Abstract
We reported a case of hepatic cancer patient with Guillain-Barré syndrome during the perioperative period of partial hepatectomy in the present study. We analyzed the clinical data and described the characteristics of this patient.
Keywords: Hepatic cancer, perioperative period, guillain-barré syndrome
Introduction
Guillain-Barré syndrome (GBS) is a demyelinating polyneuropathy of probable autoimmune pathogenesis characterized by rapidly progressive symmetric paralysis. Although the precise pathogenesis of this disease remains largely unknown, GBS is believed to result from previous bacterial or viral infections [1]. Significant antecedent events include Campylobacter jejuni (4-66%), cytomegalovirus (5-15%), Epstein-Barr virus (2-10%) and Mycoplasma pneumoniae (1-5%) infections. Recently, case reports of surgery patients suffering from GBS have accumulated, and a retrospective study suggested that surgery may increase the risk for GBS [2,3]. Furthermore, cancer patients with GBS have attracted increasing attention [4,5]. However, whether combination of cancer and surgery may constitute a new GBS trigger is not known. Here we present a case of hepatic cancer presenting with GBS just after partial hepatectomy.
Case report
The study protocol was approved by the Ethics Committees of the first affiliated hospital Guangxi medical university, Nanning (No. 2013-KY-108), and all participants provided written informed consent.
A 54 years old man was admitted to the hospital with a space occupying lesion found in his liver during a routine computer tomography (CT) examination. The patient had no complaint, trauma or history of surgery. He had a history of alcoholism for twenty years. Before the surgery, a lesion of 2.2×2.3×2.4 cm in the liver was confirmed by conventional and enhanced magnetic resonance imaging (Figure 1); small hepatic cancer was highly suspected, according to a guideline for the treatment of liver cancer [6]. Blood test showed 3.17 ng/ml alpha fetal protein (reference range: 0.00-11.00 ng/ml), 7.36 ng/ml carcinoembryonic antigen (CEA) (reference range: 0.00-5.00 ng/ml), 5.60 U/ml cancer antigen (CA) 125 (reference range: 0.00-35.00 U/ml), 20.70 U/ml CA153 (reference range: 0.00-31.30 U/ml), 38.39 U/ml CA199 (reference range: 0.00-37.00 U/ml), and 543.71 ng/ml ferritin (reference range: 21.80-247.60 ng/ml). Serum urea electrolytes, complete blood count, protein electrophoresis, head magnetic resonance scan, and chest CT scan and ultrasonic inspection for kidneys, spleen and bladder were all normal. Under general anesthesia, partial hepatectomy was carried out successfully with 600 ml blood loss and normality of heart rate, blood pressure, and arterial oxygen saturation within the procedure. The tumor in the liver tissue was found to be 3×3×2.5 cm, and highly differentiated hepatocellular carcinoma was determined by subsequent pathologic examination. The patient recovered from anesthesia and expectable anal exhaust was obtained. However, at day 4 in the afternoon following the operation, he felt a sudden weakening of his legs, which worsened the next morning (day 5); in addition, his upper limbs became weak as well at day 5 and he had trouble speaking. During breakfast, he had difficulty swallowing and experienced severe choking cough before entering a coma. He was found with cardiopulmonary arrest and received emergency treatment, including external chest compression, high concentration of oxygen, trachea cannula, elimination of food debris with fiber bronchoscope, and assisted breathing with a ventilator. He successfully recovered within fifteen minute and needed no ventilator an hour later. Afterward, a nervous system examination showed bilateral peripheral facial paralysis, dysarthria, dysphagia, pharyngeal reflex disappearance, four limb muscular loosening and symmetric paralysis at the point that he could only raise his limbs off the bed, and areflexia with pain; temperature sensory was normal. Spinal fluid examination showed increased protein level (1207.70 mmol/L), pressure (143 mmH2O), glucose amount (5.10 mmol/L), chloride concentration (145.60 mmol/L) and white blood cell number (7×106/L). Nerve conduction analyses of right median, ulnar, peroneal, and tibial nerves performed on days 9 and 14 after the operation revealed a demyelinating pattern without axonal involvement that progressively worsened with time. Increased distal motor latencies, reduced evoked motor amplitude and loss of F-waves ensued; conduction velocity remained relatively unaffected. The patient received a systemic treatment including IVIG, at a total dose of 2.0 mg/kg given as a 5-day treatment course (0.4 mg/kg per day of 6% IVIG), and other supporting therapies. The patient recovered slowly, could swallow soft food freely and facial droop improved at 11 days. At 21 days after the operation, he was discharged from the hospital.
