia (%) | 23 (36.5) | 23 (36.5) | 1.000 |
| Diabetes mellitus (%) | 13 (20.6) | 18 (28.6) | 0.301 |
| Smoking (%) | 19 (30.2) | 22 (34.9) | 0.568 |
| LVEF (%) | 61.81±3.12 | 61.51±2.97 | 0.580 |
| Durgs therapy | |||
| Calcium channel blocker (%) | 16 (25.4) | 24 (38.1) | 0.126 |
| β-Blocker (%) | 44 (69.8) | 49 (77.8) | 0.311 |
| Angiotensin converting enzyme inhibitor (%) | 23 (36.5) | 23 (36.5) | 1.000 |
rhBNP: Recombinant human brain natriuretic peptide; LVEF: Left ventricular ejection fraction.
Table 2.
Angiographic and procedural features
| rhBNP group | Hydration group | P value | |
|---|---|---|---|
| Cardiac angiographic procedure type | |||
| CAG (%) | 22 (34.9) | 18 (28.6) | 0.556 |
| PCI (%) | 41 (65.1) | 45 (71.4) | 0.444 |
| Coronary arterywith stenosis | |||
| Left main (%) | 2 (3.2) | 4 (6.3) | 0.680* |
| Left anterior descending (%) | 27 (42.9) | 24 (38.1) | 0.498 |
| Left circumflex (%) | 11 (17.5) | 9 (14.3) | 0.626 |
| Right coronary artery (%) | 13 (20.6) | 19 (30.2) | 0.302 |
| Vessels requiring intervention | |||
| 1-vessel (%) | 27 (42.9) | 34 (54.0) | 0.817 |
| 2-vessel (%) | 12 (19.0) | 9 (14.3) | 0.927 |
| 3-vessel (%) | 0 (0.00) | 2 (3.2) | 0.496* |
| 1-vessel + left main (%) | 0 (0.00) | 0 (0.00) | - |
| 2-vessel + left main (%) | 2 (3.2) | 3 (4.8) | 1.000* |
| 3-vessel + left main (%) | 0 (0.00) | 1 (1.6) | 1.000* |
| Lesion characteristics | |||
| Chronic total occlusion (%) | 2 (3.2) | 2 (3.2) | - |
| Bifurcation lesion (%) | 3 (4.8) | 4 (6.3) | 1.000* |
| Stents used per patient (n) | 0.87±0.83 | 0.84±0.60 | 0.805† |
| Type of medium | |||
| Iodixanol (%) | 28 (44.4) | 29 (46.0) | 0.858 |
| Iopromide (%) | 35 (55.6) | 34 (54.0) | |
| Total contrast dose (ml) | 145.56±75.86 | 149.36±73.28 | 0.775 |
| Catheter time (min) | 67.70±31.31 | 67.54±29.57 | 0.977 |
The P value was calculated by the exact probability method.
The P value was calculated by t’-test.
rhBNP: Recombinant human brain natriuretic peptide; CAG: Coronary angiography; PCI: Percutaneous coronary intervention.
There was no significant difference between the two groups in the occurrence of CIN (P=0.77), the study primary endpoint. There was a significant increase in SCr post-procedurally in both groups, with the peak occurring at 48 hours. The mean SCr concentration was similar in the two groups at baseline and 24 hours after the procedure (P=0.898, P=0.556 respectively). However, the SCr in the rhBNP group was significantly lower than that of the hydration group at 48 and 72 hours (82.98±22.82 μmol/L vs 91.00±21.94 μmol/L, P=0.047; 79.00±21.31 μmol/L vs 86.51±20.93 μmol/L, P=0.048 respectively) (Figure 1; Table 3). Repeat measurements using ANOVA technique showed that changes in the level of SCr after the procedure was lower in the rhBNP group when compared with the hydration group (F=56.218, P<0.001). In the hydration group, SCr tended to normalize to baseline level earlier than that of the rhBNP group.
Figure 1.
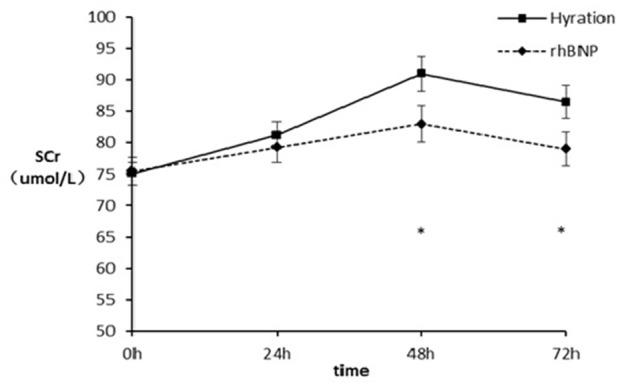
Changes in SCr level at different points. *P<0.05. SCr: serum creatinine; rhBNP: Recornb human brain natriuretic peptide.
Table 3.
Changes in serum creatinine (umol/L)
| Time | rhBNP (n=63) | Hydration (n=63) | P value |
|---|---|---|---|
| 0 h | 75.46±17.25 | 75.10±14.59 | 0.898 |
| 24 h | 79.27±19.49 | 81.19±16.96 | 0.556 |
| 48 h | 82.98±22.82 | 91.00±21.94 | 0.047* |
| 72 h | 79.00±21.31 | 86.51±20.93 | 0.048* |
Repeated measure ANOVA showed that there were significant difference among the mean change of serum creatinine of four time points (F=56.218, P<0.001). There was significant difference in the interactive effect between time and group (F=10.067, P<0.001). All results were corrected by using G-G.
P<0.05.
rhBNP: Recombinant human brain natriuretic peptide.
In both groups, the mean CCl decreased to nadir level at 48 hours after the procedure. The time-dependent CCl value from baseline to 72 hours after the procedure was higher in the BNP group when compared with the hydration group (F=64.340, P<0.001) (Figure 2; Table 4). There were significant differences at 48 hours (0.96±0.33 mL/min vs 0.83±0.25 mL/min, P=0.016) and 72 hours (1.01±0.40 mL/min vs 0.88±0.27 mL/min, P=0.019) between the two groups. Multivariate logistic regression analyses identified that smoking (OR 6.12, 95% CI 1.5 to 24.9, P=0.012) and aged >70 years old (OR 4.3, 95% CI 1.15 to 16.18, P=0.031) as significant risk factors for the development of CIN.
Figure 2.
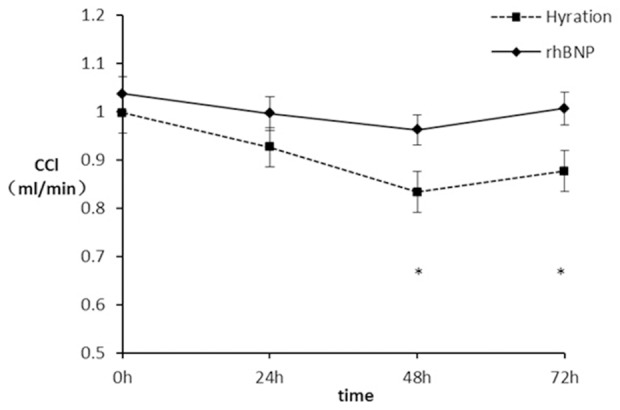
Changes in CCl level at different points. *P<0.05. CCl: creatinine clearance; rhBNP: Recombinant human brain natriuretic peptide.
Table 4.
Changes in creatinine clearance (ml/min)
| Time | rhBNP group | Hydration group | P value |
|---|---|---|---|
| 0 h | 1.04±0.33 | 1.00±0.29 | 0.476 |
| 24 h | 1.00±0.33 | 0.93±0.27 | 0.196 |
| 48 h | 0.96±0.33 | 0.83±0.25 | 0.016* |
| 72 h | 1.01±0.40 | 0.88±0.27 | 0.019* |
Repeated measure ANOVA showed that there were significant difference among the mean change of estimated glomerular filtration rate of four time points (F=64.340, P<0.001). There was significant difference in the interactive effect between time and group (F=13.305, P<0.001). All above results were corrected by using G-G.
P<0.05.
rhBNP: Recombinant human brain natriuretic peptide.
Discussion
To the best of our knowledge, this is the first study to compare rhBNP with saline hydration in preventing CIN in patients undergoing elective CAG and/or PCI. It showed that rhBNP is as effective as hydration in protecting the renal function of this group of patients.
Several renoprotective regimens have been used to reduce the incidence of CIN. These include intravenous volume expansion with isotonic saline or sodium bicarbonate [9-11], antioxidant therapy with N-acetylcysteine [12,13], ascorbic acid [14], hemofiltration [15] or dialysis [16], use of low- or iso-osmolality contrast agents [17,18] and rhBNP [8]. Periprocedural hydration remains the only measure of undisputed efficacy to date [11], while the results of other therapies have proven to be disappointing or inconclusive. Hydration acts by plasma volume expansion which contributes to the prevention of medullar hypoxia and reduces direct cellular damage [7,19,20].