Figure 1.
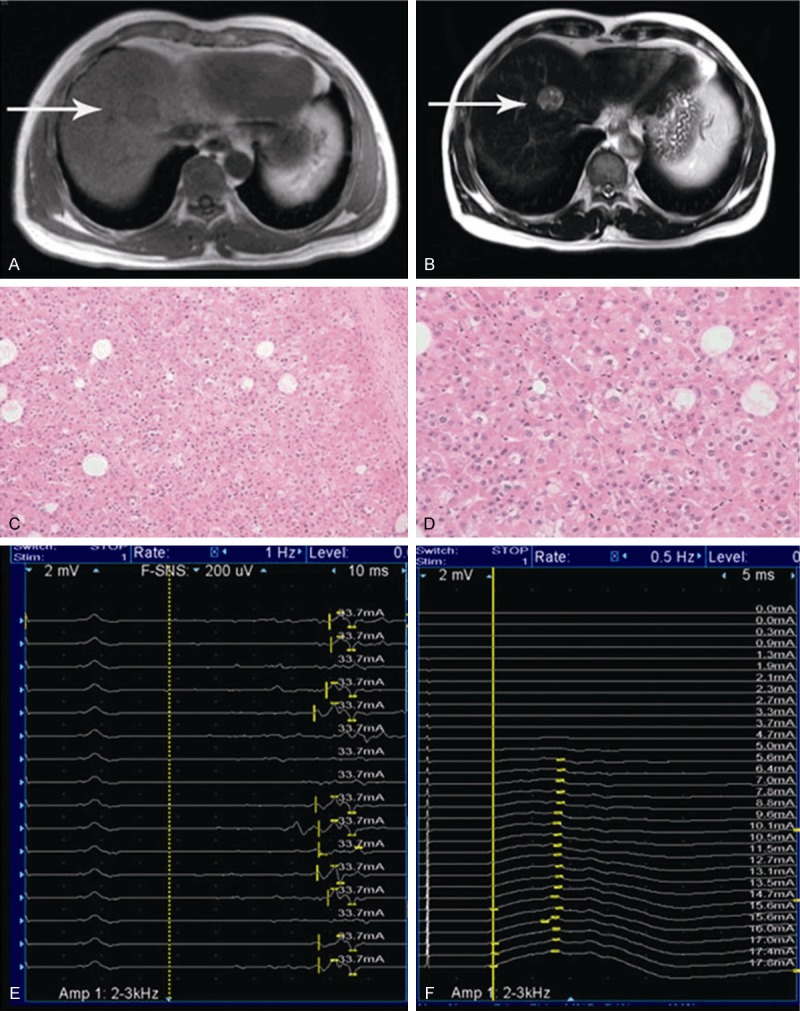
Selected liver MRI photographs of the patient (A and B). Pictures A (T1) and B (T2) showed a circular space occupying lesion on the right side of the liver (white arrows). Pictures C (×200) and D (×400) were selected from pathological examination showing hepatic cancer cells. Pictures E and F disappearance of the F wave of tibial neve latency.
Discussion
The patient’s pathological examination revealed hepatic cancer cell; his clinical GBS features included bilateral peripheral facial paralysis, weakening of all four limbs with areflexia, cytoalbuminologic dissociation in cerebral spinal, demyelinating polyneuropathy revealed by nerve conduction studies. Therefore, this patient was diagnosed with hepatic cancer and GBS.
The pathogenesis of GBS is unknown, but the ailment is generally believed to result from aberrant humoral and cellular immune response directed against components of the peripheral nervous system. This acute, inflammatory, demyelinating polyneuropathy may be most frequently triggered by virus and bacterial infections or immunizations. However, patients with malignancies suffering from GBS have been described for years. Myers and Williams [7] first reported a breast cancer patient with GBS following autologous bone marrow transplantation in 1994. Since then, multiple colon, rectal, pancreatic, bladder, lung and endometrial cancer patients have been described with GBS [8-13]. To our knowledge, the present study is the first describing a patient with hepatic cancer and GBS. The association between cancer and GBS has attracted increasing attention. To better understand the possible relationship between GBS and malignancy, Vigliani and his colleagues [14] carried out a retrospective population-based cohort study; they found that compared to the general population, the risk for cancer patients to have GBS increased 2.37-2.43 times. However, the role of cancer in GBS remains unknown. It is suggested that cancer plays a role in patients to trigger GBS.
Surgery is another probable risk factor for GBS. As early as 1972, a patient suffering from GBS was reported just after mandibular surgery [15]. Up to now, GBS is known to occur following coronary artery bypass surgery, cranial surgery, colorectal cancer surgery and thoracic spine surgery [16-18]. In addition, surgery stress is regarded as potential GBS trigger. Gensicke and his colleagues reported that surgery increases the incidence of GBS in patients: the relative risk of developing GBS during the initial 6-week period after surgery was 13.1 times higher compared to the normal incidence obtained in the study population. Although it is controversial whether surgery could increase GBS incidence, it is a definite challenge when GBS occurs during the perioperative period [17-19]. The present patient suffered from limb weakening 4 days after partial hepatectomy, which rapidly worsened the following day; he also showed facial paralysis, dysarthria, and dysphagia. In addition, his life was threatened by suffocation.
Cancer and surgery may trigger GBS, respectively; more attention should be paid that combination of surgical stress and cancer may trigger GBS, especially during the perioperative period.
Acknowledgements
This work was supported by National Natural Science Foundation of China (NSFC NO. 30860088 and 81260186 to Z.L.) and Guangxi Natural Science Foundation (GNSF No. 0832134, 0991149 to Z.L.).
Disclosure of conflict of interest
None.
References
- 1.Winer JB. An update in guillain-barré syndrome. Autoimmune Dis. 2014;2014:793024. doi: 10.1155/2014/793024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gensicke H, Datta AN, Dill P, Schindler C, Fischer D. Increased incidence of Guillain-Barré syndrome after surgery. Eur J Neurol. 2012;19:1239–1244. doi: 10.1111/j.1468-1331.2012.03730.x. [DOI] [PubMed] [Google Scholar]
- 3.Algahtani H, Moulin DE, Bolton CF, Abulaban AA. Guillain-Barre syndrome following cardiac surgery. Difficult diagnosis in the intensive care unit. Neurosciences (Riyadh) 2009;14:374–378. [PubMed] [Google Scholar]
- 4.Vatandoust S, Joshi R, Price TJ. Guillain-Barre syndrome in colorectal cancer. Asia Pac J Clin Oncol. 2012;8:205–208. doi: 10.1111/j.1743-7563.2011.01493.x. [DOI] [PubMed] [Google Scholar]