As a cardiac hormone, BNP is secreted by the ventricles during state of pressure and volume overload. By relaxing renal mesangial cells, dilating the afferent renal arterioles and constricting the efferent renal arterioles, BNP appeared to be able to increase the glomerular filtration rate [21-25]. This results in a natriuretic effect, notably in patients with chronic heart failure. The probable mechanisms of renoprotection in the rhBNP group in our study include its dilating the juxtaglomerular capillaries selectively, inhibition of therenin-angiotensin-aldosterone system and vasopressin, down-regulation of tubuloglomerular feedback, increase in local prostaglandin synthesis, maintenance of adequate renal filling pressure, dilution of the contrast media, preventing renal vasoconstriction and tubular obstruction, and increasing eGFR [26,27].
In this study, although the difference of CIN between the two groups was not statistically significant (P=0.77), rhBNP had its advantages for the protection of the renal function. In comparing with the isotonic saline hydration, the renal function in patients who were treated by intravenous infusions rhBNP recovered sooner and better. The SCr were lower and the CCl levels were higher in the rhBNP group after the procedure, especially, at 48 (P=0.047, P=0.016) and 72 hours (P=0.048, P=0.019). There was definite difference in the temporal variation of SCr and CCl in the 2 groups, as shown in Figures 1, 2; Tables 3, 4, suggestive of better renal protective function in the rhBNP group. Previous studies had showed that baseline chronic renal insufficiency [3], diabetes mellitus [28], advanced age [29], congestive heart failure [30] and higher volume of contrast media [31] were predictors for CIN. However in our study, multivariate logistic regression analysis identified smoking and aged >70 years as being the 2 predictors for CIN in our population. The reasons for the difference in risk predictors could have been due to sampling bias or inherent differences in predictors in patients with normal heart function.
The POSEIDON trial, which was presented by Brar, claimed that the left ventricular end-diastolic pressure measurements (LVEDP) guided hydration strategy can evidently reduce the CIN occurrence comparing with the standard hydration strategy to the patients who were undergoing cardiac catheterization; however, at the present time, in many developing countries (including China), the routine hydration strategy is still the most widely used method. Therefore, the routine hydration strategy was still used in this study to give guidance to the current researchers in these countries that is still using this strategy.
There were limitations in our study. First, this was a single-center study designed to generate pilot data. The study will require validation in a larger, more ethnically diverse population. Secondly, more indices such as eGFR and serum cystatin C could be used to better evaluate renal function as recommended by the European Society for Urogenital Radiology [32,33]. Thirdly, SCr was measured serially up to only 72 hours [9], with no information regarding timing of final normalization to baseline level. Finally, the fluid rate could be adjusted according to the patients’ LVEDP in the hydration group.
Conclusions
While the overall incidence of CIN was similar between the two groups, rhBNP was observed to exhibit its advantages on the protection of the renal function when compared with saline hydration, as evident by its lower increment of SCr in patients undergoing coronary diagnostic or interventional procedures.
Disclosure of conflict of interest
None.
References
- 1.Bartholomew BA, Harjai KJ, Dukkipati S, Boura JA, Yerkey MW, Glazier S, Grines CL, O’Neill WW. Impact of nephropathy after percutaneous coronary intervention and a method for risk stratification. Am J Cardiol. 2004;93:1515–1519. doi: 10.1016/j.amjcard.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Mathew V, Garratt KN, Holmes DR Jr. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–2264. doi: 10.1161/01.cir.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 3.McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368–375. doi: 10.1016/s0002-9343(97)00150-2. [DOI] [PubMed] [Google Scholar]