- 5.Cicero G, Fulfaro F, Caraceni A, Arcara C, Badalamenti G, Intrivici C, Gebbia N. A case of Guillain-Barré syndrome in a patient with non small cell lung cancer treated with chemotherapy. J Chemother. 2006;18:325–327. doi: 10.1179/joc.2006.18.3.325. [DOI] [PubMed] [Google Scholar]
- 6.Giammarile F, Bodei L, Chiesa C, Flux G, Forrer F, Kraeber-Bodere F, Brans B, Lambert B, Konijnenberg M, Borson-Chazot F, Tennvall J, Luster M. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur J Nucl Med Mol Imaging. 2011;38:1393–1406. doi: 10.1007/s00259-011-1812-2. [DOI] [PubMed] [Google Scholar]
- 7.Myers SE, Williams SF. Guillain-Barré syndrome after autologous bone marrow transplantation for breast cancer: report of two cases. Bone Marrow Transplant. 1994;13:341–344. [PubMed] [Google Scholar]
- 8.Vatandoust S, Joshi R, Price TJ. Guillain-Barre syndrome in colorectal cancer. Asia Pac J Clin Oncol. 2012;8:205–208. doi: 10.1111/j.1743-7563.2011.01493.x. [DOI] [PubMed] [Google Scholar]
- 9.Lagrange E, Veran O, Besson G. Pure motor relapsing Guillain-Barré syndrome associated with anti-GM1 antibodies revealing urinary bladder cancer. Eur J Neurol. 2007;14:e7. doi: 10.1111/j.1468-1331.2007.01990.x. [DOI] [PubMed] [Google Scholar]
- 10.Cicero G, Fulfaro F, Caraceni A, Arcara C, Badalamenti G, Intrivici C, Gebbia N. A case of Guillain-Barré syndrome in a patient with non small cell lung cancer treated with chemotherapy. J Chemother. 2006;18:325–327. doi: 10.1179/joc.2006.18.3.325. [DOI] [PubMed] [Google Scholar]
- 11.Tho LM, O’Leary CP, Horrocks I, Al-Ani A, Reed NS. Guillain-Barre syndrome occurring after adjuvant chemo-radiotherapy for endometrial cancer. Gynecol Oncol. 2006;100:615–617. doi: 10.1016/j.ygyno.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Bamberger PD, Thys DM. Guillain-Barré syndrome in a patient with pancreatic cancer after an epidural-general anesthetic. Anesth Analg. 2005;100:1197–1199. doi: 10.1213/01.ANE.0000144826.77316.ED. [DOI] [PubMed] [Google Scholar]
- 13.Christodoulou C, Anastasopoulos D, Visvikis A, Mellou S, Detsi I, Tsiakalos G, Pateli A, Klouvas G, Papadimitriou A, Skarlos DV. Guillain-Barré syndrome in a patient with metastatic colon cancer receiving oxaliplatin-based chemotherapy. Anticancer Drugs. 2004;15:997–999. doi: 10.1097/00001813-200411000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Vigliani MC, Magistrello M, Polo P, Mutani R, Chiò A. Piemonte and Valle d’Aosta Register for Guillain-Barré Syndrome. Risk of cancer in patients with Guillain-Barré syndrome (GBS). A population-based study. J Neurol. 2004;251:321–326. doi: 10.1007/s00415-004-0317-3. [DOI] [PubMed] [Google Scholar]
- 15.Shuert GT, Gamble JW. Guillain-Barré syndrome after mandibular surgery: report of case. J Oral Surg. 1972;30:913–915. [PubMed] [Google Scholar]
- 16.Renlund DG, Hanley DF, Traill TA. Guillain-Barré syndrome following coronary artery bypass surgery. Am Heart J. 1987;113:844–845. doi: 10.1016/0002-8703(87)90736-8. [DOI] [PubMed] [Google Scholar]
- 17.Parobeck V, Burnham S, Laukhuf GA. An unusual nursing challenge: Guillain-Barré syndrome following cranial surgery. J Neurosci Nurs. 1992;24:251–255. doi: 10.1097/01376517-199210000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Koc M, Ozalp N, Zulfikaroglu B. Major surgery with Guillain-Barré syndrome: a case report. J Int Med Res. 2002;30:601–604. doi: 10.1177/147323000203000609. [DOI] [PubMed] [Google Scholar]
- 19.Cheng J, Kahn DE, Wang MY. The acute motor-sensory axonal neuropathy variant of Guillain-Barré syndrome after thoracic spine surgery. J Neurosurg Spine. 2011;15:605–609. doi: 10.3171/2011.8.SPINE1159. [DOI] [PubMed] [Google Scholar]