- 4.Iakovou I, Dangas G, Lansky AJ, Mintz G, Ashby D, Losquadro M, Guiry M, Kipshidze N, Brandwein R, Limpijankit T, Forman J, Hjazi I, Stone GW, Leon MB, Mehran R. Incidence, predictors, and economic impact of contrast induced nephropathy: results in 8,628 patients treated with percutaneous coronary interventions. J Am Coll Cardiol. 2002;39:2–2. [Google Scholar]
- 5.Lindsay J, Canos DA, Apple S, Pinnow E, Aggrey GK, Pichard AD. Causes of acute renal dysfunction after percutaneous coronary intervention and comparison of late mortality rates with postprocedure rise of creatine kinase-MB versus rise of serum creatinine. Am J Cardiol. 2004;94:786–789. doi: 10.1016/j.amjcard.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180–184. doi: 10.1056/NEJM200007203430304. [DOI] [PubMed] [Google Scholar]
- 7.Thomsen HS. Guidelines for contrast media from the European Society of Urogenital Radiology. AJR Am J Roentgenol. 2003;181:1463–1471. doi: 10.2214/ajr.181.6.1811463. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Fu X, Jia X, Fan X, Gu X, Li S, Wu W, Fan W, Su J, Hao G, Jiang Y, Xue L. B-type natriuretic peptide for prevention of contrast-induced nephropathy in patients with heart failure undergoing primary percutaneous coronary intervention. Acta Radiol. 2010;51:641–648. doi: 10.3109/02841851.2010.486804. [DOI] [PubMed] [Google Scholar]
- 9.Merten GJ, Burgess WP, Gray LV, Holleman JH, Roush TS, Kowalchuk GJ, Bersin RM, Van Moore A, Simonton CA 3rd, Rittase RA, Norton HJ, Kennedy TP. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004;291:2328–2334. doi: 10.1001/jama.291.19.2328. [DOI] [PubMed] [Google Scholar]
- 10.Stacul F, Adam A, Becker CR, Davidson C, Lameire N, McCullough PA, Tumlin J, Panel CINCW. Strategies to reduce the risk of contrast-induced nephropathy. Am J Cardiol. 2006;98:59K–77K. doi: 10.1016/j.amjcard.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Mueller C, Buerkle G, Buettner HJ, Petersen J, Perruchoud AP, Eriksson U, Marsch S, Roskamm H. Prevention of contrast media-associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002;162:329–336. doi: 10.1001/archinte.162.3.329. [DOI] [PubMed] [Google Scholar]
- 12.Marenzi G, Assanelli E, Marana I, Lauri G, Campodonico J, Grazi M, De Metrio M, Galli S, Fabbiocchi F, Montorsi P, Veglia F, Bartorelli AL. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006;354:2773–2782. doi: 10.1056/NEJMoa054209. [DOI] [PubMed] [Google Scholar]
- 13.Kelly AM, Dwamena B, Cronin P, Bernstein SJ, Carlos RC. Meta-analysis: effectiveness of drugs for preventing contrast-induced nephropathy. Ann Intern Med. 2008;148:284–294. doi: 10.7326/0003-4819-148-4-200802190-00007. [DOI] [PubMed] [Google Scholar]
- 14.Spargias K, Alexopoulos E, Kyrzopoulos S, Iokovis P, Greenwood DC, Manginas A, Voudris V, Pavlides G, Buller CE, Kremastinos D, Cokkinos DV. Ascorbic acid prevents contrast-mediated nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Circulation. 2004;110:2837–2842. doi: 10.1161/01.CIR.0000146396.19081.73. [DOI] [PubMed] [Google Scholar]
- 15.Cruz DN, Perazella MA, Bellomo R, Corradi V, de Cal M, Kuang D, Ocampo C, Nalesso F, Ronco C. Extracorporeal blood purification therapies for prevention of radiocontrast-induced nephropathy: a systematic review. Am J Kidney Dis. 2006;48:361–371. doi: 10.1053/j.ajkd.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 16.Marenzi G, Lauri G, Campodonico J, Marana I, Assanelli E, De Metrio M, Grazi M, Veglia F, Fabbiocchi F, Montorsi P, Bartorelli AL. Comparison of two hemofiltration protocols for prevention of contrast-induced nephropathy in high-risk patients. Am J Med. 2006;119:155–162. doi: 10.1016/j.amjmed.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Reed M, Meier P, Tamhane UU, Welch KB, Moscucci M, Gurm HS. The relative renal safety of iodixanol compared with low-osmolar contrast media: a meta-analysis of randomized controlled trials. JACC Cardiovasc Interv. 2009;2:645–654. doi: 10.1016/j.jcin.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 18.From AM, Al Badarin FJ, McDonald FS, Bartholmai BJ, Cha SS, Rihal CS. Iodixanol versus low-osmolar contrast media for prevention of contrast induced nephropathy: meta-analysis of randomized, controlled trials. Circ Cardiovasc Interv. 2010;3:351–358. doi: 10.1161/CIRCINTERVENTIONS.109.917070. [DOI] [PubMed] [Google Scholar]
- 19.Trivedi HS, Moore H, Nasr S, Aggarwal K, Agrawal A, Goel P, Hewett J. A randomized prospective trial to assess the role of saline hydration on the development of contrast nephrotoxicity. Nephron Clin Pract. 2003;93:C29–34. doi: 10.1159/000066641. [DOI] [PubMed] [Google Scholar]
- 20.Weisbord SD, Palevsky PM. Prevention of contrast-induced nephropathy with volume expansion. Clin J Am Soc Nephrol. 2008;3:273–280. doi: 10.2215/CJN.02580607. [DOI] [PubMed] [Google Scholar]
- 21.Molina CR, Fowler MB, McCrory S, Peterson C, Myers BD, Schroeder JS, Murad F. Hemodynamic, renal and endocrine effects of atrial natriuretic peptide infusion in severe heart failure. J Am Coll Cardiol. 1988;12:175–186. doi: 10.1016/0735-1097(88)90371-3. [DOI] [PubMed] [Google Scholar]
- 22.Holmes SJ, Espiner EA, Richards AM, Yandle TG, Frampton C. Renal, endocrine, and hemodynamic effects of human brain natriuretic peptide in normal man. J Clin Endocrinol Metab. 1993;76:91–96. doi: 10.1210/jcem.76.1.8380606. [DOI] [PubMed] [Google Scholar]
- 23.Marcus LS, Hart D, Packer M, Yushak M, Medina N, Danziger RS, Heitjan DF, Katz SD. Hemodynamic and renal excretory effects of human brain natriuretic peptide infusion in patients with congestive heart failure. A double-blind, placebo-controlled, randomized crossover trial. Circulation. 1996;94:3184–3189. doi: 10.1161/01.cir.94.12.3184. [DOI] [PubMed] [Google Scholar]
- 24.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 25.Brunner-La Rocca HP, Kaye DM, Woods RL, Hastings J, Esler MD. Effects of intravenous brain natriuretic peptide on regional sympathetic activity in patients with chronic heart failure as compared with healthy control subjects. J Am Coll Cardiol. 2001;37:1221–1227. doi: 10.1016/s0735-1097(01)01172-x. [DOI] [PubMed] [Google Scholar]
- 26.Jensen KT, Carstens J, Pedersen EB. Effect of BNP on renal hemodynamics, tubular function and vasoactive hormones in humans. Am J Physiol. 1998;274:F63–72. doi: 10.1152/ajprenal.1998.274.1.F63. [DOI] [PubMed] [Google Scholar]
- 27.Burger AJ. A review of the renal and neurohormonal effects of B-type natriuretic peptide. Congest Heart Fail. 2005;11:30–38. doi: 10.1111/j.1527-5299.2005.03794.x. [DOI] [PubMed] [Google Scholar]
- 28.Toprak O, Cirit M, Yesil M, Bayata S, Tanrisev M, Varol U, Ersoy R, Esi E. Impact of diabetic and pre-diabetic state on development of contrast-induced nephropathy in patients with chronic kidney disease. Nephrol Dial Transplant. 2007;22:819–826. doi: 10.1093/ndt/gfl636. [DOI] [PubMed] [Google Scholar]
- 29.Rich MW, Crecelius CA. Incidence, risk factors, and clinical course of acute renal insufficiency after cardiac catheterization in patients 70 years of age or older. A prospective study. Arch Intern Med. 1990;150:1237–1242. [PubMed] [Google Scholar]
- 30.Dangas G, Iakovou I, Nikolsky E, Aymong ED, Mintz GS, Kipshidze NN, Lansky AJ, Moussa I, Stone GW, Moses JW, Leon MB, Mehran R. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005;95:13–19. doi: 10.1016/j.amjcard.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 31.Freeman RV, O’Donnell M, Share D, Meengs WL, Kline-Rogers E, Clark VL, DeFranco AC, Eagle KA, McGinnity JG, Patel K, Maxwell-Eward A, Bondie D, Moscucci M Blue Cross-Blue Shield of Michigan Cardiovascular Consortium (BMC2) Nephropathy requiring dialysis after percutaneous coronary intervention and the critical role of an adjusted contrast dose. Am J Cardiol. 2002;90:1068–1073. doi: 10.1016/s0002-9149(02)02771-6. [DOI] [PubMed] [Google Scholar]
- 32.Ishibashi Y, Yamauchi M, Musha H, Mikami T, Kawasaki K, Miyake F. Impact of contrast-induced nephropathy and cardiovascular events by serum cystatin C in renal insufficiency patients undergoing cardiac catheterization. Angiology. 2010;61:724–730. doi: 10.1177/0003319710379108. [DOI] [PubMed] [Google Scholar]
- 33.Filler G, Bökenkamp A, Hofmann W, Le Bricon T, Martínez-Brú C, Grubb A. Cystatin C as a marker of GFR--history, indications, and future research. Clin Biochem. 2005;38:1–8. doi: 10.1016/j.clinbiochem.2004.09.025. [DOI] [PubMed] [Google Scholar]


